Abstract
Immortalization is an important step toward the malignant transformation of human cells and is critically dependent upon telomere maintenance. There are two known mechanisms to maintain human telomeres. The process of telomere maintenance is either mediated through activation of the enzyme telomerase or through an alternative mechanism of telomere lengthening called ALT. While 85% of all human tumors show reactivation of telomerase, the remaining 15% are able to maintain telomeres via ALT. The therapeutic potential of telomerase inhibitors is currently investigated in a variety of human cancers. Gastrointestinal tumors are highly dependent on telomerase as a mechanism of telomere maintenance, rendering telomeres as well as telomerase potential targets for cancer therapy. This article focuses on the molecular mechanisms of telomere biology and telomerase activation in gastrointestinal cancers and reviews strategies of telomerase inhibition and their potential therapeutic use in these tumor entities.
Introduction
Circumvention of telomere-based senescence via activation of telomere maintenance mechanisms has been defined as a hallmark of cancer. During the last decade, our understanding of telomere biology and mechanisms of telomere maintenance in normal tissue and cancer development has improved significantly and fostered the development of telomere and telomerase-based therapeutic strategies. In this article, the genetic and molecular underpinnings of telomere maintenance in gastrointestinal cancer and their therapeutic implications are reviewed. As epithelial tumors, gastrointestinal cancers predominantly utilize telomerase to maintain telomeres. However, the molecular mechanisms that induce activation of telomerase differ remarkably among cancers of the gastrointestinal tract including transcriptional and posttranscriptional mechanisms as well as receptor tyrosine kinase signaling. Current telomerase-based therapies do either target telomerase directly to inhibit its enzyme function or use telomerase as an antigen to elicit antitumor immunity. Preclinical and early clinical trials have revealed promising results in gastrointestinal cancers and might provide novel therapeutic options in the future.
Telomere biology and mechanisms of telomere maintenance
Telomere biology
The current understanding that chromosome ends are important for the maintenance of chromosomal stability originated almost 80 years ago with two independent publications by McClintock and Muller.Citation1,Citation2 In the 1980s, it was demonstrated that the DNA sequences at the end of chromosomes consisted of DNA repeats comprising TTAGGG in humans.Citation3,Citation4 These terminal DNA sequences were named telomeres, and later experiments in yeast showed that artificial chromosomes could be stabilized by the addition of these telomere repeats, indicating their crucial role in the maintenance of chromosomal stability. To exert this function, one of two higher-order structures named T-loop and G-quadruplex, respectively, is formed by a 150–250 nucleotide long single-stranded G-rich 3′ overhang.Citation5,Citation6 The T-loop structure is stabilized by a complex of six specialized proteins known as shelterins that cap the telomeres and protect the single-stranded overhangs from DNA-damage repair and end-to-end fusionsCitation7 ().
Figure 1 Telomeres and telomerase as therapeutic targets.
Abbreviations: hTER, human telomerase RNA; hTERT, human telomerase reverse transcriptase; PARP, poly (ADP-ribose) polymerase.
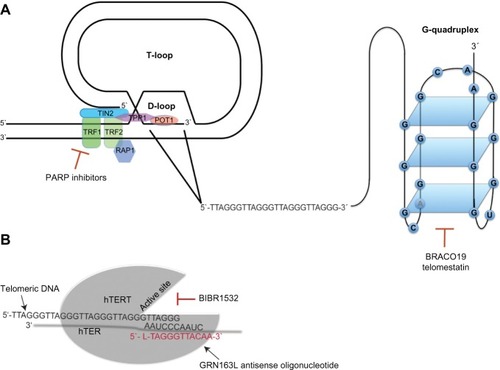
The G-quadruplex is formed by stacked guanosine quartets incorporating a 16-nucleotide d(GGGTTAGGGTTAGGGT) and a 6-nucleotide d(TAGGGT) sequence of telomeric 3′ overhang, folded via hydrogen bonding (). G-quadruplexes protect the telomeric DNA from being accessed by telomerase, regulating its catalytic activity.Citation8 Moreover, recent data indicate that G-quadruplexes can protect telomeres from DNA-damage signaling.Citation9 In normal cells, telomeres shorten by 50–150 bp with each cell division. After 60–80 cell divisions, telomeres normally reach a critical length that impairs their protective function, leading to the activation of DNA-damage responses very similar to those induced by DNA double-strand breaks.Citation10 The induction of DNA-damage responses results in cellular growth arrest called “replicative senescence”.Citation11 Thus, the stepwise loss of telomeric DNA with aging restricts the life span of each individual cell and serves as an essential mechanism of tissue homeostasis. Moreover, replicative senescence forms an important barrier against tumorigenesis that can only be overcome when cells acquire the ability to maintain their telomeres in the process of malignant transformation.
Mechanisms of telomere maintenance
The observation of immortal tumor cells with an unrestricted capacity of cell divisions implies the ability to stabilize telomere length.Citation12 In 85% of all human tumors, this process is mediated by the enzyme telomerase,Citation13,Citation14 a reverse transcriptase, which contains the catalytic subunit human telomerase reverse transcriptase (hTERT)Citation15 and its own intrinsic RNA moiety human telomerase RNA (hTER), which serves as a template for the synthesis of telomeric DNACitation16 (). The remaining 15% of telomerase-negative tumors are able to maintain their telomere length by one or more mechanisms referred to as alternative lengthening of telomeres (ALT).Citation17 While the vast majority of carcinomas expresses telomerase, ALT is frequently found in sarcomas and other tumors arising from tissues of mesenchymal origin.Citation14,Citation18 Activity of telomerase and ALT are not mutually exclusive and it has been demonstrated that telomerase and ALT can coexist in human tumors.Citation19,Citation20
Regulation of telomerase activity
Transcriptional regulation of hTERT expression
The major mechanism to regulate telomerase activity in human cells is the transcriptional control of the catalytic subunit hTERT. Analytical studies of the hTERT promoter have identified several transcription factors that directly bind to the hTERT promoter, resulting in the activation of hTERT transcription. Sp1 is a zinc finger transcription factor that binds to five GC-boxes on the hTERT promoter most likely in cooperation with the oncogenic transcription factor c-Myc.Citation21,Citation22 Cellular signaling and regulatory pathways with transcriptional factors as downstream effectors are involved in the transcriptional regulation of hTERT. In this context, phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT (PI3K/AKT) and mitogen-activated protein kinase signaling induces hTERT expression via phosphorylation of the transcriptional repressor Mad1, resulting in its ubiquitinylation and degradation.Citation23,Citation24 Moreover, the receptors of the ErbB family can act as oncoproteins and activate hTERT expression through PI3K/AKT signaling in breast and esophageal cancer as demonstrated by our group and others.Citation25,Citation26 Besides activating factors, a number of negative regulatory factors have been identified. In this context, the downstream E-box located in close proximity to the transcriptional start site was found to mediate the repression of hTERT transcription by Mad1 and USF1.Citation27
CPG-sites as potential targets of methylation can be frequently found within the hTERT promoter, suggesting an epigenetic mechanism in the regulation of hTERT expression. However, a clear correlation between promoter methylation and transcriptional activation of hTERT could not be established. While several groups have demonstrated that promoter methylation is associated with lower hTERT expression reflecting the well-described mechanism of gene silencing through hypermethylation of promoter regions,Citation28–Citation31 others have shown that hypermethylation of the hTERT promoter region leads to increased hTERT expression, whereas demethylation of this region inhibits hTERT transcription.Citation32,Citation33 This discrepancy might at least in part result from the different cell lines and methylation-specific methods that have been used in various studies.Citation31 Moreover, the local distribution of methylation events across the hTERT promoter seems to be critical for the regulation of hTERT expression. In a study by Zinn et al,Citation34 the hTERT promoter was analyzed in telomerase-positive breast, lung, and colon cancer cell lines, which displayed little or no methylation within an area close to the transcriptional start site of hTERT despite abundant methylation within promoter regions further upstream, indicating that demethylation of the core promoter might be required for the transcriptional activation of hTERT in tumor cells. In contrast, observations in normal tumor cells and tissues without hTERT expression revealed un- or hypomethylated promoters indicating that promoter methylation is not required for developmental silencing of hTERT.Citation35 To date, the available data are contradictory, suggesting that changes in the methylation status of the hTERT promoter may not represent a major mechanism regulating hTERT expression.
Posttranslational modifications
Posttranslational regulation of telomerase activity can occur via reversible phosphorylation of hTERT at specific serine/threonine or tyrosine residues.Citation36,Citation37 For example, besides its role in the transcriptional regulation of hTERT expression, AKT can also directly phosphorylate hTERT leading to increased telomerase activity.Citation26,Citation37 Moreover, studies in head and neck as well as in breast cancer cells have demonstrated that telomerase activation can be induced by protein kinase C-dependent phosphorylation of hTERT leading to increased stability of the telomerase holoenzyme.Citation38,Citation39 While phosphorylation of hTERT by AKT and protein kinase C is associated with increased telomerase activity, phosphorylation by the c-ABL kinase at specific tyrosine residues has been reported to decrease telomerase activity and telomere length.Citation40
Nuclear transport, telomerase complex assembly, and telomere binding
According to Tomlinson et al,Citation41 the two essential components of telomerase hTER and hTERT accumulate at distinct intra-nuclear sites separate from telomeres throughout most of the cell cycle. During S-phase, both components are specifically recruited to subsets of telomeres. The assembly of the telomerase complex involves multiple additional proteins that are crucial for maturation of the holoenzyme and its binding to telomeres. Heat shock protein 90 and p23 are molecular chaperones required for the assembly, maturation, and activation of the telomerase complex. Blocking the adenosine triphosphate-dependent binding of heat shock protein 90 to p23 disrupts the formation of the chaperone complex resulting in telomerase inhibition.Citation42 Moreover, interaction of telomerase with the telomere-binding proteins of the shelterin complex seems to be critical for the regulation of telomerase activity. TRF1 is a telomeric DNA-binding protein that serves as a negative regulator of telomere length by blocking access of telomerase to telomeres. Consequently, removal of TRF1 from telomeres allows telomerase to access and results in telomerase activity and elongation of telomeres.Citation8
ALT – characteristics and regulation
The one common feature of all potential ALT subtypes is the maintenance of telomeres in the absence of telomerase. The initial characteristics described in ALT cells were heterogeneous telomere length within a single cell and the presence of ALT-associated promyelocytic leukemia bodies.Citation43–Citation45 For a subgroup of ALT, more recent data suggest that telomere maintenance is based upon recombination,Citation46,Citation47 which seems to be associated with proteins involved in common reciprocal recombination.Citation48,Citation49 Moreover, ALT cells contain both linear and circular extrachromosomal telomeric repeats representing telomeric templates for recombination.Citation50 Interestingly, the presence of one of the two known telomere maintenance mechanisms varies with specific tumor type. While the majority of epithelial tumors display telomerase activity, cancers of mesenchymal origin such as osteosarcoma or astrocytoma more frequently activate ALT to maintain their telomeres.Citation18,Citation51 The molecular mechanisms involved in the regulation of ALT are not fully understood. However, mutations in genes that physiologically repress DNA recombination such as p53, ATRX, DAXX, and H3F3A seem to facilitate recombination-based telomere maintenance.Citation52 Mutations permissive for ALT include those that are involved in telomerase regulation, and our group and others have recently demonstrated that ALT can be activated by inhibition of telomerase and ALT cells that actively repress telomerase expression.Citation26,Citation53 Moreover, data from human sarcoma indicate that tumors can be mosaic for cells using either telomerase activity or ALT as mechanisms of telomere maintenance.Citation54 These findings suggest that telomerase activity and ALT are not mutually exclusive and can be present in the same tumor and possibly even in the same cell, which might have important implications for telomere-based cancer therapies.
Regulation of telomere biology and maintenance in gastrointestinal tumors
Esophageal cancer
Esophageal cancer presents in two distinct histologic subtypes, namely esophageal squamous cell cancer (ESCC) and esophageal adenocarcinoma (EAC). ESCC typically arises from the squamous epithelium in the upper two-thirds of the esophagus with alcohol and tobacco as major risk factors. EAC are frequently located in the lower third of the esophagus and arise from mucus secreting glandular tissues that are reminiscent of an intestinal epithelium and often form after long-term exposure to acid and bile reflux.
Carcinoma in situ of ESCC exhibits telomeres shorter than those in the surrounding normal epithelium, indicating that telomere attrition occurs early in the process of esophageal carcinogenesisCitation55 and is associated with chromosomal instability.Citation56 Telomerase is activated in the majority of ESCC during cancer progression leading to stabilization of telomere lengths. The molecular mechanisms involved in the activation of telomerase in ESCC development are not fully understood. Quante et alCitation57 have demonstrated that genetic alterations frequently found in early esophageal carcinogenesis such as overexpression of cyclin D1 or inactivation of p53 can independently induce transcriptional activation of hTERT through transcriptional activators that are specific for the respective genetic alteration.Citation57 These data indicate that activation of telomerase might occur relatively early in the process of cancer progression and is at least in part regulated on a transcriptional level. Activating mutations within the hTERT promoter have been described in different types of cancer, but their frequency in both types of esophageal cancer is extremely low.Citation58,Citation59 The epidermal growth factor receptor (EGFR)-signaling pathway is frequently activated in ESCC and has been linked to telomerase activation through both transcriptional and post-translational regulation of hTERT. In this context, EGFR induces hTERT transcription through the transcription factor hypoxia-inducible factor 1α as well as phosphorylation and activation of hTERT via the PI3K/AKT-signaling pathway.Citation26
Chromosomal instability is a hallmark of Barrett’s esophagus, an intestinal metaplasia in the esophagus that forms the precursor lesions of EAC, and is induced by telomere shortening.Citation60 Similar to ESCC, telomerase activity can be frequently detected in EAC and hTERT expression has been found to be gradually increasing during the Barrett’s metaplasia–dysplasia–adenocarcinoma sequence.Citation61,Citation62
HER2, a member of the EGFR family, is overexpressed in approximately 20% of EAC and is successfully used as a therapeutic target by the antibody trastuzumab. HER2 is involved in telomerase activation in breast cancerCitation63 and could play a similar role in the HER2-positive subset of EAC.
Gastric cancer
The risk for the development of gastric cancer is increased in people with shorter telomeres in peripheral blood lymphocytes. Telomere length in peripheral leukocyte DNA reflects cumulative oxidative stress and is associated with Helicobacter pylori positivity, cigarette smoking, and dietary fruit intake.Citation64 A portion of gastric cancers (10%–25%) is characterized by defects in the DNA mismatch repair, resulting in genomic instability characterized by microsatellite instability. These gastric cancers seem to preferentially utilize ALT to maintain telomere length, while tumors with proficient mismatch repair show telomerase activity.Citation65 The mechanisms by which telomerase is activated in gastric cancer remain elusive. Sequencing of the hTERT-promoter in a cohort of almost 800 patients revealed the absence of activating mutations in gastric cancer.Citation66 Similar to esophageal cancer, EGFR-signaling via AKT has been linked to telomerase activation in gastric cancer. AKT activation by epidermal growth factor increased hTERT expression and telomerase activity in gastric cancer cells, while AKT inhibition had the opposite effect. Concurrently, in gastric cancer tissues, significant correlations were found among the levels of phosphorylated AKT, hTERT expression, and telomere length.Citation67
Colorectal cancer
Like in other tumors, telomere shortening in colorectal cancer (CRC) occurs with cell proliferation in preneoplastic lesions and leads to chromosomal instability. Telomerase is activated during the progression of preneoplastic lesions as hTERT levels and telomerase activity increase with the adenoma–carcinoma sequence and are highest in carcinoma.Citation68,Citation69 Telomere lengths may then be stabilized with increasing telomerase activity during tumor progression.Citation70 Both telomere length and telomerase have been extensively studied in CRC.
Several groups have investigated the prognostic value of telomere length correlating telomere length with disease progression in CRC, but the results of these studies are inconsistent. Gertler et al observed a correlation of telomere length with disease progression and more advanced tumor stage in a cohort of 57 patients.Citation71 However, these findings could be confirmed in other cohorts.Citation70,Citation72
While the prognostic value of telomere length in CRC is still debated, it has been recently reported that telomere length might have the potential to serve as a predictive biomarker of sensitivity to anti-EGFR therapy in metastatic CRC. In their study, Bertorelle et alCitation73 demonstrated that among patients with KRAS wild-type tumors treated with anti-EGFR therapy, those with longer telomeres had a superior progression-free survival than those with shorter ones (24.9 vs 11.1 weeks; hazard ratio: 0.31; P=0.048).Citation73 Several studies have revealed that hTERT expression correlates with tumor progression and significantly higher levels of hTERT have been observed in poorly differentiated and higher stage tumors.Citation72 The prognostic value of hTERT expression in CRC has been evaluated in a cohort of 137 patients. Bertorelle et al were able to show that patients with high levels of hTERT messenger RNA (mRNA) in their tumor cells had a significantly higher risk of death during the observation period of 70 months.Citation73 Importantly, high hTERT levels inversely correlated with the disease-free survival as well as overall survival of patients with stage II tumors, indicating that telomerase might be a useful predictive tool to identify CRC patients with a stage II tumor that may benefit from adjuvant chemotherapy.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) typically develops in the setting of chronic liver inflammation leading to fibrosis and eventually cirrhosis caused by virus hepatitis, alcohol, nonalcoholic fatty liver disease, and other environmental factors. Patients with chronic hepatitis B infection may even develop HCC in the absence of cirrhosis. Telomere length progressively shortens during carcinogenesis from cirrhotic to low-and high-grade dysplastic nodules and HCC.Citation65 Telomerase activity can be detected in most HCC, and several groups have demonstrated telomerase activation already in precancerous hepatic lesions.Citation74 Oh et alCitation75 have observed shortening of telomeres and activation of telomerase in precancerous dysplastic nodules with a significant induction of hTERT expression in the transition from low-grade dysplastic nodules to high-grade dysplasia. Activating mutations within the hTERT promoter are among the most frequent genetic alterations in HCC and can be found in approximately 60% of these tumors. Consistent with increasing telomerase activity during hepatocellular carcinogenesis, their frequency rapidly increases during the different steps of the transformation from premalignant lesions into HCC.Citation76 Moreover, recent data indicate that somatic hTERT promoter mutations might serve as a biomarker to predict transformation of premalignant lesions into HCC.Citation77 hTERT mRNA can be detected in serum and has been investigated as a diagnostic marker for HCC. In cohort of 300 patients with HCC, serum levels of hTERT mRNA independently correlated with tumor size and tumor differentiation and proved to be superior to α-fetoprotein in the diagnosis of HCC. Moreover, the detection rate of small HCC was superior to α-fetoprotein warranting further evaluation of hTERT as a serum marker for HCC.Citation78
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) arises from normal pancreatic ductal cells through premalignant lesions called pancreatic intraepithelial neoplasms (PanIN). PanIN are categorized into three grades according to their histopathologic features (PanIN1–3). Telomeres are shorter in PanIN than in normal pancreatic ductal cells, but do not vary significantly between PanIN1–3. The shortest telomeres can be found in PDAC.Citation65 Telomerase activity can be detected in more than 80% of PDAC.Citation79 Similar to other gastrointestinal cancers, promoter mutations are absent in pancreatic cancer and the molecular mechanisms that lead to activation of telomerase in PDAC are largely unknown.Citation80 Activating KRAS mutations are the main driver of tumor progression in more than 90% of PDAC. Downstream effectors of RAS such as MEK and Etstranscription factors have been identified as activators of hTERT expression in other tumors and might also play a pivotal role in telomerase regulation in PDAC.
Telomerase as therapeutic target in gastrointestinal cancer
Inhibition of telomerase can be mediated either through direct targeting of its catalytic subunit hTERT or its RNA template TER inducing telomere shortening and inhibition of cell proliferation. Indirect targeting aims to block telomerase access to telomeres or binds proteins that are involved in the assembly of the telomerase complex at the telomere. Furthermore, telomerase has been utilized as an antigen for the development of immunotherapies that induces CD8+ cytotoxic T lymphocytes directed against hTERT leading to inhibition of the enzyme function.Citation8,Citation81 The efficacy of therapeutic agents targeting telomerase function in gastrointestinal cancer either by inhibition (GRN163L) or induction of anti-tumor immunity (GV1001) has been investigated in several preclinical and clinical trials over the last decade.
GRN163L (Imetelstat, Geron Corporation, Menlo Park, CA, USA) is an antisense oligonucleotide directed against the RNA template hTER and has been tested in several preclinical studies on multiple tumor models including breast and lung carcinoma as well as hematologic neoplasias in vitro and in vivo. Inhibition of telomerase by GRN163L resulted in decreased proliferation, increased senescence, and apoptosis. Moreover, reduced tumorigenicity and invasive capacity have been observed in various tumor types including gastrointestinal cancers.Citation8 In EAC cells that were microdissected from human tumor samples, GRN163L successfully inhibited telomerase, induced apoptosis, and resulted in a reduction of tumor size in mouse xenografts.Citation82 In several preclinical studies including esophageal squamous cancer and HCC, GRN163L has shown the potential to sensitize cells with critically short telomeres to radiation and chemotherapy.Citation83,Citation84
Interestingly, in PDAC, GRN163L successfully reduced the number of putative cancer stem cells, a cellular subpopulation that is thought to be involved in both resistance to chemotherapy and in metastatic progression, and significantly impaired the engraftment of PANC1 cells in nude mice.Citation85
GRN163L is currently investigated in several early clinical trials assessing its safety and efficacy in the treatment of various solid tumors and hematologic malignancies. To date, clinical data on the effect of GRN163L on gastrointestinal cancers are lacking. However in late 2014, the manufacturer announced to further develop GRN163L for oncology, including hematologic malignancies, and other human therapeutic uses.
We have recently demonstrated that alternative lengthening of telomeres can be induced by inhibition of telomerase in ESCC, indicating its role as a mechanism of resistance to telomerase inhibition.Citation20 In line with these findings, Shammas et alCitation82 demonstrated that telomerase inhibition prevented telomere elongation, but induced homologous recombination, which contributed to telomere stabilization and reduced therapeutic efficacy. The authors investigated a dual approach targeting both mechanisms in EAC. Combining inhibition of homologous recombination with telomerase inhibition rendered telomeres more vulnerable to degradation and significantly enhanced their attrition, leading to increased apoptosis.Citation86 These data indicate that future telomere-based therapeutic approaches might have to target both telomerase and ALT to inhibit tumor growth.
Despite its expression in normal human tissue, telomerase has been utilized as a tumor antigen for the development of telomere-based anticancer immunotherapies. As outlined, telomerase is present in the majority of cancers and its peptides are capable of producing strong immune responses through the induction of CD8+ cytotoxic T lymphocytes via major histocompatibility complex presentation resulting in cell lysis.Citation8 Several hTERT-based vaccination strategies have been developed over the last decade.
GV1001 is a 16 amino acid major histocompatibility complex class II-restricted hTERT peptide vaccine and is used in combination with an adjuvant to enhance immune responses. GV1001 has completed several Phase I and Phase II clinical trials in patients with advanced stage solid tumors, including HCC and pancreatic cancer. While GV1001 did not induce a significant immune response and antitumor effect in patients with HCC,Citation87 early trials in pancreatic cancer have reported promising results.Citation88,Citation89 For example, Bernhardt et alCitation88 investigated the safety and tolerability of GV1001 in combination with granulocyte-macrophage colony-stimulating factor. GV1001 was well tolerated and immune responses were observed in 63% of the patients, who had a greater median survival (218 days) than those without an immune response (88 days). Subsequently, GV1001 has been tested in patients with locally advanced or metastatic pancreatic cancer in a randomized Phase III trial in combination with chemotherapy. In this trial, the rate of immune responses was significantly lower and no effect of GV1001 on the median survival of patients with pancreatic cancer could be observed.Citation90 For telomerase vaccination strategies to be effective, an active immune response is needed. Pancreatic cancer is a highly proliferative tumor characterized by aggressive local growth and early metastasis with a short median survival time that might be insufficient to establish a stronger anti-tumor immunity. In addition, pancreatic cancer is generally poorly vascularized and less immunogenic than other tumors. In the future, hTERT vaccination studies will therefore have to focus on adjuvant strategies to enhance immune responses and antitumor effects.
Several other strategies of targeting telomeres and telomerase have been developed and are currently tested in preclinical and early clinical trials with no particular focus on gastrointestinal cancer ( and ).
Table 1 Therapeutic strategies targeting telomeres and telomerase
Conclusion
Maintenance of telomeres is an extremely complex cellular process that has been extensively studied over the last decades and our understanding of telomerase function and, to a lesser extent, ALT in normal tissue homeostasis and cancer has improved remarkably. Telomerase has been hailed as an ideal target for cancer therapies and several therapeutic strategies targeting telomerase showed potential in preclinical as well as early clinical trials. However, none of these has yet become part of standard therapeutic regimens in gastrointestinal cancer. One possible explanation could be the redundancy of telomerase activity and ALT, with ALT being activated upon inhibition of telomerase. Even though these observations have been made in esophageal cancer, it is very likely that this redundancy is also present in other cancers. In vitro data indicate that this problem might be overcome in the future by dual inhibition of telomerase and ALT.
The molecular mechanisms of telomerase activation in cancers of the gastrointestinal tract vary significantly between tumor types and most likely also between patients. These variations add another layer of complexity to the sophisticated machinery of telomere maintenance in cancer. However, the age of next-generation sequencing, transcriptomics and proteomics might open new perspectives for individualized therapeutic strategies that interfere with the molecular mechanisms of telomerase activation. As outlined in this article, receptor tyrosine kinases and their downstream signaling pathways are involved in the activation of telomerase. Inhibition of these and other receptors and pathways by agents that are already in clinical use for the treatment of cancer might serve as adjuvants or even alternatives to direct targeting of telomerase in the future.
Disclosure
The author reports no conflicts of interest in this work.
References
- McClintockBThe behavior in successive nuclear divisions of a chromosome broken at meiosisProc Natl Acad Sci U S A193925840541616577924
- MullerHJThe remaking of chromosomesCollecting Net19388182195
- BlackburnEHGallJGA tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in TetrahymenaJ Mol Biol197812013353642006
- MoyzisRKBuckinghamJMCramLSA highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomesProc Natl Acad Sci U S A19888518662266263413114
- David WilsonWPaulAKinetics and structures on the molecular path to the quadruplex form of the human telomereJ Mol Biol201442681625162824508600
- GriffithJDComeauLRosenfieldSMammalian telomeres end in a large duplex loopCell199997450351410338214
- XuLLiSStohrBAThe role of telomere biology in cancerAnnu Rev Pathol20138497822934675
- RudenMPuriNNovel anticancer therapeutics targeting telomeraseCancer Treat Rev201339544445622841437
- RaySBandariaJNQureshiMHYildizABalciHG-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA bindingProc Natl Acad Sci U S A201411182990299524516170
- AllsoppRCVaziriHPattersonCTelomere length predicts replicative capacity of human fibroblastsProc Natl Acad Sci U S A1992892110114101181438199
- BrownJPWeiWSedivyJMBypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblastsScience199727753278318349242615
- HarleyCBFutcherABGreiderCWTelomeres shorten during ageing of human fibroblastsNature199034562744584602342578
- de LangeTActivation of telomerase in a human tumorProc Natl Acad Sci U S A1994918288228858159672
- KimNWPiatyszekMAProwseKRSpecific association of human telomerase activity with immortal cells and cancerScience19942665193201120157605428
- NakamuraTMMorinGBChapmanKBTelomerase catalytic subunit homologs from fission yeast and humanScience199727753289559599252327
- Shippen-LentzDBlackburnEHFunctional evidence for an RNA template in telomeraseScience199024749425465521689074
- CesareAJReddelRRAlternative lengthening of telomeres: models, mechanisms and implicationsNat Rev Genet201011531933020351727
- HensonJDHannayJAMcCarthySWA robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomasClin Cancer Res200511121722515671549
- JohnsonJEVarkonyiRJSchwalmJMultiple mechanisms of telomere maintenance exist in liposarcomasClin Cancer Res200511155347535516061847
- QueisserAHeegSThalerMvon WerderAOpitzOGInhibition of telomerase induces alternative lengthening of telomeres during human esophageal carcinogenesisCancer Genet20132061137438624331919
- DanielMPeekGWTollefsbolTORegulation of the human catalytic subunit of telomerase (hTERT)Gene2012498213514622381618
- KyoSTakakuraMTairaTSp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT)Nucleic Acids Res200028366967710637317
- ChouCKLeeDFSunHLThe suppression of MAD1 by AKT-mediated phosphorylation activates MAD1 target genes transcriptionMol Carcinog200948111048105819526459
- ZhuJBlenisJYuanJActivation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1Proc Natl Acad Sci U S A2008105186584658918451027
- GoueliBSJanknechtRUpregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or RafMol Cell Biol2004241253514673140
- HeegSHirtNQueisserAEGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesisCancer Sci2011102235136021156006
- HorikawaICablePLMazurSJAppellaEAfshariCABarrettJCDownstream E-box-mediated regulation of the human telomerase reverse transcriptase (hTERT) gene transcription: evidence for an endogenous mechanism of transcriptional repressionMol Biol Cell20021382585259712181331
- LiuLLaiSAndrewsLGTollefsbolTOGenetic and epigenetic modulation of telomerase activity in development and diseaseGene2004340111015556289
- LiuLSaldanhaSNPateMSAndrewsLGTollefsbolTOEpigenetic regulation of human telomerase reverse transcriptase promoter activity during cellular differentiationGenes Chromosomes Cancer2004411263715236314
- ShinKHKangMKDicterowEParkNHHypermethylation of the hTERT promoter inhibits the expression of telomerase activity in normal oral fibroblasts and senescent normal oral keratinocytesBr J Cancer20038981473147814562019
- ZhuJZhaoYWangSChromatin and epigenetic regulation of the telomerase reverse transcriptase geneProtein Cell201011223221203995
- GuilleretIYanPGrangeFBraunschweigRBosmanFTBenhattarJHypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activityInt J Cancer2002101433534112209957
- RenaudSLoukinovDAbdullaevZDual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT geneNucleic Acids Res20073541245125617267411
- ZinnRLPruittKEguchiSBaylinSBHermanJGhTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start siteCancer Res200767119420117210699
- DevereuxTRHorikawaIAnnaCHAnnabLAAfshariCABarrettJCDNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) geneCancer Res199959246087609010626795
- CongYSWrightWEShayJWHuman telomerase and its regulationMicrobiol Mol Biol Rev2002663407425 table of contents12208997
- KangSSKwonTKwonDYDoSIAkt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunitJ Biol Chem199927419130851309010224060
- ChangJTLuYCChenYJhTERT phosphorylation by PKC is essential for telomerase holoprotein integrity and enzyme activity in head neck cancer cellsBr J Cancer200694687087816508638
- LiHZhaoLYangZFunderJWLiuJPTelomerase is controlled by protein kinase Calpha in human breast cancer cellsJ Biol Chem19982735033436334429837921
- KharbandaSKumarVDharSRegulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinaseCurr Biol2000101056857510837221
- TomlinsonRLZieglerTDSupakorndejTTernsRMTernsMPCell cycle-regulated trafficking of human telomerase to telomeresMol Biol Cell200617295596516339074
- HoltSEAisnerDLBaurJFunctional requirement of p23 and Hsp90 in telomerase complexesGenes Dev199913781782610197982
- BryanTMEnglezouAGuptaJBacchettiSReddelRRTelomere elongation in immortal human cells without detectable telomerase activityEMBO J19951417424042487556065
- ChungIOsterwaldSDeegKIRippeKPML body meets telomere: the beginning of an ALTernate ending?Nucleus20123326327522572954
- YeagerTRNeumannAAEnglezouAHuschtschaLINobleJRReddelRRTelomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) bodyCancer Res199959174175417910485449
- DunhamMANeumannAAFaschingCLReddelRRTelomere maintenance by recombination in human cellsNat Genet200026444745011101843
- MurnaneJPSabatierLMarderBAMorganWFTelomere dynamics in an immortal human cell lineEMBO J19941320495349627957062
- LeSMooreJKHaberJEGreiderCWRAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomeraseGenetics1999152114315210224249
- TarsounasMMuñozPClaasATelomere maintenance requires the RAD51D recombination/repair proteinCell2004117333734715109494
- CesareAJGriffithJDTelomeric DNA in ALT cells is characterized by free telomeric circles and heterogeneous t-loopsMol Cell Biol200424229948995715509797
- HensonJDReddelRRAssaying and investigating alternative lengthening of telomeres activity in human cells and cancersFEBS Lett2010584173800381120542034
- GochaARHarrisJGrodenJAlternative mechanisms of telomere lengthening: permissive mutations, DNA repair proteins and tumorigenic progressionMutat Res2013743744142150
- HuJHwangSSLiesaMAntitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancerCell2012148465166322341440
- GochaARNuovoGIwenofuOHGrodenJHuman sarcomas are mosaic for telomerase-dependent and telomerase-independent telomere maintenance mechanisms: implications for telomere-based therapiesAm J Pathol20131821414823260199
- YuHPXuSQLuWHTelomerase activity and expression of telomerase genes in squamous dysplasia and squamous cell carcinoma of the esophagusJ Surg Oncol20048629910415112252
- ZhengYLHuNSunQWangCTaylorPRTelomere attrition in cancer cells and telomere length in tumor stroma cells predict chromosome instability in esophageal squamous cell carcinoma: a genome-wide analysisCancer Res20096941604161419190333
- QuanteMHeegSvon WerderADifferential transcriptional regulation of human telomerase in a cellular model representing important genetic alterations in esophageal squamous carcinogenesisCarcinogenesis200526111879188915958520
- ZhaoYGaoYChenZHuXZhouFHeJLow frequency of TERT promoter somatic mutation in 313 sporadic esophageal squamous cell carcinomasInt J Cancer2014134249349423818232
- van NistelrooijAMZwarthoffECPostEAbsence of TERT promoter mutations in esophageal adenocarcinomaInt J Cancer201413482014201524493128
- FinleyJCReidBJOdzeRDChromosomal instability in Barrett’s esophagus is related to telomere shorteningCancer Epidemiol Biomarkers Prev20061581451145716896031
- LordRVSalongaDDanenbergKDTelomerase reverse transcriptase expression is increased early in the Barrett’s metaplasia, dysplasia, adenocarcinoma sequenceJ Gastrointest Surg20004213514210675236
- MoralesCPLeeELShayJWIn situ hybridization for the detection of telomerase RNA in the progression from Barrett’s esophagus to esophageal adenocarcinomaCancer19988346526599708927
- PapanikolaouVIliopoulosDDimouIThe involvement of HER2 and p53 status in the regulation of telomerase in irradiated breast cancer cellsInt J Oncol20093551141114919787269
- HouLSavageSABlaserMJTelomere length in peripheral leukocyte DNA and gastric cancer riskCancer Epidemiol Biomarkers Prev200918113103310919861514
- BasuNSkinnerHGLitzelmanKVanderboomRBaichooEBoardmanLATelomeres and telomere dynamics: relevance to cancers of the GI tractExpert Rev Gastroenterol Hepatol20137873374824161135
- HuangDSWangZHeXJRecurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activationEur J Cancer201551896997625843513
- SasakiTKuniyasuHLuoYAKT activation and telomerase reverse transcriptase expression are concurrently associated with prognosis of gastric cancerPathobiology2014811364123969493
- NaitoYTakagiTHandaOTelomerase activity and expression of telomerase RNA component and catalytic subunits in precancerous and cancerous colorectal lesionsTumour Biol200122637438211786731
- EngelhardtMDrullinskyPGuillemJMooreMATelomerase and telomere length in the development and progression of premalignant lesions to colorectal cancerClin Cancer Res1997311193119419815582
- RampazzoEBertorelleRSerraLRelationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancersBr J Cancer201010281300130520386541
- GertlerRRosenbergRStrickerDTelomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinomaJ Clin Oncol200422101807181415143073
- BertorelleRRampazzoEPucciarelliSNittiDDe RossiATelomeres, telomerase and colorectal cancerWorld J Gastroenterol20142081940195024616570
- BertorelleRBriaravaMRampazzoETelomerase is an independent prognostic marker of overall survival in patients with colorectal cancerBr J Cancer2013108227828423322193
- WegeHBrummendorfTHTelomerase activation in liver regeneration and hepatocarcinogenesis: Dr Jekyll or Mr Hyde?Curr Stem Cell Res Ther200721313818220890
- OhBKKimYJParkYNChoiJKimKSParkCQuantitative assessment of hTERT mRNA expression in dysplastic nodules of HBV-related hepatocarcinogenesisAm J Gastroenterol2006101483183816494581
- NaultJCMalletMPilatiCHigh frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesionsNat Commun20134221823887712
- NaultJCCalderaroJDi TommasoLTelomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosisHepatology20146061983199225123086
- MiuraNOsakiYNagashimaMA novel biomarker TERTmRNA is applicable for early detection of hepatomaBMC Gastroenterol2010104620482774
- MizumotoKTanakaMDetection of telomerase activity in patients with pancreatic cancerMethods Mol Med200510319920515542908
- VinagreJPintoVCelestinoRTelomerase promoter mutations in cancer: an emerging molecular biomarker?Virchows Arch2014465211913325048572
- BeattyGLVonderheideRHTelomerase as a universal tumor antigen for cancer vaccinesExpert Rev Vaccines20087788188718767939
- ShammasMAQaziABatchuRBTelomere maintenance in laser capture microdissection-purified Barrett’s adenocarcinoma cells and effect of telomerase inhibition in vivoClin Cancer Res200814154971498018676772
- WuXSmavadatiSNordfjällKTelomerase antagonist imetelstat inhibits esophageal cancer cell growth and increases radiation-induced DNA breaksBiochim Biophys Acta20121823122130213522906540
- DjojosubrotoMWChinACGoNTelomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatomaHepatology20054251127113616114043
- JosephITresslerRBassettEThe telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell linesCancer Res201070229494950421062983
- LuRPalJBuonLTargeting homologous recombination and telomerase in Barrett’s adenocarcinoma: impact on telomere maintenance, genomic instability and tumor growthOncogene201433121495150523604115
- GretenTFFornerAKorangyFA phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinomaBMC Cancer20101020920478057
- BernhardtSLGjertsenMKTrachselSTelomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II studyBr J Cancer200695111474148217060934
- ChoudhuryAMosolitsSKokhaeiPHanssonLPalmaMMellstedtHClinical results of vaccine therapy for cancer: learning from history for improving the futureAdv Cancer Res20069514720216860658
- MiddletonGSilcocksPCoxTGemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trialLancet Oncol201415882984024954781
- LinWHYehSHYangWJTelomerase-specific oncolytic adenoviral therapy for orthotopic hepatocellular carcinoma in HBx transgenic miceInt J Cancer201313261451146222886913
- WirthTKühnelFFleischmann-MundtBTelomerase-dependent virotherapy overcomes resistance of hepatocellular carcinomas against chemotherapy and tumor necrosis factor-related apoptosis-inducing ligand by elimination of Mcl-1Cancer Res200565167393740216103092
- MoserCLangSAStoeltzingOHeat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancerAnticancer Res20092962031204219528462
- JavleMCurtinNJThe role of PARP in DNA repair and its therapeutic exploitationBr J Cancer201110581114112221989215
- WaalerJMachonOTumovaLA novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant miceCancer Res201272112822283222440753
