Abstract
Boceprevir was the first agent, along with telaprevir, of a novel class of direct-acting antivirals that entered clinical practice for the treatment of chronic hepatitis C. Boceprevir is an antiprotease that directly blocks hepatitis C virus (HCV) replication. Two studies in patients with HCV genotype 1 infection have shown that addition of boceprevir to the standard of care, ie, pegylated interferon-alfa (PEG-IFN-α) and ribavirin, markedly increased the rate of sustained virological response. A sustained virological response was obtained in about 70% of patients who had never been treated, as well as in 69%–75% and 40% of previous relapsers and nonresponders to PEG-IFN-α-ribavirin, respectively. Side effects were observed in almost all treated patients. Anemia, the most frequent adverse event related to administration of boceprevir, occurred in about 50% of patients. The decision to add boceprevir to the standard of care is made on an individual basis, and takes into account the prognosis of the liver disease, the efficacy of therapy, as it could be at best predicted, and the side effects that may arise, taking into account the comorbidities of the patient. Ultimately, the treatment must be accepted by the patient, who should fully understand the benefits and risks. Boceprevir trials were designed with the concept of individualized and response-guided therapy which establishes treatment decisions on how rapidly patients respond to treatment. Individualized therapy for chronic hepatitis C is based on patient and viral characteristics to make the best choice about whether a person will benefit from therapy and to evaluate on-treatment predictors of response to shorten therapy in patients with a rapid response as well as in patients who did not respond sufficiently to expect HCV eradication. This review focuses on the main results obtained so far, their impact on the treatment of patients with chronic hepatitis C, and potential therapeutic perspectives.
History of HCV infection and antiviral therapy
Hepatitis C virus (HCV) was identified by Choo et al in 1989 ().Citation1 HCV infection is a major health problem and a leading cause of liver disease. More than 140–170 million people worldwide are chronically infected.Citation2,Citation3 In the United States, it is estimated that 1.3% or 3.2 million people have chronic HCV infection.Citation4 HCV is an enveloped hepatotropic, positive-stranded RNA virus of approximately 9.6 kb. HCV is highly variable and six major genotypes have been described.Citation5 Hepatitis C is transmitted primarily by the parenteral route, and sources of infection include injection drug use, and transfusions of blood or blood-derived products. The virus infects liver cells and can cause acute hepatitis, with severe inflammation of the liver and long-term complications when infection persists. In fact, in the majority (70%–80%) of people, HCV infection persists, leading to chronic hepatic infection that can progress to cirrhosis and liver cancer.Citation2,Citation6 In patients with chronic HCV infection, the risk of developing cirrhosis ranges from 5% to 25% over periods of 25–30 years. Patients with cirrhosis have increased risk of hepatic decompensation (30% over 10 years) and hepatocellular carcinoma (1%–3% per year).Citation7 So far, there is no vaccine to prevent infection.
Figure 1 Milestones in HCV research and Direct-Acting Antivirals development.
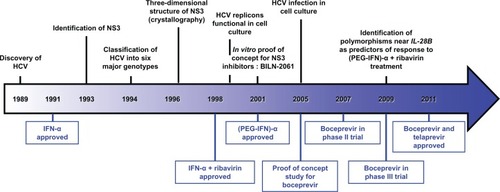
The goal of anti-HCV therapy is to prevent complications from HCV infection and death.Citation7 Milestones in HCV research and therapy are represented in . Interferon-alfa (IFN-α) was first shown in 1986 to have beneficial effects for the treatment of chronic non A-non B hepatitis as indicated by improvement in alanine transferase values and liver histology.Citation8 It was shown later that these beneficial effects were associated with a decrease in serum HCV RNA and that a long-term response was associated with sustained undetectability of serum HCV RNA. Following these initial results, it has been shown that a sustained virological response, ie, disappearance of serum HCV RNA during treatment and maintenance of the response 6 months after discontinuation of treatment, was associated with improvement in liver damage with a decrease in inflammation and hepatic fibrosis as assessed by serial liver biopsies.Citation9 Because of the slow evolution of chronic HCV infection over several decades, it has been difficult to demonstrate that treatment improves survival, although retrospective and uncontrolled studies have suggested a benefit.Citation10,Citation11 IFN-α does not act directly on HCV itself but exerts its antiviral activity through a cell membrane receptor, thereby activating IFN-stimulating genes that in turn will have intracellular antiviral effects.Citation12 It is unclear whether IFN-α, through its immunomodulatory properties, accelerates the clearance of infected cells at the same time as it inhibits viral replication.Citation13 Another major advance in the treatment of chronic HCV infection was highlighting of the increased efficacy of the combination of IFN-α and ribavirin, a nucleotide analog with a broad spectrum of activity against RNA and DNA viruses, compared with IFN-α as monotherapy. Like IFN-α, ribavirin was used empirically in 1991 for the treatment of chronic hepatitis C.Citation14 Initially, a reduction in alanine transferase values during ribavirin therapy was interpreted as a beneficial effect, but subsequent studies did not show a decrease in HCV RNA in treated patients.Citation15 Nevertheless, a study combining IFN-α and ribavirin was performed in patients with chronic hepatitis C. This study showed significantly higher efficacy of the combination therapy compared with IFN-α monotherapy.Citation16 These results were thereafter confirmed, amending the care of patients with chronic hepatitis C.Citation17 The results of combination therapy were subsequently improved by pegylation of IFN-α molecules in order to improve their pharmacokinetic and pharmacodynamic properties and to enhance their efficacy.Citation13 Since 2001, the standard of care for the treatment of chronic hepatitis C from all genotypes combines pegylated IFN-α (PEG-IFN-α) and ribavirin. While a sustained virological response can be achieved in 70%–90% of patients infected with genotype 2 or 3, only about 50% of patients infected with genotype 1 or 4 achieve a sustained virological response after standard of care therapy.Citation7 Thus, novel therapeutic strategies are urgently needed. Recent efforts to improve patient outcomes have mainly focused on antiviral therapy targeting virally encoded proteins or direct-acting antiviral agents.
Development of direct-acting antiviral agents
HCV is difficult to grow in cell culture, and the absence of robust cell culture models has long hampered the development of novel antivirals. The development of the replicon model in 1999Citation18 allowed significant progress to be made in the understanding of the mechanism of HCV replication, and thereby enabled development of specific antivirals with a direct action on HCV. After its entry into the hepatocyte, the HCV RNA genome serves as a template for cap-independent translation through its 5′ internal ribosome entry site. The resulting 3000 amino acid polyprotein undergoes proteolytic maturation by host-encoded and virally-encoded proteases, giving rise to three structural proteins (core, E1, and E2), the viroprotein p7, and six nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B), as reviewed by Lindenbach and Rice.Citation19 The nonstructural proteins coordinate the intracellular processes of the life cycle of the virus. The NS3 protease, located in the N-terminal third of the NS3 protein, forms a heterodimeric complex with the NS4A protein, a cofactor essential for the activity of NS3. NS3 encodes a chymotrypsin-like serine protease that is responsible for the cleavage of the NS3/4A, NS4A/4B, NS4B/5A, and NS5A/5B junctions.Citation19 Therefore, the RNA helicase/protease NS3 plays a central role in the RNA replication of HCV, and thus appears to be an important drug target for the treatment of HCV infection. Given the knowledge gained from the development of drugs to treat the human immunodeficiency virus (HIV) infection, research has been focused on the development of drugs inhibiting the HCV NS3 protease with the promise to block viral replication. Characterization of the atomic structure of NS3 in 1996 provided the necessary detailed insight to allow the design of NS3 inhibitors.Citation20,Citation21 BILN-2061 was the first NS3 protease inhibitor investigated in clinical studies that showed a marked antiviral effect in genotype 1-infected patients after two days of therapy.Citation22 However, further development of BILN-2061 was stopped due to cardiac toxicity in animals. In recent years, several other NS3 protease inhibitors have been designed and assessed in clinical trials, including boceprevir and telaprevir.
Boceprevir, a direct-acting antiviral for chronic HCV infection
Boceprevir and telaprevir were the first NS3 protease inhibitors that allowed significant improvement in the treatment of chronic hepatitis C. Boceprevir is a carboxamide-based oral HCV NS3/4A genotype 1 protease inhibitor.Citation23 Boceprevir provides effective inhibition by formation of a stable, covalent, and reversible complex with the viral enzyme. Initial evaluation of boceprevir was assessed in the HCV subgenomic replicon system. Continuous exposure of replicon-bearing cell lines to boceprevir for 15 days resulted in a 1.5-log to 2-log decline in RNA levels at 72 hours and a 3.5-log to 4-log reduction by day 15.Citation23 The combination of boceprevir with IFN-α was more effective in suppressing HCV replication than either compound alone indicating synergy between these two antivirals.Citation23 Furthermore, in this system, no toxicity towards hepatoma cells was observed. These promising in vitro data enabled boceprevir to enter clinical development.
Pharmacokinetics and drug interactions
Absorption and metabolism
Few pharmacokinetic data are available for boceprevir except those provided by the manufacturer (Merck, Whitehouse Station, NJ).Citation24 Boceprevir is an orally bioavail-able molecule with a short half-life of 3.4 hours, requiring its administration three times a day. After administration, the drug is rapidly absorbed, with a maximal concentration reached in approximately 2 hours. Liver impairment has been shown to be related to a maximum concentration increase of 28% and 62% in patients with moderate (Child-Pugh B) and severe hepatic failure (Child-Pugh C), respectively, in comparison with normal subjects.Citation24 End-stage renal disease requiring hemodialysis is associated with a mild decrease in the area under the concentration-time curve (AUC).Citation24 Food enhances absorption by up to 60% without effect of meal type or timing in comparison with food intake (high-fat or low-fat).Citation24 Neither gender, race, nor age (19–65 years) seem to have an impact on the pharmacokinetics of boceprevir.Citation24 An increasing trend of anemia with an increasing boce-previr AUC has been reported.Citation25 Boceprevir is primarily metabolized by the aldoketoreductase-mediated pathway to inactive ketone metabolites. Boceprevir is also metabolized by cytochrome P450 (CYP)3A4/5, which allows formation of oxidative metabolites, and is in addition an inhibitor of this enzyme.Citation26 Moreover, boceprevir is a substrate and inhibitor of the drug transporter, P-glycoprotein.Citation24
Drug interactions
Drug interactions may increase drug toxicity or decrease drug effectiveness (). Sixty percent of medications are metabolized by CYP3A. As a consequence, there are many interactions to consider with boceprevir.Citation27 CYP3A inducers, such as rifampicin, strongly reduce boceprevir exposure and therefore could be responsible for treatment failure. Furthermore, boceprevir concentration can be strongly increased by CYP3A inhibitors, such as ketoconazole, resulting in enhancement of boceprevir-mediated adverse effects. In treatment of HIV, ritonavir is used to inhibit CYP3A-mediated metabolism of other HIV protease inhibitors in order to increase their exposure to achieve a prolonged therapeutic effect. This strategy has been tested for boceprevir but unfortunately failed.Citation28 Because boceprevir is metabolized by CYP3A4/5 but is also a strong inhibitor of this cytochrome, the administration of drugs metabolized by CYP3A4/5 (eg, midazolam, a sedative drug) in association with boceprevir could prolong their therapeutic and/or adverse effects.Citation24
Table 1 Example of drug interactions with boceprevir and side effects
Drug interactions between boceprevir and antiretroviral therapy remain to be investigated, given the high proportion of patients coinfected with HCV and HIV. Among the antiretroviral drugs, it has been shown that efavirenz reduces boceprevir concentrations, while tenofovir slightly increases its concentrations. In HCV-infected patients undergoing liver transplantation, the combination of boceprevir and immunosuppressive drugs has to be tightly controlled. Indeed, boceprevir slows down the clearance of cyclosporine A and tacrolimus, but neither of these immunosuppressive drugs has an effect on the metabolism of boceprevir. Furthermore, other classes of drugs, such as oral contraceptives (eg, drosperinone), antidepressants (eg, escitalopram), or corticosteroids (eg, dexamethasone) have to be avoided or used with caution because drug interactions with boceprevir have been identified.Citation24,Citation28 Thus, a careful evaluation of drugs administered to patients who are likely to be treated with boceprevir is very important to avoid side effects, reduced efficacy of antiviral therapy, or another medication that will have its effectiveness reduced if its metabolism is impaired ().
Clinical development
Initial phase I and II studies
In 2005, a proof-of-concept, dose-escalating study over 14 days showed that boceprevir monotherapy had a dose-related antiviral effect in patients with the HCV genotype 1 who did not respond to previous IFN-α therapy.Citation29 However, subsequent emergence of HCV-resistant strains in clinical trials using boceprevir alone limited the use of this direct-acting antiviral agent as monotherapy.Citation30,Citation31 Indeed, the rapid viral kinetics and quasispecies distribution of HCV allowed emergence of resistant viral strains during antiviral therapy.Citation32 In a further Phase I study in HCV-infected patients who were nonresponsive to PEG-IFN-α, the combination of boceprevir and PEG-IFN-α resulted in a more important reduction in HCV viral load in comparison with boceprevir or PEG-IFN-α administered as monotherapy.Citation33 Phase II studies were then conducted in treatment naïve-patients and in nonresponders to the standard of care combining PEG-IFN-α and ribavirin. In treatment-naive patients, a Phase II study (n = 595) combined boceprevir with the standard of care in patients with HCV genotype 1 infection under several modalities. This study compared triple therapy of variable duration preceded or not preceded by 4 weeks of pretreatment with PEG-IFN-α-ribavirin. In addition, a group of patients received triple therapy with low doses of ribavirin to improve the tolerability of treatment. The highest sustained virological response rate was observed in patients receiving 44 weeks of triple therapy preceded by a 4-week standard of care (lead-in period). This regimen increased the treatment efficacy by two-fold as compared with patients receiving the standard of care. Indeed, 38% versus 75% of patients had a sustained virological response with double therapy and triple therapy, respectively.Citation34 A placebo-controlled randomized Phase II study was also conducted in HCV-infected patients who were nonresponders to previous standard of care therapy. In this trial, low doses of boceprevir were administered and the study included arms without ribavirin. The results showed only a slight increase in sustained virological response in 7%–14% of patients treated with boceprevir and PEG-IFN-α-ribavirin compared with 2% in the control group,Citation35 indicating the lack of efficacy of low-dose boceprevir in combination with standard of care. These results showed that, in these difficult-to-treat patients, boceprevir should be administered at higher doses, in combination with standard care at an optimal dosage to achieve optimal results.
Clinical development in Phase III studies
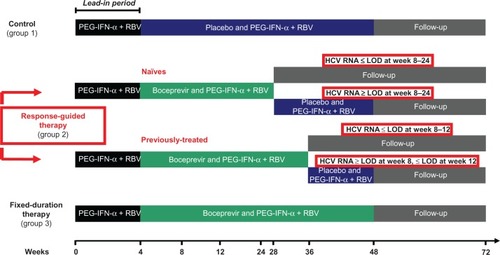
The efficacy and tolerance of boceprevir were studied in two Phase III studies in combination with PEG-IFN-α-ribavirin for treatment of chronic hepatitis C in genotype 1-infected patients. These Phase III trials were either conducted in treatment-naïve subjects, ie, the SPRINT-2 (Serine Protease Inhibitor Therapy-2) study, or in subjects who had previously failed PEG-IFN-α-ribavirin therapy, ie, the HCV RESPOND-2 (Retreatment with HCV Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol 2) study. Inclusion and exclusion criteria were the same in both studies, and exclusion criteria comprised in particular HIV infection, decompensated cirrhosis, hepatocellular carcinoma, chronic hepatitis B, and renal insufficiency.Citation36,Citation37 In both trials, the primary endpoint was sustained virological response, measured 24 weeks after the end of therapy. Plasma HCV RNA levels were measured using the TaqMan 2.0 assay, the lower limits of quantification and detection of which are 25 IU/mL and 9.3 IU/mL, respectively; the lower limit of detection was used for decision-making at various points during the study.Citation36,Citation37 The results of the two Phase III trials were published at the same time in March 2011. The treatment regimen was identical in both studies, except for the treatment duration of triple therapy that was longer in patients previously treated with standard of care than in treatment naïve-patients (). In both trials, PEG-IFN-α was administered subcutaneously at a dose of 1.5 μg/kg of body weight once weekly, and weight-based oral ribavirin was administered as 600–1400 mg/day in two divided doses. Treatment with boceprevir consisted of oral administration of 800 mg three times daily in four capsules of 200 mg each, to be taken every 7–9 hours with a light meal.
Figure 2 Phase III trials design.
Abbreviations: HCV, hepatitis C virus; PEG-IFN-α, pegylated interferon-alpha; RBV, ribavirin; LOD, limit of detection.
Efficacy in treatment-naive patients
The treatment-naïve patient trial (SPRINT-2) was a double-blind study in which previously untreated adult patients with HCV genotype 1 infection were randomly assigned to one of three groups (). In order to enroll more black subjects, two separate population cohorts were formed which enrolled nonblack and black subjects. The patients were randomized to one of the three treatment groups after stratification according to baseline HCV RNA levels and HCV genotype 1 subtypes 1a and 1b. All patients received PEG-IFN-α during the 4-week lead-in period (). Subsequently, the control group received placebo and PEG-IFN-α-ribavirin for 44 weeks; group 2 (response-guided therapy) received boceprevir and PEG-IFN-α-ribavirin for 24 weeks after the lead-in period. Treatment with PEG-IFN-α-ribavirin was continued for 44 weeks in patients with a detectable HCV RNA level between weeks 8 and 24 (absence of extended rapid virological response); and group 3 (fixed-duration therapy) received boceprevir and PEG-IFN-α-ribavirin for 44 weeks. The patients were followed after the end of therapy until week 72. Nonblack and black patients were analyzed separately.
A total of 1097 patients were included in this study, comprising 938 nonblack and 156 black patients. In the nonblack cohort, sustained virological response rates were 40% in the control group, 67% in group 2 (response-guided therapy), and 68% in group 3 (fixed-duration therapy, ). The corresponding sustained virological response rates in black patients were 23%, 42%, and 53% for groups 1, 2, and 3, respectively (). In both groups (black and nonblack patients) treated with boceprevir, response rates were significantly higher in comparison with the control group (). Importantly, in nonblack patients, response-guided therapy with individualized treatment (24 weeks of triple therapy in the event of absence of detectable HCV RNA at weeks 8 and 24) resulted in similar rates of sustained virological response in comparison with the fixed-duration group receiving triple therapy for 48 weeks (). In total, 97% of patients on response-guided therapy with an extended rapid virological response achieved a sustained virological response similar to that of group 3 patients treated for 48 weeks with triple therapy, with a sustained virological response in 96% of cases. This high rate of response observed in patients on response-guided therapy avoided the need for further administration of therapy and, importantly, precluded exposure to side effects without loss of the chance of eliminating HCV infection. The undetectability of HCV RNA in serum at week 8 was highly predictive of a sustained virological response in all three groups of patients, with rates of 85%, 88%, and 90% for groups 1, 2, and 3, respectively. In patients who responded well to PEG-IFN-α-ribavirin combination therapy, addition of boceprevir did not increase the sustained virological response rate. Thus, triple therapy with its unnecessary side effects and costs may be avoided in these patients, although an advantage would be to shorten the treatment duration. Patients with a poor response to PEG-IFN-α-ribavirin (reduction of viral load < 1 log10 IU/mL after 4 weeks of therapy) had a higher response than the control group (sustained virological response rates were 5% in the control group, 29% in the response-guided therapy group, and 39% in the fixed-duration therapy group) but, overall, the response rate was markedly reduced and appeared to be higher upon longer exposure to the triple therapy regimen in these more difficult-to-treat patients. In addition, higher rates of boceprevir-resistant strains were observed, which may have had a negative impact on subsequent treatment with another antiviral drug regimen in the event of failure on triple therapy. These patients may benefit from improved therapy once new antivirals become available and if treated with more reinforced monitoring of the virological response. Analysis of factors predicting response have shown that for boceprevir, a baseline HCV RNA level below 400,000 IU/mL, age younger than 40 years, absence of cirrhosis, and nonblack ethnicity were predictors of a sustained virological response ().Citation36 In patients with cirrhosis, the response was not different between the boceprevir and control groups, but sample sizes were small and further evaluation is needed. Furthermore, as observed for telaprevir, the response was better in patients infected with HCV subtype 1a than in those with subtype 1b ().Citation38
Table 2 SVR rates according to treatment group and cohorts in phase III clinical trials
Table 3 Predictive factors of SVR in naïve and previously non-responders patients in phase III clinical trials
Efficacy in previously treated patients
Triple therapy with PEG-IFN-α-ribavirin and boceprevir was evaluated in the RESPOND-2 trial in previous nonresponders (two-third of patients) or relapsers (one third of patients) after PEG-IFN-α-ribavirin standard of care.Citation37 The sustained virological response rate was higher in patients treated with boceprevir in combination with standard of care than in the control group (). Responses were similar for patients on fixed-duration therapy and on response-guided therapy, with rapid disappearance of serum HCV RNA, as assessed after 8 weeks of treatment. In these patients, sustained virological response rates were very high at 86% after 32 weeks of triple therapy (response-guided therapy group) and 88% after 44 weeks of triple therapy (fixed-duration therapy group). In contrast, patients who responded poorly to lead-in PEG-IFN-α-ribavirin treatment (reduction of viral load < 1 log10 IU/mL) had a markedly reduced response to triple therapy. Patients with prior relapse after PEG-IFN-α-ribavirin therapy had a higher response rate than those with previous nonresponse. The sustained virological response rate after triple therapy was 69% and 75% in prior relapsers in the response-guided therapy group and fixed-duration therapy group, respectively, and 40% and 52% in prior nonresponders in the response-guided therapy group and fixed-duration therapy group, respectively ().Citation37 With regard to treatment-naïve patients, the results overall indicated that an early response identifies patients in whom a shorter treatment period is sufficient enough to stop therapy early and avoid further potential side effects. Interestingly, preliminary results from the intermediate analysis of a Phase III study which enrolled nonresponders to PEG-IFN-α-ribavirin from previous Phase II and III trials demonstrated a sustained virological response rate of 40%,Citation39 indicating that a significant number of the most difficult-to-treat patients could benefit from triple therapy.
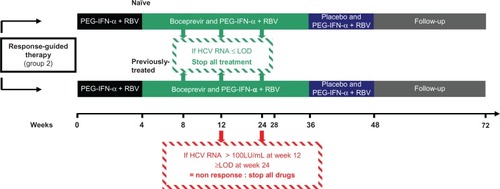
Reducing the treatment duration may not only prevent unnecessary side effects but also avoid development of resistant viral quasispecies that emerge during therapy in nonresponders. It has been shown that after discontinuation of therapy, there is a return to the initial viral population levels suggesting that patients could be retreated in the near future by interferon-free regimens combining different direct-acting antiviral agents that include a protease inhibitor.Citation40 Consequently, it seems important to prevent development of resistant strains, although this has not been assessed to date. Post hoc analyses of the SPRINT-2 and RESPOND-2 studies have shown that combination of serum HCV RNA ≥ 100 IU/mL at week 12 after starting therapy and detectable serum HCV RNA at week 24 of treatment allow the best stopping rules to avoid unnecessarily prolonging treatment (which if continued will not be effective) and to stop too early a treatment (that still allow, if continued, a viral eradication). ().Citation41
Figure 3 Duration of therapy following the registration of boceprevir by the FDA and stopping rules during boceprevir treatment in naïve and previously treated patients with response-guided therapy.
Abbreviations: HCV, hepatitis C virus; PEG-IFN-α, pegylated interferon-alpha; RBV, ribavirin; LOD, limit of detection.
Side effects
The most common adverse events reported with triple therapy combining standard of care and boceprevir were flu-like symptoms, which are characteristic of PEG-IFN-α. Serious adverse events were reported at a rate of about 10% in patients treated for the first time, with no differences between the treatment groups (). In patients previously exposed to PEG-IFN-α-ribavirin, adverse events occurred more frequently in those treated with triple therapy (). In addition to the common side effects occurring on PEG-IFN-α-ribavirin, patients treated with boceprevir had significantly more dysgeusia, rash, dry skin, anemia, and absolute neutrophil count below 750/mm3 ().Citation36,Citation37 No differences between the boceprevir treatment groups were observed. However, although the differences were mild, treatment had to be discontinued or modified more often in patients enrolled in the boceprevir arms than in the control group.
Table 4 Types of adverse events according to treatment group in phase III clinical trials
Table 5 Major adverse events related to boceprevir treatment in phase III clinical trialsTable Footnote*
Pharmacogenomics
Role of IL28B
Taking into account the individual profile of the patient to make the best therapeutic choice appears critical to establishing the most acceptable risk-to-benefit balance. Individual genetic background may influence the response to therapy as well as occurrence of side effects, because it has been shown that response to standard of care depends on race and ethnicity. Indeed, a significant difference in treatment efficacy with PEG-IFN-α-ribavirin was observed between Caucasian American and African American patients, the latter showing a lower response to therapy in comparison with Caucasian Americans.Citation42 Analysis of whole-genome single nucleotide polymorphism profiles has shown that a single nucleotide polymorphism near the interleukin-28B (IL28B) gene is associated with response to PEG-IFN-α-ribavirin treatment for chronic hepatitis C as well as with spontaneous clearance of HCV.Citation43,Citation44 IL-28B polymorphism has been shown to be the strongest baseline predictor of a sustained virological response using PEG-IFN-α-ribavirin.Citation45 In Caucasians, the CC IL-28B type was associated with an improved rapid virological response (negativity of serum HCV RNA at week 4 of therapy), complete early virological response, and sustained virological response. A sustained virological response was observed in 69% of patients with the CC IL28B type versus 33% and 27% with the CT and TT IL28B types, respectively.Citation45 African Americans had the CC IL28B type less often, which explains the lower response to PEG-IFN-α-ribavirin in these patients.Citation45 However, sustained virological response rates were lower even in African American patients with the CC genotype. In this study, African American ethnicity remained an independent negative predictor of outcome,Citation45 suggesting the presence of other as yet undetected genetic variants that influence the treatment response in African Americans. The CC IL-28B genotype also increased the proportion of patients who attained a rapid virological response. However, in patients who achieved a rapid virological response, sustained virological response rates were high, independent of IL-28B single nucleotide polymorphism. While the most important prognostic factor for sustained virological response remains rapid virological response,Citation45 this cannot be known before therapy, unlike with the IL28B polymorphism. Moreover, the CC genotype is also indicative of a sustained virological response in patients treated with PEG-IFN-α-ribavirin without a rapid virological response. Interestingly, the effect of IL28B variants was retrospectively evaluated in both Phase III studies evaluating triple therapy that were ongoing at the time of discovery of the IL28B variants.Citation39,Citation46 In both trials, IL28B CC polymorphism was a strong predictor of viral response at weeks 4 and 8. In total, 80%–90% of naïve and treatment-experienced patients with IL28B CC polymorphism qualify for a shorter duration of PEG-IFN-α-ribavirin and boceprevir. Noteworthy is that the response to lead-in treatment with PEG-IFN-α-ribavirin was the strongest predictor of a sustained virological response, with this parameter being superior to all other factors, including IL28B polymorphism. In patients with IL28B CT and TT polymorphisms who responded more poorly, addition of boceprevir significantly improved sustained virological response rates. In naïve patients, the sustained virological response increased in the most difficult-to-treat IL28B CT and TT patients from 28% to 27%, respectively, in the PEG-IFN-α-ribavirin control group, to 65% in the IL28B CT/response-guided therapy group, and to 71% in the IL28B CT/fixed-duration therapy group. The sustained virological response rate was 55% in the IL28B TT/response-guided therapy group and 59% in the IL28B TT/fixed-duration therapy group.Citation47
Inosine triphosphatase
Ribavirin-induced hemolytic anemia is a common adverse event in patients treated with standard of care and has been reported in 20% of patients.Citation48 Two single nucleotide polymorphisms of the inosine triphosphatase (ITPA) gene, which are responsible for inosine triphosphatase deficiency, have been found to be associated with protection from ribavirin-induced hemolytic anemia and a decreased need for ribavirin dose reduction during therapy. Another study did not report an association between ITPA polymorphism and sustained virological response, but the relatively small number of patients evaluated might represent a limitation.Citation49,Citation50 In the boceprevir trials, anemia increased when boceprevir was added. Anemia was reported in 29% of controls and in 49% of boceprevir groups.Citation51 In these studies, erythropoietin administration was allowed, with 43% of patients in the boceprevir arms receiving erythropoietin in comparison with 24% in the control group.Citation36 In the boceprevir studies, reduction of the ribavirin dose and administration of erythropoietin were recommended when hemoglobin fell below 10 g/dL, but decisions were made at the discretion of the investigator.
Despite the frequency of anemia, drug discontinuation due to anemia occurred in only 1% of patients included in the boceprevir trials.Citation36,Citation37 Results of the influence of ITPA gene variants, which may help to predict susceptibility to anemia, were not reported for either of the studies. The best way to manage anemia remains to be determined. Indeed, there is a possibly increased risk of thromboembolic and adverse cardiac events in patients treated with erythropoietin. A randomized trial comparing ribavirin dose reduction with administration of erythropoietin for the management of anemia in patients with chronic hepatitis C receiving boceprevir and PEG-IFN-α-ribavirin has shown similar sustained virological response rates and safety profiles in both groups.Citation51 Therefore, reduction of the ribavirin dose in patients treated with PEG-IFN-α-ribavirin and boceprevir appears to be an appropriate way to manage anemia.
Programmed cell death-1 molecule
Chronic HCV infection is characterized by impaired effector functions of virus-specific T lymphocytes. Programmed cell death-1 (PD-1) is a regulatory molecule expressed on the surface of CD4+ T cells, CD8+ T cells, and natural killer cells.Citation52 Its upregulation leads to cell exhaustion in terms of proliferation, cytokine secretion, and cytotoxic activity. PD-1 plays a major role in viral infections, because its expression is lower in individuals who resolve the infection compared with those who develop chronic disease.Citation53 Moreover, overexpression of PD-1 has been shown to be associated with failure of response to treatment with PEG-IFN-α-ribavirin.Citation52 Very recently, the PD-1.3/A allele has been shown to be strongly associated with a sustained virological response in patients treated with PEG-IFN-α-ribavirin.Citation54 PD-1.3 and IL28B seem to have a higher predictive value than HCV genotype, with a sustained virological response being achieved in 90% of genotype 1 HCV-infected patients with the IL28B CC genotype and the PD-1.3/A allele.Citation54
Implications for clinical use: patient considerations
The main findings on the use of boceprevir for the treatment of chronic hepatitis C in patients infected with genotype 1 is the marked increase in sustained virological response rates in both previously untreated patients and nonresponders to PEG-IFN-α-ribavirin. In addition, duration of therapy may be shortened to 28 weeks in the majority of previously untreated patients. Based on these results, patients with HCV genotype 1 infection are eligible for triple therapy if they have a clinical profile similar to that of patients included in the studies conducted so far. Indeed, triple therapy with boceprevir and PEG-IFN-α-ribavirin has not been evaluated until now, and only in a few studies of patients with decompensated cirrhosis, HIV coinfection, or liver transplantation.Citation55 Therefore, triple therapy including boceprevir should be avoided in these clinical situations unless in the context of carefully controlled clinical trials, due to the limited safety and efficacy data available for these difficult-to-treat patients. Although triple therapy is more effective, the side effects of boceprevir are additive to those of PEG-IFN-α-ribavirin, with an increase in potentially severe adverse events. The increasing number of adverse effects and complexity of triple therapy, with the requirement to take 12 × 200 mg capsules of boceprevir daily in three doses every 8 hours with food to increase absorption continue to limit the number of patients who can benefit from therapy. Finally, the decision to treat should be made on an individual basis, taking into account the severity and therefore the prognosis of the liver disease, the efficacy of therapy, as predicted by careful evaluation, and the side effects that may arise in a particular patient with a particular personal history. Boceprevir trials were designed with the concept of individualized and response-guided therapy and based treatment decisions on how rapidly patients responded to treatment. Predictors of response, determined at baseline, were treatment with boceprevir, nonblack ethnicity, baseline HCV RNA ≤ 400,000 UI/mL, age ≤ 40 years, absence of cirrhosis, and use of statins.Citation36
Retrospective analysis of IL28B polymorphisms showed that the IL28B CC genotype was more strongly associated with a sustained virological response than any other baseline factor, and thus identifies patients who may benefit from shorter-term treatment with a success rate higher than 80%.Citation46 These patients with a very good response profile have the best benefit-risk ratio. In such patients, the principal advantage of triple therapy is to shorten the treatment duration, although the benefit of standard PEG-IFN-α-ribavirin therapy for 24 weeks is not known, but may have similar efficacy. The discovery of IL28B polymorphism has brought the treatment of hepatitis C into the era of pharmacogenomics, with the implementation of proposals to take into account this parameter in the therapeutic algorithm 3 years after its discovery.Citation56 However, the clinical usefulness of IL28B variants needs further evaluation in prospective trials to show the relevance of the favorable IL28B genotype in clinical practice.
Another and more important predictive factor of sustained virological response was a ≥1 log10 decrease of HCV RNA at week 4. This factor was more strongly predictive of a sustained virological response than IL28B polymorphism, with 80% of patients in the different groups achieving a sustained virological response regardless of the IL28B genotype.Citation46 The lead-in period, which consists of 4 weeks of administration of PEG-IFN-α-ribavirin before adding boceprevir, has the advantage of evaluating tolerability and compliance with PEG-IFN-α-ribavirin therapy before administration of triple therapy which is more complex and difficult to endure. Finally, the decision to treat with triple therapy is also based on factors that may not have been evaluated in clinical trials, such as severity of disease, tolerability of a previous PEG-IFN-α-ribavirin regimen, and comorbidities, in particular a cardiac history in older patients.Citation57
Thus, individualized approaches to HCV therapy leading to personalized therapy are based on host factors which are prominent before therapy, as well as on viral parameters and, most importantly, on HCV genotype, which limits the efficacy of first-generation direct-acting antiviral agents for a specific genotype, such as boceprevir for genotype 1. Host factors include IL28B variants, comorbidities, adherence issues, drug–drug interactions, HIV coinfection, and decompensated liver disease. However, as mentioned earlier, some of these factors have not been evaluated in clinical trials, making the assessment of the risk-benefit ratio more difficult in routine clinical practice.
Moreover, the relevance of other important potential factors, such as the ITPA gene which may protect against anemia occurring during PEG-IFN-α-ribavirin therapy, have not been evaluated in the context of triple therapy including boceprevir, despite the high frequency of anemia in the boceprevir trials. The individualization of treatment should also take into account the rapidly changing therapeutic landscape that could indicate the potential efficacy of interferon-free regimens combining direct-acting antiviral agents only, as is the case in treatment for HIV.Citation58,Citation59 Thus, patients who have features predicting a poor response to boceprevir and PEG-IFN-α-ribavirin or who have had a poor response after the lead-in period may benefit from better therapies, once they become available.
Personalized medicine is a prerequisite for successful treatment, because boceprevir combined with PEG-IFN-α-ribavirin is effective but also has significant side effects and costs. Multiple host and virological parameters have to be assessed precisely before and during therapy. Its success will be driven by attention to the requirements of this new standard of care, which includes appropriate dosing, management, and anticipation of side effects, as well as strict adherence to rules for cessation ().Citation60 Careful patient education should give complete information on treatment to ensure good adherence and patient participation, thereby increasing the chances of treatment being successful.
Acknowledgements
We thank Mirjam Zeisel (Inserm Unit 748) for having carefully reviewed the manuscript, and for her suggestions and helpful comments.
Disclosure
FH reports receiving payment for lectures from Merck and Janssen, reimbursement for meeting expenses from Merck, Janssen, and Gilead, and consulting fees from Transgene, Gilead, BMS Cytheris, and Roche. MD reports receiving payment for medical education from MSD, Roche, BMS, and Gilead, and payment for board membership from MSD. CL and TFB do not report any potential conflicts of interest relevant to this work.
References
- ChooQLKuoGWeinerAJOverbyLRBradleyDWHoughtonMIsolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genomeScience19892443593622523562
- LauerGMWalkerBDHepatitis C virus infectionN Engl J Med2001345415211439948
- World Health Organization Hepatitis C. Fact sheet N°164. July 2012. [Webpage on the Internet] http://www.who.int/mediacentre/factsheets/fs164/en/index.html. Accessed April 14, 2012.
- ArmstrongGLWasleyASimardEPMcQuillanGMKuhnertWLAlterMJThe prevalence of hepatitis C virus infection in the United States, 1999 through 2002Ann Intern Med200614470571416702586
- MoradpourDPeninFRiceCMReplication of hepatitis C virusNat Rev Microbiol2007545346317487147
- SeeffLBNatural history of chronic hepatitis CHepatology2002365 Suppl 1S35S4612407575
- GhanyMGStraderDBThomasDLSeeffLBDiagnosis, management, and treatment of hepatitis C: an updateHepatology2009491335137419330875
- HoofnagleJHMullenKDJonesDBTreatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary reportN Engl J Med1986315157515783097544
- ShiratoriYImazekiFMoriyamaMHistologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapyAnn Intern Med200013251752410744587
- ImaiYKasaharaATanakaHInterferon therapy for aged patients with chronic hepatitis C: improved survival in patients exhibiting a biochemical responseJ Gastroenterol2004391069107715580400
- KasaharaATanakaHOkanoueTInterferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver-related deathJ Viral Hepat20041114815614996350
- KatzeMGHeYGaleMJrViruses and interferon: a fight for supremacyNat Rev Immunol2002267568712209136
- PawlotskyJMTherapy of hepatitis C: From empiricism to eradicationHepatology200643Suppl 1S207S22016447262
- ReichardOAnderssonJSchvarczRWeilandORibavirin treatment for chronic hepatitis CLancet1991337105810611673493
- Di BisceglieAMShindoMFongTLA pilot study of ribavirin therapy for chronic hepatitis CHepatology1992166496541505907
- BrillantiSGarsonJFoliMA pilot study of combination therapy with ribavirin plus interferon alfa for interferon alfa-resistant chronic hepatitis CGastroenterology19941078128177521308
- PoynardTMarcellinPLeeSSRandomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT)Lancet1998352142614329807989
- LohmannVKörnerFKochJHerianUTheilmannIBartenschlagerRReplication of subgenomic hepatitis C virus RNAs in a hepatoma cell lineScience199928511011310390360
- LindenbachBDRiceCMFlaviviridae: The viruses and their replicationFields Virology Volume 14th edPhiladelphia, PALippincott Williams & Wilkins2001
- KimJLMorgensternKALinCCrystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptideCell1996873433558861917
- LoveRAPargeHEWickershamJAThe crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding siteCell1996873313428861916
- LamarreDAndersonPCBaileyMAn NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virusNature200342618618914578911
- MalcolmBALiuRLahserFSCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cellsAntimicrob Agents Chemother2006501013102016495264
- Merck. Victrelis (boceprevir) prescribing information. May 2011. Available at: http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf. Accessed July 10, 2012.
- FDA US Food and Drug AdministrationDrug approval package Victrelis (boceprevir). Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202258Orig1s000TOC.cfm. Accessed December 14, 2011.
- GhosalAYuanYTongWCharacterization of human liver enzymes involved in the biotransformation of boceprevir, a hepatitis C virus protease inhibitorDrug Metab Dispos20113951052121123164
- FlockhartDATanus-SantosJEImplications of cytochrome P450 interactions when prescribing medication for hypertensionArch Intern Med200216240541211863472
- KiserJJBurtonJRAndersonPLEversonGTReview and management of drug interactions with boceprevir and telaprevirHepatology2012551620162822331658
- ZeuzemSSarrazinCRouzierRAntiviral activity of SCH 503034, a HCV protease inhibitor, administered as monotherapy in hepatitis C genotype-1 (HCV-1) patients refractory to pegylated interferon (PEG-IFN-alpha)Hepatology200542Suppl 1S233S234
- SusserSWelkerMWZettlerMClonal analysis of mutations selected in the HCV NS3 protease domain of genotype 1 non-responders treated with boceprevir (SCH503034)J Hepatol200848Suppl 2S29
- FlintMMullenSDeatlyAMSelection and characterization of hepatitis C virus replicons dually resistant to the polymerase and protease inhibitors HCV-796 and boceprevir (SCH 503034)Antimicrob Agents Chemother20095340141118936191
- PawlotskyJMTreatment of hepatitis C: don’t put all your eggs in one basket!Gastroenterology20071321611161517418174
- SarrazinCRouzierRWagnerFSCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonrespondersGastroenterology20071321270127817408662
- KwoPYLawitzEJMcConeJEfficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trialLancet201037670571620692693
- SchiffEPoordadFJacobsonIBoceprevir (B) combination therapy in null responders (NR): response dependent on interferon responsivenessJ Hepatol200848Suppl 2S46
- PoordadFMcConeJBaconBRBoceprevir for untreated chronic HCV genotype 1 infectionN Engl J Med20113641195120621449783
- BaconBRGordonSCLawitzEBoceprevir for previously treated chronic HCV genotype 1 infectionN Engl J Med20113641207121721449784
- BrassCBarnardRJOHoweJASustained virologic response and boceprevir resistance-associated variants observed in patients infected with HCV genotype 1a/1b when treated with boceprevir plus peginterferon alfa-2b/ribavirinJ Hepatol201154Suppl 1S471S472
- BronowickiJPDavisMFlammSSustained virologic response (SVR) in prior peginterferon/ribavirin (PR) treatment failures after retreatment with boceprevir (BOC)-+-PR: the provide study interim resultsJ Hepatol201256Suppl 2S6
- VierlingJMRalstonRLawitzEJLong-term outcomes following combination treatment with boceprevir plus peg-intron/ribavirin (P/R) in patients with chronic hepatitis C, genotype 1 (CHC-G1)J Hepatol201052Suppl 1S470S471
- JacobsonIMMarcellinPZeuzemSRefinement of stopping rules during treatment of hepatitis C genotype 1 infection with boceprevir combined with peginterferon/ribavirinHepatology5222012 [Epub ahead of print.]
- ConjeevaramHSFriedMWJeffersLJPeginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1Gastroenterology200613147047716890601
- GeDFellayJThompsonAJGenetic variation in IL28B predicts hepatitis C treatment-induced viral clearanceNature200946139940119684573
- ThomasDLThioCLMartinMPGenetic variation in IL28B and spontaneous clearance of hepatitis C virusNature200946179880119759533
- ThompsonAJMuirAJSulkowskiMSInterleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virusGastroenterology201013912012920399780
- PoordadFBronowickiJPGordonSCFactors that predict response of patients with hepatitis C virus infection to boceprevirGastroenterology5212012 [Epub ahead of print.]
- KwoPYPhase III results in genotype 1 naïve patients: predictors of response with boceprevir and telaprevir combined with pegylated interferon and ribavirinLiver Int201232Suppl 1S39S43
- FriedMWShiffmanMLReddyKRPeginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infectionN Engl J Med200234797598212324553
- FellayJThompsonAGeDITPA gene variants protect against anaemia in patients treated for chronic hepatitis CNature201046440540820173735
- ThompsonAJFellayJPatelKVariants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reductionGastroenterology20101391181118920547162
- PoordadFLawitzEJReddyKRA randomized trial comparing ribavirin dose reduction versus erythropoietin for anemia management in previously untreated patients with chronic hepatitis C receiving boceprevir plus peginterferon/ribavirinJ Hepatol201256Suppl 2S559
- Golden-MasonLKlarquistJWahedASRosenHRCutting edge: Programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differencesJ Immunol20081803637364118322167
- UrbaniSAmadeiBTolaDPD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustionJ Virol200680113981140316956940
- Vidal-CastiñeiraJRLópez-VázquezAAlonso-AriasRA predictive model of treatment outcome in patients with chronic HCV infection using IL28B and PD-1 genotypingJ Hepatol2012561230123822322230
- SulkowskiMPolSCooperCBoceprevir + pegylated interferon + ribavirin for the treatment of HCV/HIV-coinfected patients: end of treatment (week 48) interim resultsAbstract 47 presented at the 19th Conference on Retroviruses and Opportunistic InfectionsSeattle, WAMarch 5–8, 2012.
- RamachandranPFraserAAgarwalKUK consensus guidelines for the use of the protease inhibitors boceprevir and telaprevir in genotype 1 chronic hepatitis C infected patientsAliment Pharmacol Ther20123564766222296568
- IwasakiYIkedaHArakiYLimitation of combination therapy of interferon and ribavirin for older patients with chronic hepatitis CHepatology200643546316374855
- LawitzEPoordadFKowdleyKVA 12-week interferon-free regimen of ABT-450/R, ABT-072, and ribavirin was well tolerated and achieved sustained virologic response in 91% of treatment-naive HCV IL28B-CC genotype-1-infected subjectsJ Hepatol201256Suppl 2S7
- SulkowskiMRodriguez-TorresMLawitzEHigh SVR rate in treatment-naïve HCV genotype 1a and 1b patients treated for 12 weeks with an interferon-free all-oral quad regimen: interim resultsJ Hepatol201256Suppl 2S559
- Approval of Victrelis (boceprevir) by US Food and Drug Administration. May 2011. Available at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm255413.htm. Accessed July 10, 2012.
- BarrittASFriedMWMaximizing opportunities and avoiding mistakes in triple therapy for hepatitis C virusGastroenterology2012141314132322537438
- KlibanovOMVickerySBOlinJLBoceprevir: a novel NS3/4 protease inhibitor for the treatment of hepatitis CPharmacotherapy20123217319022392426
- HulkotteEGuptaSXuanFPharmacokinetic interaction between the HCV protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteersHepatology5112012 [Epub ahead of print.]