Abstract
Aim:
We evaluated single nucleotide polymorphisms (SNPs) of CYP2D6 to identify those that influence the efficiency of tamoxifen in adjuvant treatment of breast cancer through a matched case–control study.
Methods:
Peripheral blood DNA was collected from 20 patients with disease recurrence during adjuvant tamoxifen treatment and from 19 patients who had completed 5 years of tamoxifen therapy without recurrence of breast cancer. CYP2D6*4 (1846G > A; rs3892097), CYP2D6*10 (100C > T, rs1065852), and CYP2D6*5 (deletion) were genotyped. The correlation between disease-free survival (DFS) and genotype and clinical outcome were assessed using Kaplan–Meier analysis and a log-rank test.
Results:
We found the allelic frequency of CYP2D6*10 during this study. Patients with the CYP2D6*10 homozygous variant T/T genotype had a significantly shorter median of DFS than those with C/T (P = 0.036), but DFS was not significantly different from that of patients with the C/C genotype (P = 0.316). One patient who was a carrier both of CYP2D6 G/A (1846G > A) and T/T (100C > T) had DFS of 22.7 months.
Conclusions:
This study demonstrated that CYP2D6*10/*10 was significantly associated with shorter DFS in Thai breast cancer patients receiving tamoxifen. This was a pilot study investigating the correlation of CYP2D6 polymorphisms and their influence on clinical outcomes in Thai estrogen receptor-positive breast cancer patients.
Introduction
Tamoxifen is a prodrug that requires conversion to active metabolites by the cytochrome P450 enzyme CYP2D6, which is one of the key enzymes for the formation of N-desmethyltamoxifen and 4-hydroxytamoxifen.Citation1,Citation2 N-desmethyltamoxifen is further metabolized to endoxifen, which has more potent antiestrogenic effects than tamoxifen.Citation3 Breast cancer is currently the most common cancer in Thai women. Tamoxifen is the most commonly prescribed drug for the treatment and prevention of recurrence for patients with estrogen receptor (ER)-positive disease.Citation4,Citation5 However, the clinical response to tamoxifen varies widely among patients; not all ER-positive breast cancer patients benefit from tamoxifen therapy.Citation6 Recent studies suggest that inherited variations in enzymatic activity responsible for the metabolism of tamoxifen affects the risk of breast cancer recurrence in patients receiving adjuvant tamoxifen.Citation7–Citation9 Patients receiving tamoxifen who either carry genetic variants associated with low or absent CYP2D6 activity have a significantly lower plasma level of endoxifen.Citation3,Citation10–Citation12
The frequency of the CYP2D6 poor metabolizer phenotype varies across different populations.Citation13 Caucasians have the highest frequency of the poor metabolizer phenotype at a frequency of 7%–10%, with CYP2D6*4 (1846G > A) being a major variant.Citation14–Citation18 Only 1%–3% of poor metabolizers from the Asian population have CYP2D6*5 (gene deletion) as a major variant. However, the allele CYP2D6*10 (100C > T) is a major variant that has been observed with a frequency of 40%–50% in Asian populations.Citation19–Citation22
Previous studies have shown that an association exists between CYP2D6*4 genotypes and clinical outcomes in Caucasians.Citation23 Chinese women with the CYP2D6*10 (100C > T) T/T genotype have a significantly worse disease-free survival (DFS) than those with the C/C or C/T genotype.Citation20 However, the association between the CYP2D6 genotype and tamoxifen efficacy in Thai patients has never been reported. The present study examined which CYP2D6 polymorphisms influence tamoxifen efficacy in Thai patients receiving adjuvant treatment for breast cancer. We conducted a matched case–control study in breast cancer patients who received adjuvant tamoxifen to determine the association of CYP2D6 polymorphisms and DFS.
Methods
Patients and study design
We enrolled 39 breast cancer patients in Ramathibodi Hospital who met our inclusion criteria, including (1) histological diagnosis of estrogen- and/or progesterone receptor-positive breast cancer, (2) received tamoxifen as an adjuvant treatment for breast cancer, and (3) age at diagnosis > 18 years old. Exclusion criterion for patients in our study included if patients had started tamoxifen therapy along with other cytotoxic chemotherapy or any other endocrine therapy besides tamoxifen. Women taking other medicines known to inhibit CYP2D6 activity were also excluded. All subjects provided written consent before study entry. This study was approved by Ramathibodi’s ethics committee.
Patients were classified into case and control groups. Case patients consisted of patients who had recurrence of breast cancer while receiving tamoxifen; patients who had already completed 5 years of adjuvant tamoxifen treatment were included as controls. Patient data was collected from medical records. We matched one control subject per case patient by age at diagnosis of breast cancer, menstruation status, type of surgery, date of surgery, ER/progesterone receptor (PgR) status, Her-2 status, histologic grading tumor, surgery margin status, lymphovascular involvement status, T stage of tumor, nodal involvement, number of nodes dissection, start and stop date of chemotherapy either neoadjuvant or adjuvant setting, start and stop date of adjuvant radiotherapy, start and stop date of tamoxifen. Date and site of the first disease relapse were collected.
Genomic analysis
Genomic DNA was extracted and amplified by PCR. The CYP2D6*4 (1846G > A; rs3892097) allele was detected by using restriction fragment length polymorphism (RFLP) techniques with the enzyme BstNI. The CYP2D6*10 (100C > T; rs1065852) allele was detected using DNA sequencing techniques with Big Dye Terminator Cycle Sequencing Kit and the ABI 3100 Genetic Analyzer (all from Applied Biosciences, Foster City, CA). We analyzed sequences using Chroma software to compare the DNA sequence alignment with a wild type reference. Resequencing was conducted using a reverse primer. TaqMan® Copy Number Assays (Applied Biosystems) were used to genotype the CYP2D6*5 (Hs.04083572_cn). Applied Biosystems CopyCaller™ Software was used for copy number analysis.
End points and statistical analyses of associations
An association between CYP2D6 polymorphisms and any influence of tamoxifen efficacy in adjuvant treatment of breast cancer and DFS were analyzed in this study. DFS was defined as the time from surgery to the occurrence of breast event (ie, local, regional or distant occurrence or contralateral breast cancer) or death from any cause. Patients who were alive without a breast recurrence were censored at the date of their last disease evaluation. The overall distribution of DFS was estimated using Kaplan–Meier methods. A log-rank test was used to assess the association between the factor and the event of interest.
Results
Patient characteristics
Patient characteristics are shown in . Among the 39 subjects, median age was 46.12 years (range 30–66 years). Overall, patient baseline characteristics were similar, including age, menopausal status, tumor size, tumor grading, and ER status, although a higher percentage had lymphovascular involvement, progesterone receptor and Her-2 status positive in group 1. The numbers of pre- and postmenopausal patients were 31 and 8, respectively. Median DFS was 31.05 months and 96.4 months in case and control groups, respectively (P < 0.005). Median follow-up time was 52.3 and 97.4 months in case and control groups, respectively (P = 0.02).
Table 1 Patient characteristics
Genotype and allele frequency
We determined CYP2D6*10 genotypes of the 39 patients. A total of 19 patients (19/39; 48.7%) were homozygous (T/T); 11 (11/20; 55%) and 8 (8/19; 42.1%) patients were in the case and control groups, respectively. A total of 14 patients (14/39; 35.9%) were heterozygous (C/T); 4 (4/20; 20%) patients were in the case and 10 (10/19; 52.6%) patients were in the control groups. Six patients (6/39; 15.3%) were wild-type (C/C); 5 (25/20; 25%) patients were in the case group (1/19; 5.2%) and 1 patient was in the control group. No patients were homozygous for CYP2D*4 (1846G > A). We found the heterozygous CYP2D6*4 genotype in 1 patient (2.56%). Allele frequency of CYP2D6*4 (1846G > A) was 1.28%. A total of 38 patients (97.43%) carried the wild-type allele. The CYP2D6*5 (deletion) was not found in this population. Details of genotype and allele frequencies in each group are shown in .
Table 2 Genotype and allele frequencies
Clinical outcome by genotype
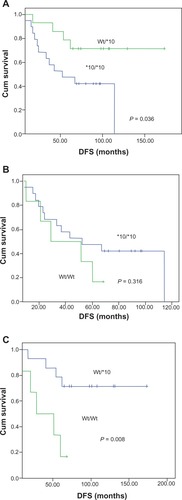
Patients with CYP2D6*10 homozygous variant (T/T; CYP2D6*10/*10) had a significantly shorter DFS than those with the heterozygous variant (C/T; CYP2D6 Wt/*10) (P = 0.036, ). Furthermore, there was a significant difference in DFS between homozygous wild-type (C/C; CYP2D6 Wt/Wt and heterozygous variant (C/T; CYP2D6 Wt/*10) (P = 0.008, ). In contrast to many previous studies, there was no significant difference in DFS between heterozygous variants, homozygous variants (T/T; CYP2D6*10/*10), and homozygous wild-type patients (C/C; CYP2D6 Wt/Wt) (P = 0.316, ). Because we found the heterozygous CYP2D6*4 G/A (1846G > A) only in one patient, we did not analyze this association. Notably, this patient also had CYP2D6*10/*10 (T/T) and was classified as an intermediate metabolizer. Her DFS was only 22.7 months.
Figure 1 Kaplan–Meier estimates of disease-free survival (DFS) with the CYP2D6*10 genotype in 39 Thai breast cancer patients receiving tamoxifen treatment. Patients with the CYP2D6*10 homozygous variant T/T genotype were compared with patients with heterozygous C/T (A) or homozygous wild-type C/C genotype (B) and heterozygous C/T compared with homozygous wild-type C/C genotype (C).
Discussion
Many recent studies have demonstrated a correlation between CYP2D6 polymorphisms and the efficacy of tamoxifen treatment in ER-positive breast cancer.Citation13,Citation24,Citation25 Most studies were in Caucasians, which found that CYP2D6*4 causes a poor metabolizer phenotype in 12%–20% of the Caucasian population. CYP2D6*4 was found in only 1%–2% of the Asian population, while CYP2D6*10 (100C > T) causes the intermediate metabolizer phenotype and is found in 40%–50% of the Asian population.Citation11,Citation26 Several studies have been performed primarily in Caucasian women.Citation14–Citation17 The relatively few studies in Asian womenCitation19,Citation20 have suggested that CYP2D6 status is associated with outcomes in prevention, adjuvant, and metastatic settings. Recently, studies in Asian populations report a correlation between CYP2D6*10 and the impact on the clinical outcome of ER-positive breast cancer.Citation19,Citation20,Citation22 However, whether CYP2D6*10 genotypes influence the clinical effect in Thai breast cancer patients is still unclear.Citation21,Citation22 This study was the first to explore this correlation in Thai patients. We designed this case–control study to investigate the relationship between CYP2D6*10 and clinical outcomes.
Our study found a high frequency of the CYP2D6*10 homozygous variant T/T genotype in 48.71% of patients, which agrees with the frequency of 40%–50% found in previous studies.Citation3 CYP2D6*4/*4 (G/G; 1846G > A) was not detected in our study, which agrees with previous reports that CYP2D6*4/*4 is rare in Asian populations (1%–3%).Citation19–Citation22 The SNP of CYP2D6*10 (100C > T) that we found in our study had previously been reported as a common SNP in Asian populations.Citation20–Citation22 Our study found that patients with the T/T genotype had a significantly shorter DFS than those with the C/T genotype, but DFS was not significantly different compared with the C/C genotype. This is in contrast to previous reports.Citation21
We then explored the baseline characteristics of all patients, including the addition of the chemotherapy regimen, which we thought might impact the outcome. We found that three of six CYP2D6 (C/C; 100C > T) patients had involvement of more than four lymph nodes and that this proportion was higher than for the other two genotypes, but not statistically significant. The regimen of chemotherapy was not a confounding factor in this study. This may be due to the small number of patients in the study. However, we matched one control subject per case patient to avoid clinical confounding factors in this analysis. Other limitations of this case–control study include our inability to control other confounding factors, such as compliance with tamoxifen treatment and the family history of breast cancer, which may influence clinical outcome. However, we still found a trend of lower DFS in patients with the CYP2D6*10 homozygous variant T/T genotype when analyzed and compared with the other two genotypes, although statistical significance was not reached. We consider that this is acceptable in a pilot study such as this, but future research should include a larger patient sample population and be prospective in order to accurately identify the correlation between CYP2D6*10, and CYP2D6*5 polymorphisms and clinical outcome in Thai ER-positive breast cancer patients.
In summary, our findings demonstrated a high frequency of the CYP2D6*10 homozygous variant T/T genotype, similarly to observations in other Asian populations. This genotype could lead to a reduced benefit of adjuvant tamoxifen and negatively affect the clinical outcome in Thai ER-positive breast cancer patients. In addition, it is reasonable to explore CYP2D6*10 rather than CYP2D6*4 due to the higher frequency of CYP2D6*10, even in a small patient sample, such as in this study.
Disclosure
The authors declare that they have no competing or conflicts of interest.
References
- HoskinsJMCareyLAMcLeodHLCYP2D6 and tamoxifen: DNA matters in breast cancerNat Rev Cancer20099857658619629072
- DestaZWardBASoukhovaNVFlockhartDAComprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6J Pharmacol Exp Ther200431031062107515159443
- LimYCLiLDestaZEndoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cellsJ Pharmacol Exp Ther2006318250351216690721
- FabianCTilzerLSternsonLComparative binding affinities of tamoxifen, 4-hydroxytamoxifen,and desmethyltamoxifen for estrogen receptor isolated from human breast carcinoma: correlation with blood levels in patients with metastatic breast cancerBiopharma Drug Dispos198124381390
- JinYDestaZStearnsVCYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatmentJ Natl Cancer Inst2005971303915632378
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- HigginsMJStearnsVUnderstanding resistance to tamoxifen in hormone receptor-positive breast cancerClin Chem20095581453145519541862
- GoetzMPRaeJMSumanVJPharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashesJ Clin Oncol200523369312931816361630
- KnoxSKIngleJNSumanVJCytochrome P450 2D6 status predicts breast cancer relapse in women receiving adjuvant tamoxifen (Tam)J Clin Oncol20062418S504
- StearnsVJohnsonMDRaeJMActive tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetineJ Natl Cancer Inst200395231758156414652237
- LimH-SJu LeeHSeok LeeKSook LeeEJangIJRoJClinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancerJ Clin Oncol200725253837384517761971
- BorgesSDestaZLiLQuantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatmentClin Pharmacol Ther2006801617416815318
- LimJSLChenXASinghOImpact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patientsBr J Clin Pharmacol201171573775021480951
- NowellSAhnJRaeJAssociation of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patientsBreast Cancer Res Treat200591324925815952058
- GoetzMPSchaidDJWickerhamDLEvaluation of CYP2D6 and efficacy of tamoxifen and raloxifene in women treated for breast cancer chemoprevention: results from the NSABP P1 and P2 clinical trialsClin Cancer Res201117216944695121880792
- SchrothWAntoniadouLFritzPBreast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypesJ Clin Oncol200725335187519318024866
- SchrothWGoetzMPHamannUAssociation between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifenJAMA2009302131429143619809024
- SchrothWHamannUFaschingPACYP2D6 polymorphisms as predictors of outcome in breast cancer patients treated with tamoxifen: expanded polymorphism coverage improves risk stratificationClin Cancer Res201016174468447720515869
- KiyotaniKMushirodaTSasaMImpact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapyCancer Sci200899599599918294285
- XuYSunYYaoLAssociation between CYP2D6*10 genotype and survival of breast cancer patients receiving tamoxifen treatmentAnn Oncol20081981423142918407954
- ToyamaTYamashitaHSugiuraHKondoNIwaseHFujiiYNo association between CYP2D6*10 genotype and survival of node-negative Japanese breast cancer patients receiving adjuvant tamoxifen treatmentJpn J Clin Oncol2009391065165619596663
- PechatananKJaruhathaiSAtivitavasTCytochrome P450 2D6 polymorphisms of Thai breast cancer patients and their outcomes of adjuvant tamoxifenJ Clin Oncol.2007Suppl Abstr e11037
- BonanniBMacisDMaisonneuvePPolymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flash: data from the Italian Tamoxifen TrialJ Clin Oncol200624223708370916877740
- TheLKMohamedNISallehMZThe risk of recurrence in breast cancer patients treated with tamoxifen: polymorphisms of CYP2D6 and ABCB1AAPS J2012141525922183189
- FleemanNMartin SaboridoCPayneKThe clinical effectiveness and cost-effectiveness of genotyping for CYP2D6 for the management of women with breast cancer treated with tamoxifen: a systematic reviewHealth Technol Assess201115331102
- RamamoorthyYTyndaleRFSellersEMCytochrome P450 2D6.1 and cytochrome P450 2D6.10 differ in catalytic activity for multiple substratesPharmacogenetics200111647748711505218