Abstract
Esophageal atresia remains one of the most challenging congenital anomalies of the newborn. In recent years, because of the advances in prenatal diagnosis, neonatal critical care, and surgical procedures, overall outcomes have improved substantially, including for premature children. Nowadays, most of the research is focused on medium- and long-term morbidity, with particular reference to respiratory and gastroesophageal problems; the high frequency of late sequelae in esophageal atresia warrants regular and multidisciplinary checkups throughout adulthood. Surprisingly, there are few studies on the impact of prenatal diagnosis and there is continuing debate over the prenatal and preoperative management of these complex patients. In this review, we analyze the literature surrounding current knowledge on the management of newborns affected by esophageal atresia, focusing on prenatal management and preoperative assessment.
Introduction
With an incidence of 2.43 cases per 10,000 births, esophageal atresia (EA), with or without tracheoesophageal fistula (TEF), remains one of the most challenging congenital anomalies of newborn babies.Citation1,Citation2 Due to advances in prenatal diagnosis, neonatal critical care, and surgical procedures, overall outcomes have improved substantially in recent years, including for premature children.Citation2–Citation4 Nevertheless, EA is still associated with a life-long risk of complications, even if mortality is currently limited to those cases with associated severe life-threatening anomalies. Many unanswered questions still remain in surgical and clinical arenas. In the latter scenario, a continuing debate over the prenatal and preoperative management of these complex patients is ongoing, and more evidence-based recommendations are impelling.Citation3–Citation5 In this review, we analyze the current knowledge on the management of newborns affected by EA, focusing on prenatal and preoperative assessment.
Classification
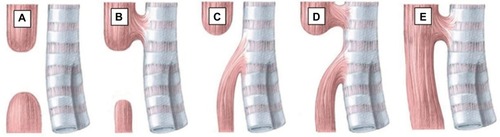
The variants of EA have been described using many anatomical classification systems.Citation1,Citation2 To avoid ambiguity, the clinician should use a narrative description. Nevertheless, Gross of Boston described the classification system that is most often cited.Citation1 According to Gross’ classification, the primary types of congenital EA are EA with distal TEF (85%, Gross C), isolated EA without TEF (8%, Gross A), TEF without atresia or H-type TEF (4%, Gross E), EA with proximal TEF (3%, Gross B), and EA with proximal and distal TEF (<1%, Gross D)Citation2 ().
Figure 1 Anatomical classification of esophageal atresia according to Gross.
Abbreviations: EA, esophageal atresia; TEF, tracheoesophageal fistula.
Antenatal considerations
Ultrasonographic prenatal diagnosis
Ultrasonographic prenatal diagnosis of EA remains difficult and challenging, with prenatal detection rates reported as varying from 10% to 50% of cases Citation5,Citation6 Multicenter studies on prenatal diagnosis are lacking, and only few national registers are currently available ().Citation7–Citation9 Traditionally, a small or absent gastric bubble in combination with maternal polyhydramnios have been the features advocated as the most sensitive sign of EA.Citation3 Nevertheless, the combination of these two signs gave only modest predictive values, ranging from 44% to 56%.Citation4,Citation6,Citation10 Moreover, in cases of TEF, the passage of amniotic fluid through the fistula fills the stomach and usually prevents the development of polyhydramnios. For this reason, in the study by Spaggiari et al, polyhydramnios and small/absent stomach bubbles were observed in 100% of cases of type A EA (pure EA without TEF) and only in 46.3% of cases with TEF (types B, C, D, and E, according to Gross’ classification.Citation1,Citation11 Similar results were reported by the 2013 French National Register, in which the prenatal suspicion of type A EA was 86% and only 12% in cases with TEF.Citation11 Considering that EA with TEF is by far the most common type of EA, this is why the overall prenatal diagnosis of EA remains quite poor.Citation12 The sensitivity of prenatal ultrasound (US) was, therefore, significantly higher for those scans performed in tertiary center hospitals compared with those performed in district general hospitals.Citation11–Citation14 The combination of a small stomach together with the so-called pouch sign has been reported to be diagnostic for EA in few studies.Citation13–Citation16 This direct finding consists of the blind-ending pouch in the fetal neck or mediastinum during fetal swallowing, and it gave excellent predictive values. Citation14 Nevertheless, the pouch sign is clearly detectable only after the 26th week of gestation (); as hypothesized by Kalache et al, in the first and early second trimester, the fetus is not able to generate sufficient pressure on swallowing to permit dilatation of the blind-ending esophagus.Citation14 The localization of the upper pouch may also be significant in predicting the outcomes of the affected fetuses: as reported by Has et al, when the blind esophagus is localized at neck level (“upper pouch sign” or “neck sign”), EA is more likely to be associated with long-gap atresia, whereas a mediastinal pouch (“lower pouch sign”) is more likely to be associated with distal TEF and short gap atresia.Citation16 Nevertheless, Solt et al reported a small series of fetuses with polyhydramnios and a pouch sign, in which the neonatal workup ruled out EA in all cases.Citation17 Eventually, Quarello et al reported an indirect sign of EA consisting of a weakness of the posterior wall of the trachea next to the esophageal defect (“tracheal print sign”); in all the six patients in their study, the length of the tracheal widening provides an estimation of the gap between esophageal pouches.Citation18
Table 1 Prenatal diagnosis in recent published data
Table 2 Prenatal findings suspected for EA
Magnetic resonance imaging (MRI)
Recently, fetal MRI has been used to identify thoracic and cardiac malformations.Citation19 In cases of EA suspicion at prenatal US, increasing evidence suggests that the second-line MRI is required in order to refine the diagnosis.Citation9,Citation11,Citation20–Citation22 The two most sensitive and specific features of EA at MRI are the nonvisualization of the intrathoracic portion of the esophagus and the pouch sign.Citation11 The direct visualization of a TEF is pathognomonic, although extremely uncommon.Citation20 Nevertheless, according to Hochart et al, in patients in whom a pouch sign is visualized, distension of the lower esophagus indicates the possible presence of a TE fistula, whereas its absence favors type A.Citation21 Salomon et al proposed the use of rapid dynamic T2-weighted sequences in order to visualize the swallowing fetus more dynamically (“Fast Imaging Employing Steady-State Acquisition”).Citation22 As these dynamic sequences should be repeated as many times as necessary in order to observe sufficient fetal swallowing, MRI is a relatively lengthy examination.Citation21 Moreover, the quality of the MRI images is related to the amount of amniotic fluid, which potentially increases fetal motion. Fetal MRI may not be also routinely available in all centers.Citation22 Despite these limitations, prenatal MRI could also focus on associated malformations that are reported in up to 50% of patients with EA, contributing to the overall evaluation through the use of specific sequences, as required, for the evaluation of the nervous system, the chest, or the abdomen.Citation1,Citation21
Biochemistry of amniotic fluid
Only a few studies have evaluated the biochemistry of the amniotic fluid in fetuses with EA.Citation23–Citation27 Morin et al first reported that amniotic fluid from pregnancies with fetuses with intestinal obstruction presented diminished microvillar enzyme activities, as compared to normal fetuses.Citation23 In subsequent years, abnormal levels of gamma-glutamyl transpeptidase (GGT) in amniotic fluid were reported in some series.Citation24,Citation25 In 2011, Czerkiewicz et al proposed the EA index, corresponding to the multiplication of GGT and alpha-fetoprotein, both expressed in multiples of median; by using a threshold of EA index >3, a sensibility of 98% and a specificity of 100% were found in the diagnosis of EA.Citation24 Less encouraging results were reported by Garabedian et al, who with the same EA index observed 90% of sensibility and 60% of specificity.Citation25 The evidence with regard to routine amniotic fluid analysis in fetuses with EA is poor, including due to the fact that it has only been assessed in cases of polyhydramnios.Citation26
The impact of prenatal diagnosis
There has been little study of the impact of prenatal diagnosis on the prognosis of newborns with EA.Citation1,Citation6,Citation27–Citation29 Garabedian et al, analyzing data from the French National Register for infants with EA born from 2008 to 2010, concluded that patients with prenatal diagnosis have a higher morbidity rate related to the EA type (types A, B, and/or long gap), even if no difference in mortality was found.Citation9 Nevertheless, prenatal diagnosis enables the research of associated abnormalities that could affect morbidity.Citation27 Stringer et al demonstrated a high incidence of other major anomalies, particularly trisomy 18, in fetuses with suspected EA, and they indicated much poorer outcomes and a higher mortality in these patients, as compared to children with postnatal diagnosis of EA.Citation29 According to Fallon et al, when EA is suspected in a woman with polyhydramnios, prenatal management with amniotic fluid reduction should be considered to reduce the risk of preterm labor, thus exposing the infant to prematurity-related morbidity.Citation28 Moreover, prenatal diagnosis allows the parents to choose for the birth to take place close to a neonatal surgery unit to avoid the problem of postnatal transfer.Citation9 When an antenatal diagnosis of EA is suspected, cesarean section is recommended in the presence of associated anomalies and not to EA itself. In the Italian National Register, vaginal delivery was reported in 18 (42%) patients with prenatal suspicion of EA, with no statistically significant differences between those with and without antenatal diagnosis of EA.Citation7
Perinatal considerations
Confirmation of the diagnosis
At birth, a 10–12 French gauge nasogastric (NG) tube should be passed through the mouth into the esophagus for any infant born of a pregnancy complicated by polyhydramnios; failure to pass the NG tube beyond 11 or 12 cm has been universally recognized as the classical sign of EA.Citation2,Citation6 A simple X-ray of the chest and abdomen shows the tip of the catheter halted in the superior mediastinum, while gas in the stomach and intestine signifies the presence of a distal TEF.Citation2 Nevertheless, in very rare instances, radiological demonstration of a catheter reaching the stomach does not exclude the diagnosis of EA, as the NG tube may take an alternative route (through the laryngeal inlet, trachea, TEF, and distal esophagus to reach the stomach), which is a rare but well-known scenario.Citation30,Citation31
Preoperative care
The patient should be positioned sitting upright and a sump catheter should be positioned in the upper esophageal pouch to aspirate saliva continuously under low-pressure suction, in order to decrease the risk of pneumonia from aspiration of upper pouch secretions.Citation2,Citation6 Broad-spectrum antibiotics (ampicillin and gentamicin) and vitamin K analog are traditionally administered.Citation2,Citation4 Routine endotracheal intubation should be avoided because of the risk of iatrogenic gastric perforation and of increasing respiratory distress, as the abdomen becomes distended from ventilation through the TEF.Citation2,Citation4 In these patients, a rectal probe should be inserted to facilitate the evacuation of air and to minimize the intestinal overdistension leading to respiratory impairment.
Associated anomalies
A careful clinical examination should be conducted in order to rule out associated abnormalities, which are mainly responsible for the medium- and long-term prognosis in these patients.Citation4 Their unequal distribution between EA patients is also important from a clinical perspective, as newborns with isolated EA without TEF (Gross A EA) exhibit anomalies in as many as 65% of cases, compared with a 10% observed in patients with TEF without atresia (Gross E EA).Citation4,Citation7 All patients should have an echocardiogram prior to surgery, in order to identify any structural anomaly of the heart, which is reported in up to 25% of patients.Citation1,Citation8,Citation9 Moreover, preoperative echocardiogram could accurately detect the correct side of the aortic arch: this anomaly, although only reported in 3.6% of newborns with EA, poses a dilemma in terms of optimal surgical approach, namely the side of the thoracotomy for EA repair.Citation2,Citation32 When a prenatal suspicion is evident, the presence of life-threatening anomalies, including Potter’s syndrome, cerebral hypoplasia, and chromosomal anomalies (trisomy of chromosomes 13, 14, and 18), should be accurately investigated.Citation1
Preoperative assessment
Contrast esophagram
Contrast esophagram has been traditionally performed in order to detect the location of the dilated upper esophageal pouch in relation to the thoracic inlet and to detect a proximal TEF. Nevertheless, the need for contrast esophagram is still debated, as it can give false-negative results (when the fistula is occluded by mucus) or false-positive results (when the contrast identifies the tracheobronchial tree, which is more likely to be aspiration through the larynx rather than through a proximal TEF).Citation2,Citation33 Moreover, this procedure involves radiation hazards and may be associated with complications, including aspiration pneumonia.Citation33
US scan, computed tomographic (CT) scan, and MRI
Increasing evidence suggests that US scans are a useful noninvasive tool for the diagnostic assessment of newborns with EA in order to outline the tracheoesophageal anatomy,Citation34,Citation35 Recently, CT scans and MRIs have been proposed for newborns with EA, to identify the position of the TE fistula and anomalies of the aortic arch.Citation36,Citation37 Nevertheless, experience with these diagnostic tools in the preoperative assessment of newborns with EA is very limited, also for concerns regarding neonatal transportation to the radiology department, the need for sedation, and CT-related radiation hazards.Citation37 Moreover, the routine use of preoperative CT scans or MRIs in these patients is controversial, as the limited information acquired that may contribute to modifying the surgical plan can be easily obtained by preoperative tracheobronchoscopy (TBS) or intraoperatively.Citation38 The only exception is represented by the extremely small subset of patients with preoperative diagnosis of right-side aortic arch and long-gap EA, in whom preoperative MRI is recommended in order to define the most appropriate surgical approach.Citation32
TBS
In recent years, preoperative TBS has received increasing attention in the evaluation of the presence of proximal TEF.Citation33,Citation38–Citation40 However, a recent European survey demonstrated that only 43% of the respondent pediatric surgeons surveyed routinely perform preoperative TBS before EA repair.Citation41 Beyond confirming the presence and location of most commonly observed lower pouch TEF, TBS enables the evaluation of vocal cord motility, to assess the presence of other specific foregut-associated anomalies (tracheomalacia, tracheal clefts, and so on), and the preoperative determination of the gap between esophageal pouches. Moreover, TBS allows the detection of rare upper pouch TEF, which could be unnoticed during surgery. Therefore, today, endoscopic assessment is an essential part of surgical planning.Citation38,Citation39
Assessment of the gap
Accurate preoperative determination of the gap between esophageal pouches is mandatory in order to define the most appropriate surgical plan.Citation1,Citation4,Citation32,Citation42 Gap measurement has been largely reported in detail and should be performed at the time of preoperative TBS.Citation42,Citation43 When a distal TEF is evident, a rigid catheter is inserted into the upper esophageal pouch, while the tip of the bronchoscope is positioned at the level of tracheal opening of distal fistula. Chest fluoroscopy demonstrates the distance between them, which is highly representative of the gap length.Citation32,Citation42 Bagolan et al reported that a standardized and reproducible preoperative protocol of gap measurement enhanced the possibility of preserving the native esophagus in cases of long-gap EA.Citation42
Future perspectives on prenatal and preoperative assessment of patients with EA
EA remains one of the most challenging disorders in newborns and, although improvements have been made in the diagnosis and treatment of these patients over the years, much remains to be understood. Nowadays, research is focused on medium- and long-term morbidity, with particular reference to respiratory and gastroesophageal problems; the high frequency of late sequelae in EA warrants regular and multidisciplinary checkups throughout adulthood. Surprisingly, there are few studies on the impact of prenatal diagnosis. An accurate prenatal diagnosis is necessary in order to offer an early multidisciplinary prenatal counseling and to allow parents to choose for the birth to take place close to a high-volume tertiary-level center to offer the best of care and to avoid the problem of postnatal transfer. Unfortunately, despite recent advancements in prenatal diagnosis, the suspicion of EA is stably reported in <50% of cases of EA, with higher rate reserved only for uncommon EA variants (types A and B). Efforts should be made in order to find new signs alternative when maternal polyhydramnios is absent. Increasing evidence suggests that in case of ultrasonographic suspicion of EA, second-level MRI (including real-time MRI with cine mode) should be offered to parents. Moreover, in cases of pregnancy with polyhydramnios and suspected EA, amniotic fluid reduction should be considered to reduce the risk of preterm labor, thus exposing the infant to prematurity-related morbidity. The type of delivery (cesarean section vs vaginal delivery), according to associated anomalies, should be considered to plan appropriate perinatal care with the prompt availability of the multidisciplinary team (obstetrician, neonatologist, radiologist, neonatal surgeon, and neonatal anesthetist). At birth, an early and accurate preoperative diagnosis including TBS and, in selected case, magnetic resonance imaging is necessary to detect anatomical details such as the side of aortic arch and “long-gap” EA, in order to plan the more appropriate surgical approach before surgical repair. An early and accurate evaluation of associated anomalies, which are mainly responsible for the medium- and long-term prognosis in these patients, is also mandatory.
Author contributions
FP, ALB, SB, and DA conceptualized and designed the study, designed the data collection instruments, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted. Authors declare that this manuscript has never been published and it would not be submitted to any other journal while under consideration for publication in your journal.
Disclosure
The authors report no conflicts of interest in this work.
References
- PedersenRNCalzolariEHusbySGarneEOesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regionsArch Dis Child201297322723222247246
- SpitzLOesophageal atresiaOrphanet J Rare Dis200722417498283
- SulkowskiJPDeansKJAstiLMatteiPMinneciPCUsing the Pediatric Health Information System to study rare congenital pediatric surgical diseases: development of a cohort of esophageal atresia patientsJ Pediatr Surg20134891850185524074656
- PinheiroPFSimões e SilvaACPereiraRMCurrent knowledge on esophageal atresiaWorld J Gastroenterol201218283662367222851858
- BrantbergABlaasH-GKHaugenSEEik-NesSHEsophageal obstruction-prenatal detection rate and outcomeUltrasound Obstet Gynecol200730218018717625804
- HoubenCHCurryJICurrent status of prenatal diagnosis, operative management and outcome of esophageal atresia/tracheo-esophageal fistulaPrenat Diagn20082866767518302317
- Pini PratoACarlucciMBagolanPA cross-sectional nationwide survey on esophageal atresia and tracheoesophageal fistulaJ Pediatr Surg20155091441145625783403
- LeonciniEBowerCNassarNOesophageal atresia and tracheo-oesophageal fistula in Western Australia: prevalence and trendsJ Paediatr Child Health201551101023102925976171
- GarabedianCSfeirRLangloisCDoes prenatal diagnosis modify neonatal treatment and early outcome of children with esophageal atresia?Am J Obstet Gynecol20152123340.e1340.e725265404
- SfeirRBonnardAKhen-DunlopNEsophageal atresia: data from a national cohortJ Pediatr Surg20134881664166923932604
- SpaggiariEFaureGRousseauVSonigoPMillischer-BellaicheAEPerformance of prenatal diagnosis in esophageal atresiaPrenat Diagn201535988889326058746
- HollandAJFitzgeraldDAOesophageal atresia and tracheo-oesophageal fistula: current management strategies and complicationsPaediatr Respir Rev201011210010620416546
- KalacheKDChaouiRMauHBollmannRThe upper neck pouch sign: a prenatal sonographic marker for esophageal atresiaUltrasound Obstet Gynecol19981121381409549842
- KalacheKDWauerRMauHChaouiRBollmannRPrognostic significance of the pouch sign in fetuses with prenatally diagnosed esophageal atresiaAm J Obstet Gynecol2000182497898110764484
- SatohSTakashimaTTakeuchiHKoyanagiTNakanoHAntenatal sonographic detection of the proximal esophageal segment: specific evidence for congenital congenital esophageal atresiaJ Clin Ultrasound19952374194237560155
- HasRGünaySUpper neck pouch sign in prenatal diagnosis of esophageal atresiaArch Gynecol Obstet20042701565812827385
- SoltIRotmenschSBronshteinMThe esophageal ‘pouch sign’: a benign transient findingPrenat Diagn201030984584820582925
- QuarelloESaadaJDesbriereRRousseauVDe LagausiePBenachiAPrenatal diagnosis and evaluation of defect length in esophageal atresia using direct and indirect (tracheal print) signsUltrasound Obstet Gynecol201138222522821105018
- LevineDBarnewoltCEMehtaTSTropIEstroffJWongGFetal thoracic abnormalities: MR imagingRadiology2003228237938812821772
- LangerJCHussainHKhanAPrenatal diagnosis of esophageal atresia using sonography and magnetic resonance imagingJ Pediatr Surg200136580480711329594
- HochartVVerpillatPLangloisCThe contribution of fetal MR imaging to the assessment of oesophageal atresiaEur Radiol201525230631425304819
- SalomonLJSonigoPOuPVilleYBrunelleFReal-time fetal magnetic resonance imaging for the dynamic visualization of the pouch in esophageal atresiaUltrasound Obstet Gynecol200934447147419746445
- MorinPRMelançonSBDallaireLPotierMPrenatal detection of intestinal obstructions, aneuploidy syndromes, and cystic fibrosis by microvillar enzyme assays (disaccharidases, alkaline phosphatase, and glutamyltransferase) in amniotic fluidAm J Med Genet19872624054152880507
- CzerkiewiczIDreuxSBeckmezianABiochemical amniotic fluid pattern for prenatal diagnosis of esophageal atresiaPediatr Res201170219920221522036
- GarabedianCVerpillatPCzerkiewiczIDoes a combination of ultrasound, MRI, and biochemical amniotic fluid analysis improve prenatal diagnosis of esophageal atresia?Prenat Diagn201434983984224706336
- MullerCCzerkiewiczIGuimiotFSpecific biochemical amniotic fluid pattern of fetal isolated esophageal atresiaPediatr Res201374560160523942557
- EyheremendyEPfisterMAntenatal real-time diagnosis of esophageal atresiasJ Clin Ultrasound19831173953976415126
- FallonSCEthunCGOlutoyeOOComparing characteristics and outcomes in infants with prenatal and postnatal diagnosis of esophageal atresiaJ Surg Res2014190124224524768139
- StringerMDMcKennaKMGoldsteinRBFillyRAAdzickNSHarrisonMRPrenatal diagnosis of esophageal atresiaJ Pediatr Surg1995309125812638523220
- KutiKPatelRChapmanSJawaheerGA rare pitfall in the diagnosis of oesophageal atresiaPediatr Radiol201343890290420461367
- KumarMThomasNAppearances are deceptive – passing a nasogastric tube does not always rule out oesophageal atresiaJ Clin Diagn Res2016104SD01SD02
- ParoliniFArmelliniABoroniGBagolanPAlbertiDThe management of newborns with esophageal atresia and right aortic arch: a systematic review or still unsolved problemJ Pediatr Surg201651230430926592954
- ParoliniFBoroniGStefiniSRole of preoperative tracheobronchoscopy in newborns with esophageal atresia: a reviewWorld J Gastrointest Endosc201461048248725324919
- SuPYuanYZhangZHuangYWangWApplication of high-frequency ultrasound in esophageal atresia with distal fistulaDis Esophagus20142794325329
- GassnerIGeleyTESonographic evaluation of oesophageal atresia and tracheo-oesophageal fistulaPediatr Radiol200535215916415480618
- FitozSAtasoyCYagmurluAAkyarSErdenADindarHThree-dimensional CT of congenital esophageal atresia and distal tracheoesophageal fistula in neonates: preliminary resultsAJR Am J Roentgenol200017551403140711044052
- GargeSRaoKLBawaMThe role of preoperative CT scan in patients with tracheoesophageal fistula: a reviewJ Pediatr Surg20134891966197124074676
- AtzoriPIacobelliBDBotteroSPreoperative tracheobronchoscopy in newborns with esophageal atresia: does it matter?J Pediatr Surg20064161054105716769333
- ParoliniFMorandiAMacchiniFEsophageal atresia with proximal tracheoesophageal fistula: a missed diagnosisJ Pediatr Surg2013486e13e17
- ShoshanyGVatzianAIlivitzkiASmolkinTHakimFMakhoulIRNear-missed upper tracheoesophageal fistula in esophageal atresiaEur J Pediatr2009168101281128419194723
- ZaniAEatonSHoellwarthMEInternational survey on the management of esophageal atresiaEur J Pediatr Surg20132413923934626
- BagolanPValfrèLMoriniFConfortiALong-gap esophageal atresia: traction-growth and anastomosis – before and beyondDis Esophagus201326437237923679026
- ChanKLSaingHCombined flexible endoscopy and fluoroscopy in the assessment of the gap between the two esophageal pouches in esophageal atresia without fistulaJ Pediatr Surg19953056686707623224
