Abstract
The epidermal growth factor receptor (EGFR) is expressed in the majority of non-small-cell lung cancer (NSCLC). However, only a restricted subgroup of NSCLC patients respond to treatment with the EGFR tyrosine kinase inhibitor (EGFR TKI) gefitinib. Clinical trials have demonstrated that patients carrying activating mutations of the EGFR significantly benefit from treatment with gefitinib. In particular, mutations of the EGFR TK domain have been shown to increase the sensitivity of the EGFR to exogenous growth factors and, at the same time, to EGFR TKIs such as gefitinib. EGFR mutations are more frequent in patients with particular clinical and pathological features such as female sex, nonsmoker status, adenocarcinoma histology, and East Asian ethnicity. A close correlation was found between EGFR mutations and response to gefitinib in NSCLC patients. More importantly, randomized Phase III studies have shown the superiority of gefitinib compared with chemotherapy in EGFR mutant patients in the first-line setting. In addition, gefitinib showed a good toxicity profile with an incidence of adverse events that was significantly lower compared with chemotherapy. Therefore, gefitinib is a major breakthrough for the management of EGFR mutant NSCLC patients and represents the first step toward personalized treatment of NSCLC.
Keywords:
Introduction
Non-small-cell lung cancer (NSCLC) is the major cause of cancer death worldwide. Conventional chemotherapy offers only a modest benefit for patients with advanced NSCLC, slightly prolonging survival, but at the cost of clinically significant adverse events (AEs). Novel target-based agents, which act on specific signaling pathways involved in cell proliferation and survival, have emerged as effective agents in treating NSCLC.Citation1
The epidermal growth factor receptor (EGFR) (HER1, ErbB-1) is a tyrosine kinase receptor belonging to the ErbB family of receptors. The EGFR possesses an extracellular ligand-binding domain, a single hydrophobic transmembrane domain that is involved in interaction among the ErbB receptors, and an intracellular domain in which the tyrosine kinase (TK) activity resides. Ligands that belong to the EGF family of growth factors are able to activate the EGFR by inducing the formation of homo- or heterodimers with other ErbB receptors, with subsequent phosphorylation of the intrinsic TK domain.Citation2,Citation3 In turn, ErbB receptors activate several intracellular signaling pathways, including the RAS/RAF/MAPK pathway, which plays an important role in regulating cell proliferation, migration, and differentiation, and the PI3K/AKT pathway, which is involved in cell survival.
The EGFR is overexpressed in a variety of common solid tumors, including NSCLC.Citation3 The observation that the EGFR is expressed in the majority of human NSCLC led to the hypothesis that activation of this receptor might play an important role in NSCLC growth and therefore might represent a suitable therapeutic target. In this regard, several anti-EGFR agents are in an advanced phase of clinical development. Two classes of EGFR inhibitors have been approved: anti-EGFR monoclonal antibodies and small-molecule inhibitors of the EGFR tyrosine kinase activity (EGFR TKIs).Citation4 Monoclonal antibodies directed against the EGFR target the extracellular domain of the receptor to prevent ligand binding and subsequent receptor activation. EGFR TKIs compete for adenosine triphosphate (ATP) binding in the TK domain of the receptor, thus blocking EGFR autophosphorylation and downstream signaling pathways. The EGFR TKIs gefitinib and erlotinib have been approved for the treatment of NSCLC.Citation3 In particular, erlotinib was approved as second- and third-line treatment of patients with advanced NSCLC on the basis of the results of the Phase III trial BR.21 in which a survival advantage in unselected patients treated with the drug, compared with patients treated with placebo, was demonstrated.Citation5 Gefitinib, in the ISEL (Iressa Survival Evaluation in Lung Cancer) trial, failed to show an improvement of survival in an unselected population but showed a benefit in never-smokers and Asian patients.Citation6 These results, as well as findings from Phase II studies of gefitinib in NSCLC, led to the hypothesis that only patients with well-defined clinicopathological and molecular features might benefit from EGFR TKIs.
In 2004, three landmark papers identified in NSCLC patients mutations in the TK domain of the EGFR that predicted response to erlotinib and gefitinib.Citation7–Citation9 These novel EGFR mutations rendered tumors dramatically more sensitive to the effects of EGFR TKIs compared with tumors carrying the wild-type receptor. As we will detail in the next paragraphs, the results of clinical trials have clearly demonstrated that treatment with EGFR TKIs produces an extremely high response rate and a significant improvement of survival in NSCLC patients with EGFR mutations. These data induced the European Medicines Agency (EMEA) to approve the use of gefitinib in all lines of therapy for patients carrying a mutant EGFR. As a matter of fact, this decision has established a new paradigm for the treatment of NSCLC patients and represents the first step toward personalized therapy for lung cancer patients.
EGFR mutations in NSCLC
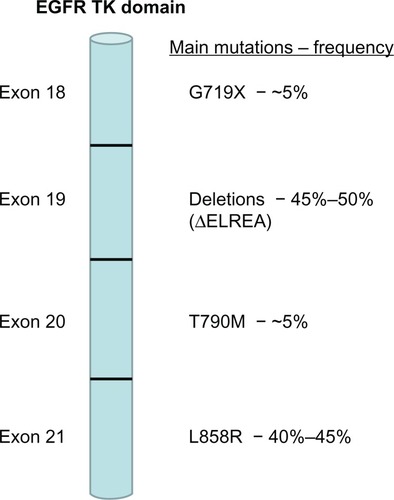
EGFR mutations were discovered following sequencing of the EGFR gene in NSCLC patients who responded to treatment with gefitinib or erlotinib in early clinical trials. Mutations were found in the first four exons (18 through 21) of the TK domain of the EGFR (). A number of different mutations have been described.Citation10 However, only nine mutations have been reported with a frequency higher than 1%, and approximately 160 mutations have been described only once.Citation10 The most frequent EGFR mutations are a group of small in-frame deletions in exon 19 and a single-point mutation in exon 21. The exon 19 deletions include amino acid residues around codons 746 to 750 (ΔELREA) and account for 45%–50% of all EGFR TK mutations.Citation11–Citation14 In exon 21, a single-point mutation that substitutes an arginine for a leucine at codon 858 (L858R) represents 40%–45% of all EGFR TK-activating mutations. Other less frequent EGFR-activating mutations include a glycine-719 change to serine, alanine, or cysteine (G719X) in exon 18 and in-frame duplications, insertions, or point mutations in exon 20 ().
Figure 1 Frequency of main epidermal growth factor receptor (EGFR) mutations in non-small-cell lung cancer.
Kinase domain mutations of the EGFR are referred to as “activating mutations” because they lead to more persistent activation of the TK in response to exogenous growth factors compared with the wild-type receptor.Citation7 Activating EGFR mutations involve the ATP-binding pocket in the receptor TK domain, which is the binding site for erlotinib and gefitinib. In this regard, it has been demonstrated that activating EGFR mutations also lead to increased affinity of gefitinib and erlotinib for the mutant receptor compared with the wild-type receptor.Citation9 As a consequence, mutant EGFR is sensitive to lower concentrations of gefitinib compared with wild-type receptor. Similar data were obtained for erlotinib.Citation9
NSCLC carrying a mutant EGFR is highly dependent on the EGFR pathway for its growth, ie, an “oncogenic addiction” to the EGFR pathway occurs.Citation12 In contrast, current evidence suggests that lung tumors that carry a wild-type EGFR are not highly sensitive to inhibition of this pathway. The relationship between EGFR mutations and clinical responses to EGFR TKIs has been extensively characterized in NSCLC. Overall, activating EGFR mutations are strongly associated with increased response to EGFR TKIs.Citation12 However, it must be emphasized that the majority of available clinical data are related to the most frequent mutations, ie, the exon 19 deletions and the L858R-point mutation. Exon 18 G719X mutations are also likely associated with a response to EGFR TKIs. However, data on survival following treatment with EGFR TKIs in patients carrying these mutations are not available. In contrast, a group of mutations in exon 20 has been associated with resistance to these agents.Citation12 These mutations are often associated with sensitizing mutations in exons 18, 19, or 21. The most characterized resistance mutation is the T790M-point mutation in exon 20, which increases the affinity of the EGFR for ATP, thus reducing the binding of EGFR TKIs to the ATP pocket.Citation15
An interesting feature of EGFR mutations in NSCLC is that they are strongly associated with particular clinical and pathological features. In fact, EGFR mutations are far more frequent in female patients compared with male patients (38.7% vs 10%), in the adenocarcinoma subtype compared with other histologic types (29.4% vs 1.8%), in nonsmokers compared with smokers or former smokers (45.8% vs 7.1%), and in East Asian NSCLC patients (33.4%) compared with non-East Asian patients (5.5%).Citation2 These clinical, pathological, and molecular characteristics identify a specific, uncommon subtype of lung cancer. The growth of this type of tumor is strictly dependent on the activation of the EGFR pathway, which might represent the leading pathway in inducing cellular transformation in these selected patients. Indeed, expression of activating EGFR mutations (either exon 19 deletions or the L858R mutation) in the lung tissue of transgenic mice results in the development of lung tumors that histologically resemble human NSCLC carrying the EGFR mutations and that are extremely sensitive to EGFR inhibition.Citation16
Efficacy of gefitinib
The activity of gefitinib as a single agent in pretreated advanced NSCLC patients was first evaluated in the randomized Phase II IDEAL 1 (Iressa Dose Evaluation in Advanced Lung Cancer) and IDEAL 2 trials.Citation17,Citation18 An objective response rate of 18% in IDEAL 1% and 10% in IDEAL 2 was observed. Median survival was 7 months, and 1-year overall survival (OS) was 27%–35%, similar to that expected with chemotherapy. These trials led to the US Food and Drug Administration (FDA) approving gefitinib as salvage third-line therapy for NSCLC. Two randomized Phase III clinical trials (INTACT 1 [Iressa NSCLC Trials Assessing Combination Treatment] and INTACT 2) examined the efficacy of first-line treatment with a combination of platinum-based chemotherapy and gefitinib.Citation19,Citation20 The combined treatment of chemotherapy plus gefitinib was well tolerated. However, both INTACT studies failed to demonstrate that the addition of gefitinib to chemotherapy produces a survival benefit. A placebo-controlled randomized Phase III trial (ISEL) of gefitinib as second- or third-line treatment in chemotherapy-refractory NSCLC patients failed to show that gefitinib was effective in improving survival.Citation6 Median survival was 5.6 months in the gefitinib group and 5.1 months in the placebo group, and 1-year OS was 27% versus 21%, respectively. In June 2005, on the basis of the lack of survival benefit of the ISEL study, the FDA restricted the use of gefitinib to patients participating in a clinical trial or continuing to benefit from treatment already initiated. Four randomized Phase III trials compared gefitinib and docetaxel as second-line treatment of advanced NSCLC patients. The largest study was INTEREST (Iressa NSCLC Trial Evaluating Response and Survival Against Taxotere), which evaluated the effectiveness of gefitinib versus docetaxel in patients with locally advanced or metastatic NSCLC pretreated with platinum-based chemotherapy.Citation21 The trial enrolled 1466 patients from 149 centers in 24 countries. The results of this study demonstrated the noninferiority of gefitinib in terms of OS: median OS was 7.6 months in the gefitinib group and 8.0 months in the docetaxel group, and 1-year survival was 32% and 34%, respectively.Citation21
Although the results of the INTEREST study propose that gefitinib might have activity in unselected NSCLC patients, different observations suggest that only patients carrying EGFR mutations are highly sensitive to gefitinib and are likely to significantly benefit from treatment with this drug. In the IDEAL 1 trial, a higher response rate was seen in Japanese patients than in a predominantly European-derived population (27.5% vs 10.4%).Citation17 In several studies in Caucasian patients, partial clinical responses to gefitinib have been observed most frequently in women, in nonsmokers, and in patients with adenocarcinoma.Citation22,Citation23 Subgroup analyses of patients in the ISEL trial identified a unique subpopulation of patients (Asian, female, never-smokers, adenocarcinoma histology) who were most likely to respond to EGFR TKIs.Citation6 All these features are clearly related to a higher frequency of EGFR mutations in subgroups of patients with specific clinical and pathological features, as previously specified. In this regard, a subgroup analysis of the INTEREST trial showed that treatment with gefitinib in patients carrying mutant EGFR produced a significant improvement in progression-free survival (PFS) (hazard ratio [HR] 0.16; P = 0.001) and response rate (42.1% vs 21.1%) compared with docetaxel.Citation24 In patients with EGFR mutations, an improvement in survival in both gefitinib and docetaxel groups (median survival 14.2 and 16.6 months, respectively) compared with EGFR wild-type patients (6.4 and 6.0 months, respectively) was also observed. The difference for survival between the two treatments in EGFR mutant patients was not statistically significant (HR 0.83, P = 0.60). In contrast, in patients with wild-type EGFR, the response rate was higher in the docetaxel arm (9.8% vs 6.6%), whereas no significant difference was found for PFS (HR 1.24; P = 0.14) and OS (HR 1.02, P = 0.91). In addition, a series of Phase II clinical trials has specifically selected patients with documented EGFR mutations to enrich the population of subjects who are most likely to benefit from first-line treatment with EGFR TKI therapy.Citation25–Citation29 These studies have uniformly demonstrated impressive response rates in the range of 50%–70% with excellent PFS and OS rates. These trials also exhibit notably improved treatment tolerance compared with conventional platinum-based doublet chemotherapy regimens, despite the inclusion in some studies of elderly patients with poor performance status.
The superiority of gefitinib compared with chemotherapy in patients with EGFR mutations in the first-line setting was confirmed in four randomized Phase III studies that enrolled NSCLC patients on the basis of molecular or clinical characteristics (). The randomized Phase III IPASS (Iressa Pan-Asia Study) compared gefitinib monotherapy with intravenous carboplatin (C) and paclitaxel (P) chemotherapy as first-line treatment in 1217 clinically selected chemotherapy-naïve Asian patients (never or light smokers and with adenocarcinoma histology) with advanced NSCLC.Citation30 In this study, gefitinib produced a significantly superior PFS compared with C/P (HR 0.74), exceeding the primary objective that was the noninferiority of gefitinib versus chemotherapy. However, the most interesting data from this study derived from the analysis of EGFR mutations that were carried in approximately one-third of the enrolled patients (n = 437). In this clinically selected population of patients, the mutation rate was high, with 261 of 437 available samples (60%) harboring an EGFR mutation. In the subgroup of patients with mutant EGFR, PFS was significantly longer (HR 0.48) and the response rate was significantly higher with gefitinib compared with C/P (71.2% vs 47.3%). In contrast, in patients carrying the wild-type receptor, PFS was significantly shorter (HR 2.85) and response rate was significantly lower with gefitinib (1.1% vs 23.5%).Citation30 Recently, OS data have been reported showing no difference between gefitinib and chemotherapy in the whole population (18.8 months with gefitinib vs 17.4 months with chemotherapy, HR 0.90, P = 0.11) and in the mutation-positive subgroup (21.6 months with gefitinib vs 21.9 months with chemotherapy, HR 1.00) ().Citation31 However, this result might be due to the crossover of mutant patients from the chemotherapy to the gefitinib arm. In fact, after discontinuation of the assigned treatment, 39.5% of the patients in the C/P group received an EGFR TKI.
Table 1 Phase III clinical trials of gefitinib versus chemotherapy in non-small-cell lung cancer patients harboring epidermal growth factor receptor (EGFR) mutations
In First-SIGNAL (First-line Single Agent Iressa versus Gemcitabine and Cisplatin Trial in Never-smokers with Adenocarcinoma of the Lung), 309 Korean never-smoker patients with untreated lung adenocarcinoma were randomized to receive either Iressa at 250 mg daily or cisplatin/gemcitabine. The study failed to reach its primary endpoint of OS. Molecular analyses showed that the mutation rate in this study was 43.8%. Importantly, in patients carrying an EGFR mutation, the response rate was 84.6% for gefitinib versus 37.5% for chemotherapy (P = 0.002), whereas in the mutation-negative subgroup the response rate was 29.9% for gefitinib and 51.9% for chemotherapy (P = 0.051). Furthermore, in the EGFR-mutant subgroup, the PFS was longer for patients receiving gefitinib compared with those having chemotherapy (8.5 versus 6.7 months), although such difference was not statistically significant (HR 0.613, P = 0.0849). No significant difference in OS was observed between gefitinib and chemotherapy in the EGFR mutant and in the whole population of patients, presumably due to the poststudy use of EGFR TKIs in 80% of the patients in the chemotherapy arm ().Citation32
The results of two randomized Phase III studies that enrolled Japanese, EGFR-mutant patients with advanced NSCLC have recently been reported. In the WJTOG3405 (West Japan Oncology Group) trial, 172 EGFR-mutant patients were randomly assigned to receive gefitinib (250 mg daily) or chemotherapy (cisplatin plus docetaxel).Citation33 The study showed a median PFS of 9.2 months in the gefitinib group versus 6.3 months in the chemotherapy group (HR 0.489). The response rate was 62.1% and 32.2% with gefitinib and chemotherapy, respectively (P < 0.0001) ().
The Phase III NEJ002 (North East Japan Gefitinib Study Group) trial compared gefitinib with chemotherapy with carboplatin and paclitaxel as first-line treatment in advanced NSCLC patients selected for EGFR mutation.Citation34 The study was stopped by an independent data and safety monitoring committee after the preplanned interim analysis, because it showed a significant difference in PFS between the two treatment groups (median PFS 10.4 months for gefitinib versus 5.5 months for chemotherapy, HR 0.36). The final analysis confirmed these results, showing a median PFS of 10.8 versus 5.4 months for gefitinib and chemotherapy, respectively (HR 0.30), and a response rate significantly higher in the gefitinib group compared with in the chemotherapy group (73.7% vs 30.7%, P < 0.001). The OS did not differ significantly between the two treatment groups (median survival time 30.5 months for the gefitinib group and 23.6 months for chemotherapy). However, among 112 patients who completed chemotherapy, 106 (94.6%) received second-line gefitinib, and 58.5% of these patients had a response ().Citation34
Safety and tolerability
The safety profile of gefitinib was first defined in the Phase II IDEAL 1 and 2 trials.Citation17,Citation18 No unexpected AEs were observed at dosages of 250 mg/day and 500 mg/day, confirming the results obtained in Phase I trials. The most frequent grade 1/2 drug-related AEs were diarrhea and skin reactions. Grade 3/4 drug-related AEs were nausea, vomiting, and liver enzyme elevation. Although the profile of grade 1/2 AEs was similar for both doses, grade 1/2 AEs were more frequently reported with the gefitinib dosage of 500 mg/day. Furthermore, in both IDEAL trials, a higher incidence of grade 3/4 drug-related AEs was reported in patients receiving gefitinib 500 mg/day than in those receiving the lower dose. Dose interruptions were mainly due to skin reactions, gastrointestinal disturbances, and elevated transaminases. Withdrawal due to drug-related AEs was observed in 1.9% of patients receiving gefitinib 250 mg/day and 9.4% of patients receiving 500 mg/day in the IDEAL 1 trial, and in 1% of patients receiving gefitinib 250 mg/day and 4% of those receiving 500 mg/day in the IDEAL 2 trial.Citation17,Citation18
Because the two doses of gefitinib (250 mg/day and 500 mg/day) showed a similar response rate but toxicity was greater at 500 mg, the lower dose was chosen for further clinical studies.
Data from the large Phase III randomized trials ISEL, INTEREST, and IPASS confirmed the tolerable toxicity profile of gefitinib when administered at the dosage of 250 mg/day ( and ).
Table 2 Most frequent adverse events observed in non-small-cell lung cancer patients treated with gefitinib
Table 3 Frequency of adverse events occurring in non-small-cell lung cancer patients treated with gefitinib 250 mg/day in randomized Phase III clinical trials
In the ISEL trial, the most common AEs in the gefitinib group were grade 1/2 rash and diarrhea.Citation6 The overall frequency of grade 3/4 AEs was 30% for gefitinib and 27% for placebo-treated patients (). The frequency of interstitial lung disease was similar in the two treatment groups (1%). Few patients experienced AEs necessitating withdrawal (5% in the gefitinib group and 2% in the placebo group), and few patients died as a result of AEs events (5% in the gefitinib group and 4% in the placebo group).
The majority of AEs associated with gefitinib in the INTEREST trial were mild in nature, and those most commonly reported were grade 1/2 diarrhea and skin reactions.Citation21 Common toxicity grade 3/4 AEs were reported in 9% of patients receiving gefitinib and 41% of patients receiving docetaxel (). AEs leading to drug discontinuation were reported in 4% of patients in the gefitinib group and in 11% of patients in the docetaxel group. AEs leading to death occurred in 1% versus 2%, respectively. Lung interstitial disease was reported in 1% of patients treated with gefitinib and in 1% of patients in the docetaxel arm.
In the IPASS study, gefitinib was associated with a lower rate of grade 3/4 AEs compared with C/P (28.7% vs 61.0%), a lower rate of AEs leading to drug discontinuation (6.9% vs 13.6%), and a lower rate of dose modification due to toxic effects (16.1% vs 35%–37% for C/P).Citation30 AEs leading to death occurred in 3.8% of patients treated with gefitinib and in 2.7% of patients treated with the combination C/P. The most common treatment-related AEs were skin rash, diarrhea, and elevated liver aminotransferase levels in the gefitinib group, and neurotoxic effects, alopecia, and hematologic effects in the C/P arm. Interstitial lung disease occurred in 2.6% of patients treated with gefitinib and in 1.4% of patients receiving chemotherapy.
Data from the WJTOG3405 trial confirmed the good profile of toxicity of gefitinib.Citation33 The most common AEs in the gefitinib group were skin rash, liver dysfunction, dry skin, and diarrhea. AEs of grade 3 or more were infrequent with the exception of liver dysfunction. In the cisplatin plus docetaxel group, the most common AEs, which occurred in more than half of patients, were nausea, myelosuppression, fatigue, and alopecia. Interstitial lung disease was observed in 2.3% of patients in the gefitinib group.
In agreement with the other previously described trials, the NEJ002 study showed that skin rash and elevated levels of aspartate aminotransferase or alanine aminotransferase were the most common AEs in the gefitinib-treated group.Citation34 The incidence of severe toxic effects (grade 3/4) was shown to be significantly higher in the chemotherapy group than in the gefitinib group (71.7% vs 41.2%) (). Interstitial lung disease was reported in 5.3% of patients in the gefitinib group.
Overall, these data demonstrated that gefitinib 250 mg/day has a favorable safety profile with a low incidence of grade 3/4 AEs. The treatment was well tolerated because interruption of the administration of the drug was observed only in a low percentage of patients (<7%). The only serious AE associated with the gefitinib treatment that has been reported in several studies is interstitial lung disease. However, the incidence of gefitinib-induced interstitial lung disease is higher in Japanese patients (4%–6%) compared with those from other countries such as the US (0.3%), although the reason for this geographic difference is unclear.Citation35 Analysis of Japanese patients with NSCLC treated with gefitinib showed that positive smoking history, male gender, the coincidence of interstitial pneumonia, an older age, and a poor performance status are significantly associated with gefitinib-induced interstitial lung disease.Citation36,Citation37
Implications for enhanced patient care, quality of life, and patient satisfaction/acceptability
Treatment of patients with advanced NSCLC is aimed at obtaining a prolongation in survival and an improvement of disease symptoms without additive side effects. For this reason, assessment of quality of life (QoL) is considered important in clinical trials. QoL was determined in the gefitinib trials with the use of the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire and the Trial Outcome Index (TOI), which is the sum of the physical well-being, functional well-being, and Lung Cancer Subscale (LCS) scores of FACT-L. Symptoms were assessed with the use of the LCS score.Citation38
The IDEAL 1 and 2 trials first showed an improvement of QoL in patients who received gefitinib.Citation17,Citation18 An improvement in disease-related symptoms was observed both in patients with tumor regression and in those with stable disease. In the group of patients receiving gefitinib 250 mg/day, the rate of disease-related symptom improvement was 40.3% and 43.1% in IDEAL 1 and 2, respectively. The median time to symptom improvement was short, occurring within a few days following the start of the treatment.Citation17,Citation18 Importantly, in the cohort of patients receiving 250 mg of gefitinib in the IDEAL 2 trial, a correlation was found between OS and symptom improvement. In fact, patients with symptom improvement had a median survival time of 13.6 months versus 4.6 months of patients with no improvement. The median survival of patients with symptom improvement without tumor response was 9.7 months.Citation18
In the INTEREST study, significantly more patients had sustained and clinically relevant improvement in QoL with gefitinib than with docetaxel, as assessed by FACT-L total score (25.1% for gefitinib vs 14.7% for docetaxel) and the FACT-L TOI (17.3% for gefitinib vs 10.3% for docetaxel).Citation21 Improvement in lung cancer symptoms, as assessed on the basis of the LCS scores, was observed both in patients treated with gefitinib (20.4%) and in those treated with docetaxel (16.8%).
An improvement of QoL in patients receiving gefitinib was observed in the IPASS study.Citation30 Significantly more patients in the gefitinib group than in the chemotherapy group had a clinically relevant improvement in QoL, as assessed by scores on the FACT-L questionnaire (48.0% for gefitinib vs 40.8% for chemotherapy) and scores on the TOI (46.4% for gefitinib vs 32.8% for chemotherapy). Rates of reduction in symptoms were similar between patients who received gefitinib (51.5%) and those who received chemotherapy (48.5%), as determined on the basis of LCS scores. As expected, analysis of subgroups revealed that EGFR mutation-positive patients treated with gefitinib showed a greater improvement of symptoms compared with EGFR mutation-negative patients (70.2% vs 14.6%, FACT-L score; 70.2% vs 12.4%, TOI scores; 75.6% vs 20.2%, LCS). Within mutation-positive patients, treatment with gefitinib produced a higher improvement of symptoms compared with chemotherapy (70.2% vs 44.5%, FACT-L score; 70.2% vs 38.3%, TOI scores; 75.6% vs 53.9%, LCS). In contrast, patients with no mutations of the EGFR showed an improvement of symptoms inferior with gefitinib than with chemotherapy (14.6% vs 36.3%, FACT-L score; 12.4% vs 28.8%, TOI scores; 20.2% vs 47.5%, LCS).
Conclusion
The decision of the EMEA to approve the use of gefitinib only for patients with advanced NSCLC who have mutations of the EGFR represents a milestone for the treatment of this disease. As a matter of fact, gefitinib is the first drug that has been approved for NSCLC on the basis of mutational analysis. The approval by EMEA was based on the evidence that treatment with gefitinib produces a significant effect on the course of the disease only in patients who carry activating mutations of the EGFR, as previously described.
Although the correlation between EGFR mutations and activity of gefitinib has been clearly demonstrated in several studies, several points still need to be addressed in order to improve the use of this drug. For example, some studies have suggested that patients carrying deletions of exon 19 have a better prognosis compared with those who have the L858R-point mutation in exon 21 when treated with an EGFR TKI.Citation29 However, this correlation has not been confirmed in two trials in East Asian patients; therefore, it needs to be further explored in both Caucasian and East Asian subgroups.Citation33,Citation34 Furthermore, data on the activity of EGFR TKIs are available for the most common mutations, such as the deletions of exon 19 and the L858R mutations, whereas little information is available on the clinical outcome of patients carrying rarer mutations of exons 18 and 20. Interestingly, activity of gefitinib on the mechanisms involved in the progression of bone metastases in NSCLC patients has also been reported.Citation39–Citation42 However, it is not clear whether this phenomenon might be related to the presence and type of EGFR mutation.
Some patients harboring activating EGFR mutations that are associated with activity of EGFR TKIs do not respond to gefitinib or erlotinib. In this regard, it will be important to improve the methods of detection of the EGFR mutations. In fact, it has been shown that artifacts might frequently occur when mutations are investigated with direct sequencing of the polymerase chain reaction product and a low amount of tissue/DNA is available.Citation43,Citation44 Therefore, it will be important to assess whether lack of response is due to molecular mechanisms that limit the efficacy of gefitinib or to false positive results of the mutational analysis.
Finally, another important issue that needs to be addressed is the phenomenon of acquired resistance to gefitinib. Although the majority of patients with EGFR mutations respond to this drug and have a prolonged PFS, inevitably, all patients will experience progression of the disease. In this regard, it has been shown that approximately 50% of the patients who progress following initial response to an EGFR TKI do have the T790M mutations, whereas 25% of the patients show amplification of the gene that encodes for the MET receptor.Citation3 In both cases, this event seems to be due to selection of tumor cells that harbor the T790M mutation or that have amplification of MET at the diagnosis.Citation45–Citation47 Because new agents that are able to block the activation of the T790M mutant EGFR or of MET will soon be available for clinical use, it will be important to develop techniques for the molecular monitoring of NSCLC patients who are treated with gefitinib. As a matter of fact, for most of these patients, at least two lines of therapy with biologic agents will be available in the near future.
In conclusion, the introduction of gefitinib in the therapy of EGFR-mutant NSCLC patients is a major breakthrough for the management of these patients and represents the first step toward personalized treatment for NSCLC patients.
Acknowledgements
Nicola Normanno is supported by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), the Italian Department of Health and Department of Research of the Campania Region.
Disclosure
The authors report no conflicts of interest in this work.
References
- JankuFStewartDJKurzrockRTargeted therapy in non-small-cell lung cancer: is it becoming a reality?Nat Rev Clin Oncol20107740141420551945
- NormannoNDe LucaABiancoCEpidermal growth factor receptor (EGFR) signaling in cancerGene200636621616377102
- De LucaANormannoNPredictive biomarkers to tyrosine kinase inhibitors for the epidermal growth factor receptor in non-small-cell lung cancerCurr Drug Targets201011785186420388064
- NormannoNBiancoCDe LucaATarget-based agents against ErbB receptors and their ligands: a novel approach to cancer treatmentEndocr Relat Cancer200310112112653668
- ShepherdFARodrigues PereiraJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- ThatcherNChangAParikhPGefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer)Lancet200536694961527153716257339
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A200410136133061331115329413
- PaezJGJannePALeeJCEGFR mutations in lung cancer: correlation with clinical response to gefitinib therapyScience200430456761497150015118125
- LinardouHDahabrehIJBafaloukosDSomatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLCNat Rev Clin Oncol20096635236619483740
- SequistLVBellDWLynchTJHaberDAMolecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancerJ Clin Oncol200725558759517290067
- SharmaSVBellDWSettlemanJHaberDAEpidermal growth factor receptor mutations in lung cancerNat Rev Cancer20077316918117318210
- PaoWChmieleckiJRational, biologically based treatment of EGFR-mutant non-small-cell lung cancerNat Rev Cancer2010101176077420966921
- GazdarAFActivating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitorsOncogene200928Suppl 1S24S3119680293
- PaoWMillerVAPolitiKAAcquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domainPLoS Med200523e7315737014
- JiHLiDChenLThe impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapiesCancer Cell20069648549516730237
- FukuokaMYanoSGiacconeGMulti-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial) [corrected]J Clin Oncol200321122237224612748244
- KrisMGNataleRBHerbstRSEfficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trialJAMA2003290162149215814570950
- GiacconeGHerbstRSManegoldCGefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1J Clin Oncol200422577778414990632
- HerbstRSGiacconeGSchillerJHGefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2J Clin Oncol200422578579414990633
- KimESHirshVMokTGefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trialLancet200837296521809181819027483
- JännePAGurubhagavatulaSYeapBYOutcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access studyLung Cancer200444222115084387
- MillerVAKrisMGShahNBronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancerJ Clin Oncol20042261103110915020612
- DouillardJYShepherdFAHirshVMolecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trialJ Clin Oncol201028574475220038723
- SequistLVMartinsRGSpigelDFirst-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutationsJ Clin Oncol200826152442244918458038
- AsahinaHYamazakiKKinoshitaIA phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutationsBr J Cancer2006958998100417047648
- InoueASuzukiTFukuharaTProspective phase II study of gefitinib for chemotherapy-naive patients with advanced non- small-cell lung cancer with epidermal growth factor receptor gene mutationsJ Clin Oncol200624213340334616785471
- InoueAKobayashiKUsuiKFirst-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapyJ Clin Oncol20092791394140019224850
- RosellRMoranTQueraltCScreening for epidermal growth factor receptor mutations in lung cancerN Engl J Med20093611095896719692684
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- YangCHFukuokaMMokTSFinal overall survival results from a phase III, randomised, open-label, first-line study of gefitinib vs carboplatin/paclitaxel in clinically selected patients with advanced non-small cell lung cancer in Asia (IPASS)Presented at European Society of Medical Oncology Meeting2010 (Abstract LBA2).
- LeeJSParkKKimSWA randomized phase III study of gefitinib (IRESSA) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lungPresented at: World Conference on Lung Cancer2009 (Abstract PRS4).
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- CohenMHWilliamsGASridharaRFDA drug approval summary: gefitinib (ZD1839) (Iressa) tabletsOncologist20038430330612897327
- AndoMOkamotoIYamamotoNPredictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinibJ Clin Oncol200624162549255616735708
- KudohSKatoHNishiwakiYInterstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control studyAm J Respir Crit Care Med2008177121348135718337594
- CellaDFBonomiAELloydSRReliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrumentLung Cancer19951231992207655830
- NormannoNDe LucaAAldinucciDGefitinib inhibits the ability of human bone marrow stromal cells to induce osteoclast differentiation: implications for the pathogenesis and treatment of bone metastasisEndocr Relat Cancer200512247148215947117
- ZampaGMoscatoMBranniganBWProlonged control of bone metastases in non-small-cell lung cancer patients treated with gefitinibLung Cancer200860345245418079016
- PluquetECadranelJLegendreAOsteoblastic reaction in non-small cell lung carcinoma and its association to epidermal growth factor receptor tyrosine kinase inhibitors response and prolonged survivalJ Thorac Oncol20105449149620195171
- GarfieldDNormannoNOsteoblastosis and activating epidermal growth factor receptor mutations: a relationship?J Thorac Oncol20105441541620357615
- MarchettiAFelicioniLButtittaFAssessing EGFR mutationsN Engl J Med20063545526528 author reply52652816452569
- MarchettiANormannoNPintoCRecommendations for mutational analysis of EGFR in lung carcinomaPathologica2010102311912621171518
- MaheswaranSSequistLVNagrathSDetection of mutations in EGFR in circulating lung-cancer cellsN Engl J Med2008359436637718596266
- Godin-HeymannNBryantIRiveraMNOncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutationCancer Res200767157319732617671201
- TurkeABZejnullahuKWuYLPreexistence and clonal selection of MET amplification in EGFR mutant NSCLCCancer Cell2010171778820129249