Abstract
Background
Rheumatoid arthritis (RA) is a common autoimmune disease with the main symptoms being joint swelling and pain. In severe cases, joint deformity or even complete loss of function occurs. Technetium methylene diphosphonate (99Tc-MDP) is widely used for RA treatment in China, but there are no studies on the effects of 99Tc-MDP on intestinal flora.
Objective
To explore the effects of 99Tc-MDP treatment on the composition and function of the intestinal flora and to provide new information on the mechanism of 99Tc-MDP in RA treatment.
Methods
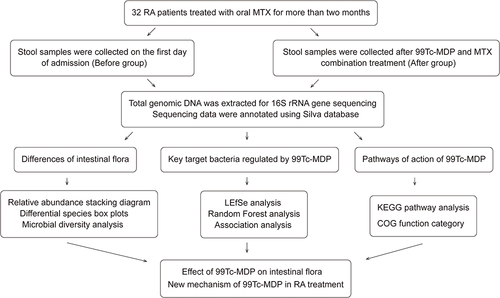
Stool samples from RA patients before and after 99Tc-MDP treatment were collected to form two groups (Before and After). Total genomic DNA of the samples was extracted for 16S rRNA gene sequencing. The altered composition of the intestinal flora, the key target bacteria regulated by 99Tc-MDP, and the pathways of action of 99 Tc-MDP were analyzed by bioinformatics.
Results
A total of 64 fresh stool samples were collected from 32 RA patients. Compared to the Before group, the After group showed increased Bacteroidetes abundance and decreased Firmicutes abundance. At the genus level, Prevotella increased whereas Escherichia decreased. Both α and β diversity analyses showed that 99Tc-MDP treatment did not affect gut microbial diversity in RA patients. LEfSe analyses and random forest analyses showed Bacteroidetes, Prevotella, Enterococcus, Escherichia and Ruminococcaceae were the main 99Tc-MDP regulating bacteria. Functional enrichment analysis revealed that the functional differences in gut flora of the two groups centered on Metabolism and Genetic Information Processing.
Conclusion
This study revealed differences in the composition of the gut microbiota in RA patients before and after 99Tc-MDP treatment. The therapeutic effect of 99Tc MDP is mainly achieved through Bacteroidetes, Prevotella, and Enterococcus. Regulating metabolism and genetic information processing of gut flora may be the mechanism of 99Tc-MDP in treating RA.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease. As the disease progresses, it will cause the gradual loss of joint function, which will seriously affect the life and work of patients. The global prevalence of RA is about 1%, with a much higher incidence in women than in men.Citation1 Although the pathogenesis of RA is unclear, scholars agree that it is closely related to environmental factors, genetic susceptibility, and immune dysfunction. Gut microbes happen to interact closely with all the three.Citation2–4 Lifestyle, dietary preferences, and gut flora metabolites affect the development and prognosis of RA.Citation3,Citation5 Meanwhile, the RA susceptibility gene HLA-DRB1 favors a more inflammatory gut microbiome.Citation6 In addition, gut microbes are indispensable for the maturation of the immune system, especially mucosa-associated lymphoid tissue.Citation7 In recent years, with the development of high-throughput sequencing technologies, more and more evidence has shown that the pathogenesis of RA is closely related to the host gut microbiota, especially in the induction of chronic inflammation and activation of autoimmunity.Citation8,Citation9
Technetium methylene diphosphonate (99Tc-MDP) is a nuclide preparation, and its main component is the complex formed by the trace element technetium and methylene diphosphonic acid (Indications: RA and orthopaedic diseases). 99Tc-MDP has strong anti-inflammatory and bone repair effects, and at the same time has high safety. It is often used in the treatment of RA in China.Citation10–12 99Tc-MDP can also reduce the secretion of inflammatory cytokines such as TNF-α and IL-6 by regulating immune cells.Citation13,Citation14 Clinically, 99Tc-MDP is often used in combination with methotrexate (MTX), the basic drug for RA. The results of a multicenter, double-blind RCT showed that 99Tc-MDP and MTX combination therapy was more effective in RA patients and did not increase the incidence of adverse events compared with 99Tc-MDP or MTX monotherapy.Citation15 There have been some studies on MTX and gut microbial interactions, but there are no studies on the effect of 99Tc-MDP on the gut flora of RA patients.Citation16,Citation17 Therefore, we investigated the effect of 99Tc-MDP on the gut microbiota using 16S rRNA gene sequencing and performed bioinformatics analysis. This study provides new information on the mechanism of 99Tc-MDP in RA treatment.
Materials and Methods
Participants
All inpatients who were definitively diagnosed with RA based on clinical, laboratory and imaging status from September to November 2019 at the Department of Rheumatology and Immunology, the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine were screened. The RA diagnoses also fulfilled the 2010 American College of Rheumatology/ European League Against Rheumatism (ACR/EULAR) classification criteria for RA.Citation18 The age, tender joint count (TJC), swollen joint count (SJC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), anti-cyclic citrullinated peptide antibody (ACPA), and other clinical conditions of all the participants were recorded. The patients were advised to maintain their usual diet, lifestyle, and exercise throughout the study period. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (GK2019004), and all participants signed an informed consent form. All study procedures involving human participants were in accordance with the 1964 Declaration of Helsinki and its subsequent revisions.
The patients were enrolled according to the following criteria: (1) Lived in Guizhou Province in the previous year. (2) Aged less than 75 years old and more than 18 years old, and were able to take care of themselves. (3) Oral MTX (10 mg/week) monotherapy for more than 2 months. (4) Disease activity score DAS28-ESR > 3.2.Citation19 (5) After evaluation by two senior rheumatologists, 99Tc-MDP could be added to the treatment and there was no drug contraindication. The patients were excluded based on any of the following factors: (1) History of severe gastrointestinal diseases such as gastroenteritis, peptic ulcer, and gastric tumor. (2) Antibiotics, glucocorticoids, disease-modifying antirheumatic drugs (DMARDs) except MTX and other immunosuppressive drugs were used within 2 months. (3) Special diet within 2 weeks, such as Chinese herbal medicine, probiotics, yogurt, coffee, and wine. (4) Complicated with hypertension, hyperlipidemia, diabetes, and other serious cardiovascular diseases or metabolic diseases. (5) Combined with other autoimmune diseases such as systemic lupus erythematosus, ankylosing spondylitis, and multiple sclerosis. (6) Children, pregnant or lactating women, and patients with mental illness. (7) Those who discontinued medication without authorization or were lost to follow-up.
Sample Collection
Fresh faecal samples were collected from RA patients on the first day of admission and transported by cold chain to a freezer at −80°C for storage within 10 minutes. These samples were labelled as before the 99Tc-MDP treatment group (Before). After 10 days of treatment with the combination of 99Tc-MDP and MTX, fresh stool samples were collected again using the same method and labelled as after the 99Tc-MDP treatment group (After).
Details of treatment for RA patients: (1) 99Tc-MDP: agent A (5 mL, containing technetium [99Tc] 0.05 μg) and agent B (containing methylene diphosphonic acid of 5 mg and stannous chloride of 0.5 mg, 3 vials, a total of 16.5 mg) fully mixed and then set for 5 minutes. Added to 250 mL of 0.9% sodium chloride solution, intravenous drip, once a day for 10 consecutive days. (2) MTX: Patients had been treated with MTX for more than 2 months before hospitalization; therefore the dosage remained unchanged according to the previous dosage (10 mg/week orally).
DNA Extraction, Amplification, Library Construction and Sequencing
Total genomic DNA from samples was extracted using the TIANamp Stool DNA kit (TLANGEN Co., China). DNA concentration and integrity were measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and agarose gel electrophoresis, respectively. PCR amplification of the V3-V4 hypervariable regions of the bacterial 16S rRNA gene using universal primer pairs (343F: 5′-TACGGRAGGCAGCAG-3′; 798R:5′-AGGGTATCTAATCCT-3′). The reverse primer contained a sample barcode and both primers were connected with an Illumina sequencing adapter.
The PCR products were purified with Agencourt AMPure XP beads (Beckman Coulter Co., USA) and quantified using Qubit dsDNA assay kit. Sequencing was performed on an IlluminaNovaSeq6000 with two paired-end read cycles of 250 bases each. (Illumina Inc., San Diego, CA, USA; OEBiotech Company, Shanghai, China). Paired-end reads were filtered low-quality sequences, denoised, merged, and detect and cut off the chimera reads using DADA2 with the default parameters of QIIME2.Citation20,Citation21 Last, the software output the representative reads and the ASV abundance table. All representative reads were annotated and blasted against the Silva database (Version 138) using q2-feature-classifier with the default parameters.
Bioinformatics Analysis
Differences between the two groups were also counted at the levels of phylum, class, order, family, and genus. To evaluate the 99Tc-MDP treatment infection on the diversity of gut microbiota, the alpha-diversity that includes Chao1 index and Shannon index was calculated.Citation22 The similarity of bacterial communities was estimated using beta diversity. The Bray-Curtis dissimilarity and Weighted UniFrac dissimilarity were calculated.
LEfSe analysis, linear discriminant analysis (LDA) coupled with effect size measurements, was used to identify the main differential bacteria. LDA score indicates the magnitude of the difference contribution, the larger the absolute value the greater the difference contribution. Random Forest is a machine learning classification model that can efficiently and accurately classify microbial community samples. Mean Decrease Gini is used to compare the importance of variables by calculating their effect on the heterogeneity of the classification model. A higher value indicates that the variable (species) is more important.
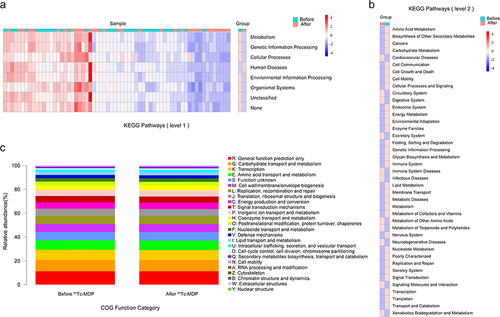
Association analysis was performed on the top 30 genera with relative abundance in the gut microbiota to determine the correlation between species (Spearman correlation coefficient). KEGG pathway enrichment analysis was performed at three levels. The Clusters of Orthologous Groups of proteins (COG) database was used to infer the main biological functions of the microbiota. The brief process is shown in .
Statistical Analysis
Statistical analyses of clinical status of the RA patients were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA). Continuous variables with a normal distribution and homogeneity of variance are represented by mean ± standard deviation (SD), and comparisons between groups were performed by t-test. Continuous variables that do not conform to the normal distribution are represented by the median (interquartile range) and comparisons between groups were performed by the Mann–Whitney U-test. Categorical variables are expressed in terms of quantity and percentage (n, %). The above test methods were all statistically significant with P < 0.05.
Results
Clinical Status
A total of 64 fresh stool samples were collected from 32 RA patients. After the combined treatment of 99Tc-MDP and MTX, the ESR, CPR, RF, and DAS28-ESR of RA patients were significantly decreased (P < 0.05). It indicated that the patients had reduced disease activity and improved disease severity after treatment ().
Table 1 Clinical Status Before and After Treatment
Sequencing Data
We sequenced a total of 64 stool samples and obtained 3,198,961 clean tags after quality control. After removing chimeras, 2,888,964 valid tags (90.3%) were obtained. The valid tags of each sample were from 29,578 to 49,022. The mean length of the valid tags was from 409 bp to 424 bp. The number of OTUs of each sample was from 331 to 1680. Annotated information based on matching OTUs included 19 phyla, 47 classes, 101 orders, 175 families, 413 genera, and 138 species of gut microbes.
99Tc-MDP Therapy Regulated the Abundance of Gut Microbiota
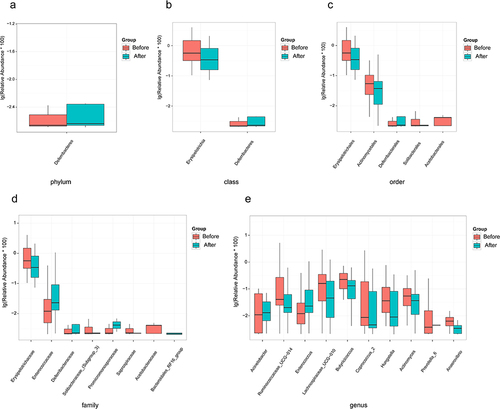
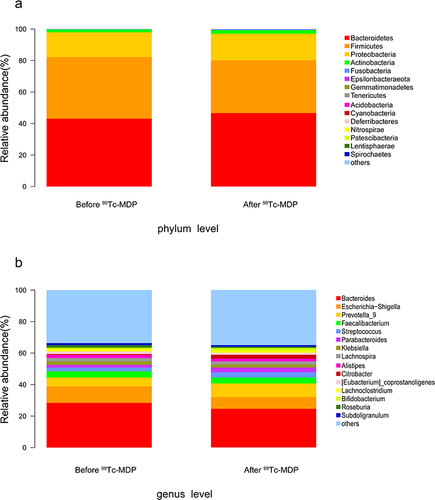
The gut microbial composition of RA patients before and after 99Tc-MDP treatment is shown in . At the phylum level (), Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, and Fusobacteria were the five most abundant phyla, accounting for over 98% of all the microorganisms. Of these, Bacteroidetes abundance increased while Firmicutes abundance decreased. At the genus level (), Bacteroides, Escherichia-Shigella, Prevotella_9, Faecalibacterium, and Streptococcus were the five most abundant genera. Of these, Escherichia-Shigella decreased by 30% and Prevotella_9 increased by 55%.
Figure 2 Compositional of gut microbes. (a) Relative abundance at the phylum level. (b) Relative abundance at the genus level.
Compared to the Before group, the Deferriactors increased significantly at the phylum level (). At the class level, the abundance of Deferribacteres increased significantly, while Erysipelotrichia decreased (). At the order level, Deferribacterales and Solibacterales increased, while Erysipelotrichales, Actinomycetales, and Acetobacterales decreased (). At the family level, Enterococcaceae, Deferribacterales, Solibacteraceae_(Subgroup_3), Promicromonosporaceae, and Bacteroidales_RF16_group increased, while Erysipelotrichales, Saprospiraceae, and Acetobacterales decreased (). At the genus level, Acinetobacter, Enterococcus, and Prevotella_6 increased, whereas Ruminococcaceae_UCG-014, Lachnospiraceae_UCG-010, Butyricicoccus, Coprococcus_2, Hungatella, Actinomyces, and Anaerovibrio decreased ().
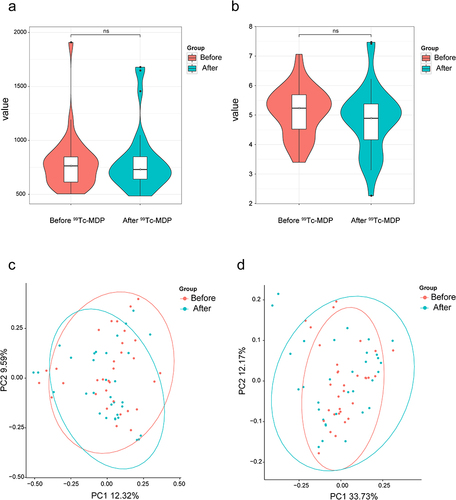
Gut Microbial Diversity Unaffected with 99Tc-MDP Therapy
The alpha-diversity results showed that the Chao index and Shannon index had no significant differences between the two groups (P > 0.05; and ). The similarity of bacterial communities was estimated using beta diversity. Similarity analysis showed that there was no significant difference between the two groups (Bray-Curtis, R2=0.0129, P > 0.05; Weighted UniFrac, R2=0.0107, P > 0.05). Principal Coordinates Analysis (PCoA) was performed based on Bray–Curtis distances and weighted UniFrac distances to visualize the similarity of the bacterial community structure in the two groups. As shown in and , the clustering and bacterial composition of the two groups were similar.
LEfSe Analysis to Identify Main Differential Bacteria
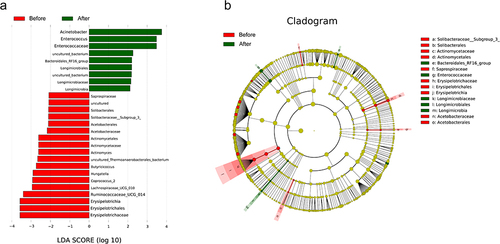
LEfSe analysis was used to reveal the composition of species that differed between two groups of biomes. It included the magnitude of the species’ contribution to the difference () and cladogram annotation of the differential species (). The results showed that Acinetobacter, Enterococcus, Erysipelotrichaceae, and Ruminococcaceae_UCG_014 were the main microorganisms responsible for the differences between the two groups and were the main regulatory bacteria for 99Tc-MDP treatment.
Figure 5 LEfSe analysis. (a) The magnitude of the species’ contribution to the difference. LDA SCORE indicates the magnitude of the difference contribution; the larger the absolute value, the greater the difference contribution. (b) Cladogram annotation of the differential species. Nodes at each layer indicate phylum/class/order/family/genus from the inside out. Node diameter is proportional to relative abundance. Red, rich in the Before group; Green, rich in the After group; Yellow, the difference is not significance.
Random Forest Analysis to Identify Key Classified Bacteria
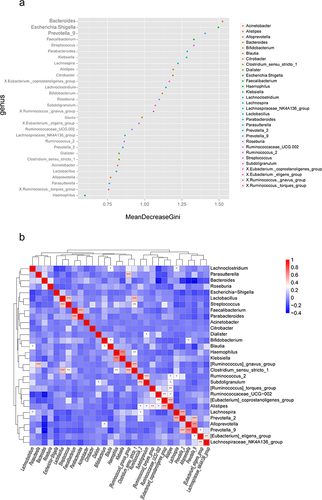
Random Forest model can efficiently and accurately classify microbial community samples. Mean Decrease Gini is used to assess the importance of variables. A higher value indicates that the variable (species) is more important. The results showed that Bacteroides, Escherichia-Shigella, and Prevotella_9 were the most important variables in the classification model and were the key bacteria to distinguish 99Tc-MDP before and after treatment ().
Association Analysis of Gut Microbiota
Association analysis was performed on the top 30 genera with relative abundance in the gut microbiota. As shown in , Bacteroides was negatively correlated with Prevotella_9 and Ruminococcaceae_UCG-002. Prevotella_9 was positively correlated with Alloprevotella and Prevotella_2.
Functional Enrichment Analysis
KEGG pathway enrichment analysis was performed at three levels. At level 1 (), microbial functions in Metabolism, Genetic Information Processing, Cellular Processes, Human Diseases, Environmental Information Processing, and Organismal Systems were significantly different (P < 0.05) after 99Tc-MDP treatment. At level 2 (), there were 41 significantly different functions between the two groups, of which 43% (18 items) were Metabolism and Genetic Information Processing related. At level 3 (Supplementary Table 1), we found 293 differential functional pathways of which 30% (88 items) were related to Metabolism. COG database was used to infer the function of microbiota. The results showed that Carbohydrate transport and metabolism, Transcription, Amino acid transport and metabolism were the functions with the largest percentage ().
Discussion
The human microbiome is involved in the development and regulation of the host immune system, and dysbiosis of the intestinal flora is considered a key predisposing factor for RA.Citation23,Citation24 An increasing number of studies have showed significant differences in the composition of gut microbiota in patients with RA compared to healthy controls. In China, 99Tc-MDP is often combined with MTX for the treatment of RA with anti-inflammatory, analgesic and immunomodulatory effects.Citation17,Citation25,Citation26 However, the relationship between 99Tc-MDP and gut microbes has not been reported. Therefore, we clarified the effect of 99Tc-MDP on the gut microbiota by 16S rRNA gene sequencing.
Consistent with recent reports, Bacteroidetes, Firmicutes, and Proteobacteria are the main dominant phylum in patients with RA and show an increase in Bacteroidetes and a decrease in Firmicutes.Citation7,Citation27,Citation28 A study on the prediction of MTX efficacy showed that the ratio of Firmicutes/Bacteroidetes in MTX non-responder is high.Citation17 However, after 99Tc-MDP treatment, Bacteroidetes increased, Firmicutes decreased, and the ratio decreased. This suggests that 99Tc-MDP and MTX may have a synergistic effect in regulating intestinal flora and that 99Tc-MDP treatment can increase the response rate of MTX. Notably, our study found that 99Tc-MDP treatment did not alter gut microbial diversity in RA patients. This suggests that 99Tc-MDP is not simply an “antibiotic” or a “culture medium”, but specifically regulates the target flora to alleviate RA.
In patients with RA, there are large amounts of secreted immunoglobulins (IgA and IgM) such as RF, ACPA, and anti-carbamylated protein antibodies. Studies have found that these immunoglobulins are usually produced in mucosal areas and are associated with Prevotella.Citation29 An HLA-DR presenting peptide was identified in Prevotella’s 27-kDa protein (Pc-p27) that stimulated Th1 cell responses in 42% of patients with new-onset RA.Citation30 It was also shown patients with RA (both new-onset and chronic) either had IgA-like antibody responses to Pc-p27 or Prevotella or presented anti-Prevotella IgG antibodies.Citation4 Random Forest analysis also showed that Prevotella is a key classified bacterium.
Enterococcus can have a broad inhibitory effect on pathogenic bacteria by producing bacteriocins.Citation31 Enterococcus may be a potential probiotic for RA patients, enhancing the anti-inflammatory effects of MTX and alleviating arthritic symptoms in adjuvant-induced arthritis rat model.Citation32 Our study showed a significant increase in Enterococcus after 99Tc-MDP treatment, while LEfSe analysis also identified Enterococcus as the main differential bacteria. Therefore, the regulation of Enterococcus abundance may be one of the mechanisms of 99Tc-MDP for RA treatment. In addition, several studies have found an increased proportion of Escherichia and Ruminococcaceae in fecal samples from RA patients.Citation33,Citation34 And 99Tc-MDP treatment can reverse the regulation and reduce the abundance of these bacteria.
Functional enrichment analysis showed the effects after 99Tc-MDP treatment were focused on the Metabolism and Genetic Information Processing functions of gut microbiota. Short-chain fatty acids (SCFAs) are metabolites of intestinal flora. While providing energy for the host, SCFAs also promote the activation of Tregs by inhibiting histone deacetylase (HDAC).Citation35,Citation36 According to studies, levels of valproic acid, butyric acid and propionic acid were reduced in RA.Citation37–39 However, a high-fibre diet increases these SCFAs, reduces pro-inflammatory cytokines (IL-33, IL-18 and CCL2), and prevents inflammation-induced bone loss. This coincides with the role of 99Tc-MDP in bone metabolism. In addition, overactivation of tryptophan metabolism contributes to RA, and dysregulation of tryptophan metabolism is closely associated with Escherichia.Citation33,Citation40–43 On the other hand, the gut microbiome also interacts with many RA-related genes. RA susceptibility gene HLA-DRB1 favors a more inflammatory gut microbiome that drives aberrant immunity in RA.Citation6,Citation44,Citation45 The relative abundance of Prevotella in RA is negatively correlated with the presence of HLA-DRB1.Citation46
Conclusions
In conclusion, this study revealed differences in the composition of gut microbiota before and after 99Tc-MDP treatment. 99Tc-MDP exerts therapeutic effects by modulating the abundance of specific bacteria, especially Bacteroidetes, Prevotella, and Enterococcus. Influencing metabolism and genetic information processing of gut flora may be one of the mechanisms of how 99Tc-MDP treats RA.
Ethical Statement
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (GK2019004), and all participants signed an informed consent form. All study procedures involving human participants were in accordance with the 1964 Declaration of Helsinki and its subsequent revisions.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Laboratory Department of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine. The author is very grateful to the serum analysts in the laboratory. The authors would like to thank all the nurses who assisted in collecting stool samples.
Data Sharing Statement
The raw sequencing data are deposited into the NCBI BioProject (http://www.ncbi.nlm.nih.gov/bioproject/871753) under accession number: PRJNA871753. The study protocol and the datasets generated during analysis during the current study will be available from the corresponding author on reasonable request.
Additional information
Funding
References
- Aziz AUR, Farid S, Qin K, et al. PIM kinases and their relevance to the PI3K/AKT/mTOR pathway in the regulation of ovarian cancer. Biomolecules. 2018;8(1):7. doi:10.3390/biom8010007
- Miyauchi E, Shimokawa C, Steimle A, et al. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. 2023;23(1):9–23. doi:10.1038/s41577-022-00727-y
- Wolte M, Grant ET, Boudaud M, et al. Leveraging diet to engineer the gut microbiome. Nat Rev Gastroenterol Hepatol. 2021;18(12):885–902. doi:10.1038/s41575-021-00512-7
- Zaiss MM, Joyce Wu H-J, Mauro D, et al. The gut-joint axis in rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(4):224–237. doi:10.1038/s41584-021-00585-3
- Badsha H. Role of diet in influencing rheumatoid arthritis disease activity. Open Rheumatol J. 2018;12:19–28. doi:10.2174/1874312901812010019
- Asquith M, Sternes PR, Costello M-E, et al. HLA alleles associated with risk of ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthritis Rheumatol. 2019;71(10):1642–1650. doi:10.1002/art.40917
- Zhao Q, Maynard CL. Mucus, commensals, and the immune system. Gut Microbes. 2022;14(1):2041342. doi:10.1080/19490976.2022.2041342
- Yuan L, Zhang S-X, Yin X-F, et al. The gut microbiota and its relevance to peripheral lymphocyte subpopulations and cytokines in patients with rheumatoid arthritis. J Immunol Res. 2021;2021:6665563. doi:10.1155/2021/6665563
- Kang Y, Cai Y, Yang Y, et al. The gut microbiome and hepatocellular carcinoma: implications for early diagnostic biomarkers and novel therapies. Liver Cancer. 2021;11(2):113–125. doi:10.1159/000521358
- Mu R, Liang J, Sun L, et al. A randomized multicenter clinical trial of 99 Tc-methylene diphosphonate in treatment of rheumatoid arthritis. Int J Rheum Dis. 2018;21(1):161–169. doi:10.1111/1756-185X.12934
- Chen J, Lan Y, Yue H, et al. 99Tc-MDP-induced human osteoblast proliferation, differentiation and expression of osteoprotegerin. Mol Med Rep. 2017;16(2):1801–1809. doi:10.3892/mmr.2017.6839
- Wu Q, Ni Y, Yang Q, et al. 99 Tc-MDP treatment for the therapy of rheumatoid arthritis, choroidal neovascularisation and Graves’ ophthalmopathy. Biomed Rep. 2016;4(4):400–402. doi:10.3892/br.2016.609
- Wu Y-G, Ma Q-L, Liu G-F, et al. Effect of 99Tc-MDP on cytokine production by peripheral blood mononuclear cells of patients with rheumatoid arthritis. Hunan Yi Ke Da Xue Xue Bao. 2002;27(2):173–175.
- Su D, Shen M, Gu B, et al. (99) Tc-methylene diphosphonate improves rheumatoid arthritis disease activity by increasing the frequency of peripheral γδ T cells and CD4(+) CD25(+) Foxp3(+) Tregs. Int J Rheum Dis. 2016;19(6):583–593. doi:10.1111/1756-185X.12292
- Fu Q, Feng P, Sun L-Y, et al. A double-blind, double-dummy, randomized controlled, multicenter trial of 99Tc-methylene diphosphonate in patients with moderate to severe rheumatoid arthritis. Chin Med J. 2021;134(12):1457–1464. doi:10.1097/CM9.0000000000001527
- Nayak RR, Alexander M, Deshpande I, et al. Methotrexate impacts conserved pathways in diverse human gut bacteria leading to decreased host immune activation. Cell Host Microbe. 2021;29(3):362–377.e11. doi:10.1016/j.chom.2020.12.008
- Artacho A, Isaac S, Nayak R, et al. The pretreatment gut microbiome is associated with lack of response to methotrexate in new-onset rheumatoid arthritis. Arthritis Rheumatol. 2021;73(6):931–942. doi:10.1002/art.41622
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
- England BR, Tiong BK, Bergman MJ, et al. 2019 Update of the American college of rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res. 2019;71(12):1540–1555. doi:10.1002/acr.24042
- Callahan BJ, Mcmurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference fromIllumina amplicon data. Nature Methods. 2016;13(7):581–583. doi:10.1038/nmeth.3869
- Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnol. 2019;37(8):852–857. doi:10.1038/s41587-019-0209-9
- Chao A, Bunge J. Estimating the number of species in a stochastic abundance model. Biometrics. 2002;58(3):531–539. doi:10.1111/j.0006-341x.2002.00531.x
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi:10.1056/NEJMra1004965
- Edilova MI, Akram A, Abdul-Sater AA. 9 Innate immunity drives pathogenesis of rheumatoid arthritis. Biomed J. 2021;44(2):172–182. doi:10.1016/j.bj.2020.06.010
- Li Y, Cai M, Zhang R, et al. Investigating the preventive effects of 99tc-methylene diphosphonate on a glucocorticoid-induced osteoporosis rabbit model. Curr Top Med Chem. 2021;21(26):2425–2433. doi:10.2174/1568026621666210804114744
- Scher JU, Nayak RR, Ubeda C, et al. 9 Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat Rev Rheumatol. 2020;16(5):282–292. doi:10.1038/s41584-020-0395-3
- Mei L, Yang Z, Zhang X, et al. Sustained drug treatment alters the gut microbiota in rheumatoid arthritis. Front Immunol. 2021;12:704089. doi:10.3389/fimmu.2021.704089
- Kishikawa T, Maeda Y, Nii T, et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann Rheum Dis. 2020;79(1):103–111. doi:10.1136/annrheumdis-2019-215743
- van Delft MAM, van der Woude D, Toes REM, et al. Secretory form of rheumatoid arthritis-associated autoantibodies in serum are mainly of the IgM isotype, suggesting a continuous reactivation of autoantibody responses at mucosal surfaces. Ann Rheum Dis. 2019;78(1):146–148. doi:10.1136/annrheumdis-2018-213724
- Pianta A, Arvikar S, Strle K, et al. Evidence of the immune relevance of prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69(5):964–975. doi:10.1002/art.40003
- Hanchi H, Mottawea W, Sebei K, et al. The genus enterococcus: between probiotic potential and safety concerns-an update. Front Microbiol. 2018;9:1791. doi:10.3389/fmicb.2018.01791
- Rovenský J, Svík K, Matha V, et al. Combination treatment of rat adjuvant-induced arthritis with methotrexate, probiotic bacteria enterococcus faecium, and selenium. Ann N Y Acad Sci. 2005;1051:570–581. doi:10.1196/annals.1361.101
- Yu D, Du J, Pu X, et al. The gut microbiome and metabolites are altered and interrelated in patients with rheumatoid arthritis. Front Cell Infect Microbiol. 2022;11:763507. doi:10.3389/fcimb.2021.763507
- Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. doi:10.1038/nm.3914
- Rasouli-Saravani A, Jahankhani K, Moradi S, et al. Role of microbiota short-chain fatty acids in the pathogenesis of autoimmune diseases. Biomed Pharmacother. 2023;162:114620. doi:10.1016/j.biopha.2023.114620
- Attur M, Scher JU, Abramson SB, et al. Role of intestinal dysbiosis and nutrition in rheumatoid arthritis. Cells. 2022;11(15):2436. doi:10.3390/cells11152436
- Yao Y, Cai X, Zheng Y, et al. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br J Pharmacol. 2022;179(17):4315–4329. doi:10.1111/bph.15852
- Dürholz K, Hofmann J, Iljazovic A, et al. Dietary short-term fiber interventions in arthritis patients increase systemic SCFA levels and regulate inflammation. Nutrients. 2020;12(10):3207. doi:10.3390/nu12103207
- Lucas S, Omata Y, Hofmann J, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. doi:10.1038/s41467-017-02490-4
- Stoll ML, Kumar R, Lefkowitz EJ, et al. Fecal metabolomics in pediatric spondyloarthritis implicate decreased metabolic diversity and altered tryptophan metabolism as pathogenic factors. Genes Immun. 2016;17(7):400–405. doi:10.1038/gene.2016.38
- Kang KY, Lee SH, Jung SM, et al. Downregulation of tryptophan-related metabolomic profile in rheumatoid arthritis synovial fluid. J Rheumatol. 2015;42(11):2003–2011. doi:10.3899/jrheum.141505
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi:10.1016/j.chom.2018.05.003
- Rosser EC, Piper CJM, Matei DE, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837–851.e10. doi:10.1016/j.cmet.2020.03.003
- Liu X, Zou Q, Zeng B, et al. Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Curr Microbiol. 2013;67(2):170–176. doi:10.1007/s00284-013-0338-1
- Gomez A, Luckey D, Yeoman CJ, et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One. 2012;7(4):e36095. doi:10.1371/journal.pone.0036095
- Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi:10.7554/eLife.01202