Abstract
Background
Pharmacogenetic study of cytochrome P450 (CYP) gene CYP2D6 and tamoxifen outcomes remain controversial. Apart from CYP2D6, other drug-metabolizing enzymes and transporters also play a role in tamoxifen metabolic pathways. The aim of this study is to investigate the impact of CYP3A4/5, ABCB1, and ABCC2 polymorphisms on the risk of recurrence in Thai patients who received tamoxifen adjuvant therapy.
Methods
Patients with early-stage breast cancer who received tamoxifen adjuvant therapy were recruited in this study. All six single-nucleotide polymorphisms (SNPs), including CYP3A4*1B (−392 A>G)/*18(878 T>C), CYP3A5*3(6986 G>A), ABCB1 3435 C>T, ABCC2*1C(−24 C>T), and ABCC2 68231 A>G, were genotyped using real-time polymerase chain reaction assays. The impacts of genetic variants on disease-free survival (DFS) were analyzed using the Kaplan–Meier method and Cox regression analysis.
Results
The ABCB1 3435 C>T was found to have the highest allele frequency among other variants; however, CYP3A4*1B/*18 could not be found in this study. Patients with heterozygous ABCB1 3435 CT genotype showed significantly shorter DFS than those with homozygous 3435 CC genotype (P = 0.041). In contrast, patients who carried homozygous 3435 TT genotype showed no difference in DFS from wild-type 3435 CC patients. Cox regression analysis showed that the relative risk of recurrence was increased by five times (P = 0.043; hazard ratio = 5.11; 95% confidence interval: 1.05–24.74) in those patients carrying ABCB1 3435 CT genotype compared to those with ABCB1 3435 CC.
Conclusion
ABCB1 3435 C>T is likely to have a clinically significant impact on recurrence risk in Thai patients with breast cancer who receive tamoxifen adjuvant therapy.
Introduction
Tamoxifen, a selective estrogen receptor modulator (SERM), is the standard prescribed drug for the treatment of breast cancer in patients with estrogen and/or progesterone receptor positive disease.Citation1 Tamoxifen is extensively metabolized by cytochrome P450 (CYP) enzyme 2D6 in the liver to produce pharmacologically active metabolites such as endoxifen and 4-hydroxytamoxifen.Citation2 It is well documented that CYP2D6 polymorphisms play an important role in tamoxifen effectiveness;Citation3 however, some findings have been inconsistent.Citation4–Citation7 To date, there is no consensus whether CYP2D6 genotyping is definitely essential before receiving the drug regimen. In addition to CYP2D6, tamoxifen could be metabolized by other metabolizing enzymes such as CYP3A4/5.Citation8 Recently, it was reported that drug transporters such as ABCB1 are involved in the transport of endoxifen and 4-hydroxytamoxifen, active metabolites of tamoxifen.Citation9 Furthermore, overexpression of ABCC2, an efflux transporter, has been reported in tamoxifen-resistant breast cancer.Citation10 Therefore, genetic variants of these metabolizing enzymes and drug transporters are likely to be associated in variable degree with clinical outcome observed in patients treated with tamoxifen. The impact of CYP3A4/5, ABCB1, and ABCC2 polymorphisms on tamoxifen effectiveness in Thai populations has not yet been reported. In this study, genetic variants of CYP3A4*1B (−392 A>G)/*18(878 T>C), CYP3A5*3(6986 G>A), ABCB1 3435 C>T, ABCC2*1C (−24 C>T), and ABCC2 68231 A>G in Thai patients with early-stage breast cancer were investigated. The risk of recurrence within 3 years among Thai women after receiving tamoxifen adjuvant therapy was evaluated.
Materials and methods
Patients
This study was retrospectively conducted in 30 breast cancer patients who visited Ramathibodi Hospital, Bangkok, Thailand, during the time between February 1997 and January 2008. All patients were estrogen and/or progesterone receptor positive and received tamoxifen as an adjuvant treatment for breast cancer. All patients had previously been treated with cyclophosphamide/methotrexate/5-fluorouracil (CMF) chemotherapy prior to tamoxifen treatment. The prognostic clinical factors known to affect the clinical outcome, such as age, tumor size, and lymph node status were matched between recurrence and nonrecurrence groups. Exclusion criteria included concurrent medications that induce or inhibit CYP2D6, CYP3A, and efflux transporters. Patients’ data were collected from medical records. The clinical data included in this study are given in . All analyzed patients had uniform diagnostic, management, and follow-up protocols. Blood samples were collected (5 mL) in an ethylenediaminetetraacetic acid (EDTA) tube and stored at −20°C until isolation of genomic DNA for genotype analysis. The study was approved by Ramathibodi Hospital’s ethics committee. All patients gave informed consent.
Table 1 Baseline characteristics of patients with and without recurrence (N = 30)
Genotyping
The criteria for candidate single-nucleotide polymorphism (SNP) selection in this study are that CYP3A4*1B/18Citation11 and CYP3A5*3Citation12,Citation13 have been reported to be involved in variable metabolism of CYP3A4/5 substrates. ABCB1 3435 C>T is the common SNP associated with altered P-glycoprotein (P-gp) expression and/or function.Citation14,Citation15 It has been reported that ABCC2 was overexpressed in tamoxifen-resistant breast cancer cells.Citation10 Thus, the possibility of active metabolites being pumped out from breast cancer cells by ABCC2 was suggested.Citation10ABCC2 68231 A>G (*1A, −1774delG linkage disequilibrium)Citation16 and ABCC2*1C (−24 C>T)Citation16,Citation17 have been reported to be associated with decreased ABCC2 promoter activity. All polymorphisms, except CYP3A4*1B/18, have been shown by HapMap (http://hapmap.ncbi.nlm.nih.gov/) to have minor allele frequency (≥5%) in a Han Chinese population.
In brief, genomic DNA was isolated from 5 mL venous blood stored in an EDTA tube by the standard phenol-chloroform method. The genotype of each candidate SNP was determined using TaqMan® drug metabolism genotyping assays (Applied Biosystems®; Life Technologies, Carlsbad CA, USA) as follows: CYP3A4*1B (5’-flanking region –392 A>G, reference sequence [rs]2740574) (assay ID: AHPAJVY); CYP3A4*18(c.878 T>C, rs28371759) (assay ID: C_27859823_20); CYP3A5*3(g.6986 G>A, rs776746) (assay ID: C_26201809_30); ABCB1 (c.3435 C>T, rs1045642) (assay ID: C_7586657_20); ABCC2*1C (5’-flanking region −24 C>T, rs717620) (assay ID: C_2814642_10); and ABCC2 (g.68231 A>G, rs3740065) (assay ID: C_22271640_10). The geno typing experiments were carried out using allele-specific Taqman® MGB probe 5’ nuclease assay with real-time PCR (polymerase chain reaction) Viia™ 7 system (Applied Biosystems®; Life Technologies). Each 20 μL PCR mixture contained 4 μL of genomic DNA (5 ng/μL), 10 μL of Taqman® Genotyping Mastermix, 1 μL of allele-specific Taqman® MGB probe and sequence-specific primer kit, 5 μL of DNase-free H2O. The thermal cycler program was set up as follows: at 95°C for 10 minutes, repeated 50 cycles at 92°C for 15 seconds and 60°C for 90 seconds. The Allelic Discrimination Plot was analyzed by Viia™ 7 software (Applied Biosystems®; Life Technologies).
Statistical analysis
The association between genetic variants and their influences to disease-free survival (DFS) was examined. DFS time was defined as the period from surgery to the date at first disease recurrence (local, regional, or contralateral breast cancer or distant recurrence). Patients who survived without any recurrence during tamoxifen treatment for 3 years were grouped as the nonrecurrence group, whereas patients who relapsed within 3 years were grouped as the recurrence group. The overall distribution of DFS was estimated using the Kaplan-Meier method. Statistical significance of a relationship between outcome and each of the genetic polymorphisms was assessed by log-rank test. Independent contribution of genetic factors to DFS was evaluated by Cox regression analysis. The result was considered to be statistically significant at bilateral P-values ≤ 0.05. Statistical tests were performed using Stata software (version 12; StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
All patients were estrogen receptor positive except one patient, who was estrogen receptor negative but progesterone receptor positive. There were no statistically significant differences between baseline characteristics of the two groups. The patient characteristics are listed in . Ten patients had either local or distant recurrence of within during 3 years of tamoxifen treatment. The mean DFS time of the recurrence group was 1.73 ± 0.74 years. The nonrecurrence had an average DFS time of 6.61 ± 1.73 years.
CYP3A4/5, ABCB1 and ABCC2 genotype and allele frequency
ABCB1 3435 T was found to have the highest allele frequency among the variants. However, CYP3A4*1B/*18 variants could not be investigated in all patients. CYP3A5*3, ABCB1 3435 C>T, ABCC2*1C, and ABCC2 68231 A>G allele frequency were found to be within Hardy–Weinberg equilibrium. The frequency of CYP3A5*1/*1,*1/*3 and *3/*3 genotypes were 63% (n = 19), 33% (n = 10) and 4% (n = 1), respectively. The frequency of ABCB1 3435 CC, CT and TT genotypes were 43% (n = 13), 40% (n = 12) and 17% (n = 5), respectively. The frequency of ABCC2*1/*1 and *1/*1C genotypes were 57% (n = 17) and 43% (n = 13), respectively. The frequency of ABCC2 68231 AA and AG genotypes were 47% (n = 14) and 53% (n = 16), respectively. Genotype and allele frequency of CYP3A5, ABCB1, and ABCC2 are shown in .
Table 2 Genotype and allele frequency of CYP3A5, ABCB1, and ABCC2
CYP3A5, ABCB1 and ABCC2 genetic variants and clinical outcomes
Genetic polymorphisms of all patients were evaluated for DFS association. Kaplan–Meier analysis showed that patients with heterozygous ABCB1 3435 CT genotype had significantly shorter DFS than those with homozygous 3435 CC genotype (P = 0.041) (). In contrast, homozygous 3435 TT genotype did not show different clinical outcomes than in wild-type 3435 CC patients (data not shown). Furthermore, patients with 3435 CT genotype also had significantly shorter DFS than others (CC+TT genotypes), as shown in (P = 0.011). No statistical association between CYP3A5*3, ABCC2*1C, and ABCC2 68231 A>G with clinical outcome was observed.
Figure 1 Kaplan–Meier estimates of disease-free survival of patients with 3435 ABCB1 genotype.
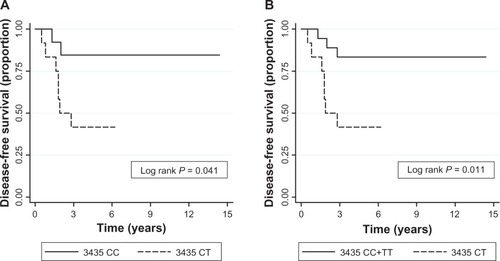
Cox regression analysis revealed that 3435 CT was associated with increased recurrence risk compared to 3435 CC (P = 0.043; hazard ratio [HR] = 5.11; 95% confidence interval [CI]: 1.05–24.74) and 3435 CC+TT genotypes (P = 0.023; HR = 4.83; 95% CI: 1.24–18.8) ().
Table 3 HRs in breast cancer patients treated with tamoxifen
Discussion
Intermediate and poor metabolizer-related CYP2D6 genotypes have been associated with unfavorable outcomes in estrogen positive breast cancer patients who received tamoxifen adjuvant therapy.Citation18–Citation22 However, several studies with large sample sizes have shown contradictory results.Citation4–Citation7 In Asian (including Thai) studies, CYP2D6*10, which is the most common variant, has been associated with short DFS.Citation20,Citation23–Citation25 However, it could not be indicated as a recurrent predictive marker in tamoxifen treatment.Citation23–Citation26 It has been suggested that genetic polymorphisms of other CYPs and drug transporters are involved in the variable effectiveness of tamoxifen.Citation21,Citation27 In this study, we evaluated the additional genetic variants associated with tamoxifen effectiveness in Thai patients with early-stage breast cancer.
CYP3A4*1B/18 could not be found in our limited sample size due to the fact that the frequency of both alleles is only around 1% in the Asian population;Citation28 however, the allele frequency of CYP3A5*3 (non-function variant) was observed to be comparable to that in other reports.Citation29,Citation30 Several studies, including one by our group, investigated the association of CYP3A5 genotype with tamoxifen clinical outcomes; no significant association was observed.Citation27,Citation31–Citation35 It has been demonstrated that CYP3A5*3/*3 does not affect endoxifen level in vitro and in vivo.Citation30,Citation36 Moreover, tamoxifen can be metabolized by other CYP enzymes such as CYP3A4 and CYP2C19 in the liver or by CYPs that are expressed in breast cancer cells.Citation2,Citation37,Citation38
Research in Japanese women has suggested that the A allele of ABCC2 68231 A>G is at risk for recurrence after 5 years of tamoxifen treatment but not ABCC2*1C (−24 C>T) and ABCB1 3435 C>T.Citation21 In contrast, we found that neither ABCC2 68231 A>G nor ABCC2*1C (−24 C>T) appear to influence tamoxifen adjuvant treatment. However, we found an association between ABCB1 3435 C>T and impact on recurrence risk in patients with 3435 CT genotype, which occurred more than in those with 3435 CC. Surprisingly, homozygous 3435 TT is not associated with the risk of recurrence whilst heterozygous 3435 CT is. This can be explained from evidence that 3435 C>T polymorphism is associated with certain changes in P-gp expression.Citation14 No difference in P-gp messenger (m)RNA and protein levels was observed in non-tumor cells with either 3435 CT or TT polymorphism.Citation15 However, a previous study in humans found that liver tumors with 3435 CT genotype expressed higher levels of P-gp protein compared to CC and TT genotype.Citation39 The increased P-gp protein expression limits drug penetration into intratumor cells. Furthermore, ABCB1 3435 CT genotype has previously been identified as an independent factor for DFS in breast and other cancers.Citation40–Citation42 However, this correlation needs to be verified based on an individual’s complete haplotype of the ABCB1 gene. Although in our study the sample size is not large, the association can still be observed. Therefore, our findings are interesting and warrant further investigation in a larger sample.
Conclusion
The findings of this study suggest that ABCB1 is a potential predictive marker of tamoxifen therapy outcomes.
Author contributions
All authors contributed to the interpretation of the results and read and approved the final manuscript.
Acknowledgments
This research project was financially supported by the Faculty of Science and Faculty of Medicine, Ramathibodi Hospital, Mahidol University. Additional support for this work has been provided by the Ramathibodi Cancer Center, Ramathibodi Hospital, Bangkok, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- DestaZWardBASoukhovaNVFlockhartDAComprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6J Pharmacol Exp Ther200431031062107515159443
- HoskinsJMCareyLAMcLeodHLCYP2D6 and tamoxifen: DNA matters in breast cancerNat Rev Cancer20099857658619629072
- GoetzMPSchaidDJWickerhamDLEvaluation of CYP2D6 and efficacy of tamoxifen and raloxifene in women treated for breast cancer chemoprevention: results from the NSABP P1 and P2 clinical trialsClin Cancer Res201117216944695121880792
- RaeJMDrurySHayesDFATAC trialistsCYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patientsJ Natl Cancer Inst2012104645246022395643
- ReganMMLeyland-JonesBBouzykMBreast International Group (BIG) 1–98 Collaborative GroupCYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trialJ Natl Cancer Inst2012104644145122395644
- BrooksJDTeraokaSNMaloneKEWECARE Study Collaborative GroupBernsteinJLFigueiredoJCVariants in tamoxifen metabolizing genes: a case-control study of contralateral breast cancer risk in the WECARE studyInt J Mol Epidemiol Genet201341354823565321
- ManiCGelboinHVParkSSPearceRParkinsonAKupferDMetabolism of the antimammary cancer antiestrogenic agent tamoxifen. I. Cytochrome P-450-catalyzed N-demethylation and 4-hydroxylationDrug Metab Dispos19932146456568104124
- IusufDTeunissenSFWagenaarERosingHBeijnenJHSchinkelAHP-glycoprotein (ABCB1) transports the primary active tamoxifen metabolites endoxifen and 4-hydroxytamoxifen, and restricts their brain penetrationJ Pharmacol Exp Ther2011337371071721378205
- ChoiHKYangJWRohSHHanCYKangKWInduction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cellsEndocr Relat Cancer200714229330317639045
- DaiDTangJRoseRIdentification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifosJ Pharmacol Exp Ther2001299382583111714865
- HustertEHaberlMBurkOThe genetic determinants of the CYP3A5 polymorphismPharmacogenetics200111977377911740341
- HesselinkDAvan SchaikRHvan der HeidenIPGenetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimusClin Pharmacol Ther200374324525412966368
- HaenischSZimmermannUDazertEInfuence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortexPharmacogenomics J200771566516788565
- Kimchi-SarfatyCOhJMKimIWA “silent” polymorphism in the MDR1 gene changes substrate specificityScience2007315581152552817185560
- ChoiJHAhnBMYiJMRP2 haplotypes confer differential susceptibility to toxic liver injuryPharmacogenet Genomics200717640341517502832
- LaecheltSTurriniERuehmkorfASiegmundWCascorbiIHaenischSImpact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and functionPharmacogenomics J2011111253420351751
- SchrothWHamannUFaschingPACYP2D6 polymorphisms as predictors of outcome in breast cancer patients treated with tamoxifen: expanded polymorphism coverage improves risk stratifcationClin Cancer Res201016174468447720515869
- MadlenskyLNatarajanLTchuSTamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomesClin Pharmacol Ther201189571872521430657
- XuYSunYYa oLAssociation between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatmentAnn Oncol20081981423142918407954
- KiyotaniKMushirodaTImamuraCKSignificant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patientsJ Clin Oncol20102881287129320124171
- ParkHSChoiJYLeeMJAssociation between genetic polymorphisms of CYP2D6 and outcomes in breast cancer patients with tamoxifen treatmentJ Korean Med Sci20112681007101321860550
- ToyamaTYamashitaHSugiuraHKondoNIwaseHFujiiYNo association between CYP2D6*10 genotype and survival of node-negative Japanese breast cancer patients receiving adjuvant tamoxifen treatmentJpn J Clin Oncol2009391065165619596663
- SukasemCSirachainanEChamnanphonMImpact of CYP2D6 polymorphisms on tamoxifen responses of women with breast cancer: a microarray-based study in ThailandAsian Pac J Cancer Prev20121394549455323167378
- ChamnanphonMPechatananKSirachainanEAssociation of CYP2D6 and CYP2C19 polymorphisms and disease-free survival of Thai post-menopausal breast cancer patients who received adjuvant tamoxifenPharmgenomics Pers Med20136374823776391
- OkishiroMTaguchiTJin KimSShimazuKTamakiYNoguchiSGenetic polymorphisms of CYP2D6*10 and CYP2C19*2,*3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifenCancer2009115595296119156902
- SchrothWAntoniadouLFritzPBreast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypesJ Clin Oncol200725335187519318024866
- ZhouQYuXShuCAnalysis of CYP3A4 genetic polymorphisms in Han ChineseJ Hum Genet201156641542221412247
- SupanyaDTassaneeyakulWSirivongsDPrevalence of CYP3A5 polymorphism in a Thai populationThai Journal of Pharmacology20093119597
- LimJSLChenXASinghOImpact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharma-cokinetics in Asian breast cancer patientsBr J Clin Pharmacol201171573775021480951
- JinYDestaZStearnsVCYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatmentJ Natl Cancer Inst2005971303915632378
- MurdterTESchrothWBacchus-GerybadzeLGerman Tamoxifen and AI Clinicians GroupEichelbaumMSchwabMBrauchHActivity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasmaClin Pharmacol Ther201189570871721451508
- GoetzMRaeJSumanVPharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashesJ Clin Oncol200523369312931816361630
- TuckerANTkaczukKALewisLMTomicDLimCKFlawsJAPolymorphisms in cytochrome P4503A5 (CYP3A5) may be associated with race and tumor characteristics, but not metabolism and side effects of tamoxifen in breast cancer patientsCancer Lett20052171617215596297
- GjerdeJGeislerJLundgrenSAssociations between tamoxifen, estrogens, and FSH serum levels during steady state tamoxifen treatment of postmenopausal women with breast cancerBMC Cancer201010131320565970
- MugunduGMSallansLGuoYShaughnessyEADesaiPBAssessment of the impact of CYP3A polymorphisms on the formation of α-hydroxytamoxifen and N-desmethyltamoxifen in human liver microsomesDrug Metab Dispos201240238939622096084
- RooneyPHTelferCMcFadyenMCMelvinWTMurrayGIThe role of cytochrome P450 in cytotoxic bioactivation: future therapeutic directionsCurr Cancer Drug Targets20044325726515134533
- MurrayGIPatimallaSStewartKNMillerIDHeysSDProfiling the expression of cytochrome P450 in breast cancerHistopathology201057220221120716162
- BaldisseraVDde MattosAACoralGPEvaluation of the C3435T polymorphism in the MDR1 gene in patients with hepatocellular carcinomaAnn Hepatol201211689990623109454
- KafkaASauerGJaegerCPolymorphism C3435T of the MDR-1 gene predicts response to preoperative chemotherapy in locally advanced breast cancerInt J Oncol20032251117112112684679
- ChangHRhaSYJeungH-CAssociation of the ABCB1 gene polymorphisms 2677G.T/A and 3435C.T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patientsAnn Oncol200920227227718836089
- IllmerTSchulerUSThiedeCMDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patientsCancer Res200262174955496212208746