Abstract
Infantile hemangiomas (IHs) are the most common benign tumor of infancy. As our understanding of their pathobiology has evolved, treatment has become more focused and tailored to specifically treat IH while minimizing adverse effects. Propranolol has gained FDA approval as the first medical therapy for a traditionally surgical disease. This review provides readers with an overview of IH, treatment modalities, and addresses specific considerations in IH disease management.
Introduction
Infantile hemangiomas (IHs) are the most common benign tumor of infancyCitation1–Citation3 with an incidence of approximately 4–5%.Citation2,Citation4 It is more common in females and Caucasians.Citation4,Citation5 IHs are also associated with low birth weight and premature infants.Citation5–Citation7 Preterm infants have an increased incidence of developing IHs and can be as high as 30% in infants with birth weight <1000 g.Citation6,Citation8 Other risk factors associated with IH development include placental abnormalitiesCitation5,Citation9–Citation11 and infants whose mother underwent either amniocentesis or chorionic villus sampling.Citation11–Citation13 However, none of these factors have been proven to have a causal relationship with IH development.
Preterm infants have been shown to have an increased incidence of IHs that is associated with birth weight.Citation6 The incidence of IH is increased with decreasing gestational age with a female and Caucasian predominance.Citation8
IHs have a distinct natural history involving 3 stages. An early proliferative phase is characterized by rapid growth of abnormal blood vessels during the first 6 months of life and extends to the first 10–12 months of life. The hemangioma appears bright red and bosselated (). This proliferative phase is followed by an involuting phase starting at about 1 year of age and can take years for complete involution.Citation2,Citation4 An involuted IH may shrink resulting in anetoderma of the involved area. Otherwise, an involuted IH can result in a fibrofatty residuum with contour deformity ().Citation4,Citation14–Citation16 Coarse telangiectasia can also be seen on the skin surface.Citation4,Citation16,Citation17 Although IHs are benign and undergo spontaneous regression,Citation18 their clinical course during any phase, but in particular during the proliferative phase, can lead to significant morbidity and mortality to patients. Intervention is indicated if the IH is expected to cause morbidities.
Figure 1 (A) A proliferating IH on the cheek of an infant. Note the ulceration in the center of the IH. (B) A proliferating IH is highly cellular with poorly defined vascular spaces (black arrowheads).
Abbreviation: IH, infantile hemangioma.
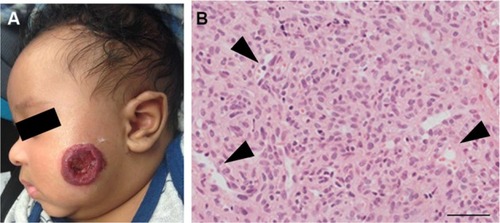
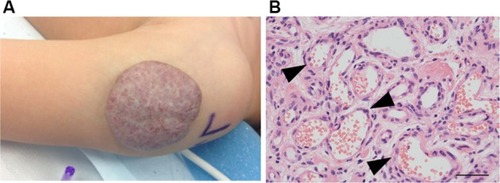
Figure 2 (A) An involuting IH on the arm of a toddler. Note the graying red color when compared to the proliferating IH. (B) Histology: involuting IHs have fewer cells and organized vascular tubular structures (black arrowheads).
Abbreviation: IH, infantile hemangioma.
Morbidities associated with IHs
Despite being a “benign” tumor with eventual involution and possible resolution, IHs can develop complications and require medical intervention. One study reports 24% of patients presented with a complication and 38% of patients required treatment.Citation7 Different morbidities are associated with proliferative phase versus involuting/involuted phase IH. Therefore, the treatment modalities may be different based on the morbidity that has developed. However, the timing of intervention may or may not correlate with the time that a morbidity has developed. For example, an urgent ulceration or bleeding of a proliferating IH will require intervention at the time of development of the complication. By contrast, a large IH that results in a residual contour deformity can be treated more electively, with less urgency to immediate intervention.
Proliferative phase
Morbidities during proliferation include ulceration, bleeding, and obstruction of vital structures. Surgical biopsy may be indicated in the case of a diagnostic dilemma. In particular, preterm infants have an increased risk of ulceration of their IHs.Citation19 In fact, preterm birth predisposes infants to ulceration of IHs, with an odds ratio of 2.29.Citation19
Involuting/involuted phase
During the involuting/involuted phase, the major morbidities are disfigurement and contour deformity. If there was ulceration that healed secondarily, there will be disfigurement from the residual scar.
Hepatic hemangiomas (HHs)
A special subset of IHs are visceral IHs, of which HHs are the most common.Citation20–Citation22 There are 3 subtypes of HHs: solitary, multifocal, and diffuse. Only the multifocal HHs are similar to IHs. HH lesions in some patients had similarities to both multiple and diffuse HHs, suggesting that there is a link between multifocal and diffuse HH.Citation23 The solitary HH is thought to be similar to a congenital hemangioma, whereas a diffuse HH would almost completely replace liver parenchyma, and the infant may develop abdominal compartment syndrome and severe hypothyroidism. Affected infants can develop high out cardiac failure from shunting as well. These can be life-threatening conditions.
Indications for treatment
Proliferative phase
There are few indications for treatment during the proliferative phase. They are divided into the broad categories of 1) prevention of, or limitation of extent, of morbidities, and 2) treatment of morbidities that have already developed.
Prevention of morbidities
Systemic medical therapies are indicated to prevent the development of morbidities associated with both proliferative and involuting/involuted phase morbidities. Medical therapies are also indicated as a treatment modality even after the development of some morbidities.Citation24 If the IH is located in an anatomically sensitive area, such as the face, or if rapid proliferation leading to disfigurement is expected, or if there are concerns for potential functional impairment if the IH growth is left unchecked, then medical therapy is indicated.Citation25 The goal of medical therapies is to prevent or limit IH proliferation to decrease the risk of ulceration/bleeding, or the subsequent disfigurement that may result without intervention.
Common morbidities and diagnostic dilemma
Treatment is indicated for ulcerationCitation18,Citation24,Citation26 () and bleeding.Citation15,Citation27 Ulcerated IH may require wound care with or without concurrent medical therapies, and antibiotics may be needed if there is superinfection. Laser therapy may improve healing time and decrease pain.Citation28 Refractory ulceration non-responsive to conservative management may require surgical resection and closure. Oozing may occur with an ulcerated IH and will stop after replacement of a dressing. Occasionally, arterial bleeding can occur with or without concurrent ulceration. These are true surgical emergencies, and the source of bleeding – the IH – needs to be surgically resected.Citation15,Citation27
Less commonly, an IH may present with less typical feature. If the natural history or imaging modalities are not completely consistent with an IH, and there are concerns of a different lesion in the differential diagnosis, excisional or incisional biopsy may be indicated. Differential diagnoses include a wide range of benign and malignant lesions, including glioma (glabella IH), other vascular tumors such as tufted angiomas, hemangiopericytoma, and kaposiform hemangioendothelioma (KHE), vascular malformations such as venous and lymphatic malformations, to malignant lesions including infantile fibrosarcoma, rhabdomyosarcoma, and neuroblastoma.Citation25
Special considerations: airway and subglottic IHs
Subglottic IHs may obstruct the airway. Infants do not usually present until the IH has proliferated large enough to produce biphasic stridor.Citation29,Citation30 If an infant presents with a beard distribution IH, there is a high risk (>60%) for a concurrent subglottic IH.Citation31,Citation32 Patients with beard distribution IH should be investigated by fiberoptic bronchoscopy. The patient may require multimodality treatments including medical, laser, and surgical therapies.
Special considerations: periorbital IHs
Periorbital IHs may obstruct the visual axis.Citation33,Citation34 If left untreated, astigmatism can occur as a result of deformation of the cornea from pressure exerted by an upper eyelid IH. Deprivation amblyopia can occur from a large periorbital IH obstructing input to the retina at a critical period of development of the visual axis. If the IH is untreated, permanent blindness in the affected eye will result. Medical and/or surgical treatment is indicated. Every infant who develops a periorbital IH should be referred to a pediatric ophthalmologist for evaluation and management as well.
Special considerations: PHACE syndrome
PHACE syndrome was first described in 2005 and consists of a Posterior fossa brain malformations, large segmental facial Hemangioma, Arterial anomalies, Cardiac anomalies, and Eye anomalies.Citation2,Citation35,Citation36 The central nervous system (CNS) abnormality has since been defined to include other structure brain anomalies not limited to the posterior fossa.Citation36 Arterial anomalies include atresia or absence of the ipsilateral cerebral circulatory system. These patients are at risk for ischemic infarcts.Citation35,Citation37–Citation39 PHACE syndrome is diagnosed when 2 or more major criteria are met.Citation40 When a patient presents with a large segmental facial IH, there is ~20% risk of PHACE syndrome.Citation41 Interventions unrelated to the hemangiomas may be indicated for these patients. For example, cardiac surgery may be indicated if there is coarctation of the aortaCitation42 or a encephaloduroarteriosynangiosis, where the superficial temporal artery is grafted directly onto the pia mater of the CNS, which may be indicated to provide adequate perfusion to the brain.Citation38,Citation43
Special considerations: HH
There are 3 subtypes of HH: solitary, multifocal, and diffuse. The solitary HH is thought to be a rapidly involuting congenital hemangioma and will resolve without intervention and is often an incidental finding. The multifocal HH has glucose transporter 1 (GLUT1) positivity and is thought to be similar to IHs. Multifocal HHs do not always have physiological symptoms but are thought to respond to propranolol or corticosteroids. Diffuse HH can be life-threatening. Liver parenchyma is almost completely replaced by HH, resulting in hepatomegaly, compression of the vena cava, abdominal compartment syndrome, high output cardiac failure from shunting, and severe, life-threatening hypothyroidism from the production of type 3 iodothyronine deiodinase by the HH.Citation20–Citation22
Involuting/involuted phase
During the involuting/involuted phase, the indication for treatment is to treat residual stigmata. Laser and surgical treatments are the main modalities. If there is coarse telangiectasia, laser therapy is indicated.Citation44,Citation45 If the elasticity of the skin is destroyed, anetoderma results.Citation16 This can be treated with surgical resection if it is clinically obvious. If there is gross disfigurement resulting from rapid proliferation resulting in contour deformity, then surgical resection and debulking are the only treatment options. All surgical treatment options will result in scars. Therefore, the objective is to produce a scar that is more esthetically pleasing than the deformity.
Treatment modalities: during the proliferative phase
Laser
Laser treatment has been shown to be efficacious in correcting discoloration.Citation44,Citation45 A hypothesis has developed that laser treatment in the early proliferative phase, before the bulk develops and IH thickens, may contain and disrupt IH proliferation. Unfortunately, a randomized controlled trial demonstrated that early laser treatment was unable to prevent IH proliferation, although it did treat children who had more skin atrophy and hypopigmentation.Citation46 Therefore, there are no indications for laser treatment for discoloration during the proliferative phase, and early treatment may have less desirable esthetic appearances of the skin.
However, there is a role for laser treatment to improve ulceration.Citation47,Citation48 A dual wavelength pulsed dye laser (PDL) can be used. Laser treatment resulted in more rapid healing of the ulcer and decreased pain.Citation28
Another role for laser therapy during proliferation is for the treatment of airway hemangiomas. If a large subglottic IH obstructs the airway, and medical treatment alone is not sufficient, resection using laser is the treatment of choice. Unlike laser treatments for the superficial aspects of IH, laser treatment of airway IHs use ablative lasers such as CO2.Citation49,Citation50 Other ablative lasers have also been used, such as the potassium titanyl phosphate (KTP) laserCitation51 and the Nd:Yag laser.Citation52 However, it is important to note that an obstructing subglottic hemangioma is potentially life-threatening, and it is common to treat with multiple modalities, including medical therapy, and laser ablation.
Medical therapy
The most common treatment modality during the proliferative phase is medical treatment. Medical intervention is indicated when an IH is in an anatomically critical area, and rapid proliferation can cause destruction and/or obstruction and ulceration.Citation53 The goal is to limit or disrupt the IH proliferative process, to either minimize or prevent development of morbidities altogether. Effective medical treatment during proliferation could minimize or prevent the future need for surgical debulking and recontouring (subsequently). If an IH is not expected to proliferate to the extent to cause significant disfigurement, or if an IH is small, or in an anatomically noncritical area (trunk, arm, and leg), and expected to have no lasting sequelae after involution, it is acceptable to monitor conservatively with no intervention.Citation25
Propranolol
Propranolol is now considered the first-line medical treatment. Hemangeol®, a liquid formulation of propranolol, obtained FDA approval for the treatment of IH in March 2014. This was the first time that a medication was approved for the treatment of any type of infantile vascular tumor.
Propranolol efficacy was first discovered when 2 infants with large IHs had signs of cardiac failure. Propranolol was initiated for their cardiac indications, but the IHs regressed rapidly.Citation54 A subsequent follow-up study confirmed a response rate of 100% in 32 patients.Citation55 Since the first reports, there have been multiple small series and case reports of propranolol efficacy.Citation56–Citation59 A meta-analysis demonstrated that propranolol was more effective than laser, steroids, and vincristine.Citation60
The first randomized controlled trial for propranolol demonstrated propranolol efficacy in the discoloration and volume as early as 4 weeks after treatment initiation. However, the authors reported rebound growth if treatment was stopped before the patient turned 1 year of age.Citation61 Infants with PHACE syndrome were excluded. Subsequent large-scale studies, including a meta-analysis of 1264 patients, demonstrated a response rate of 82–100%, rebound growth in 17% of patients, and adverse event incidence of 31%, although the majority were minor, including sleep disturbances and acrocyanosis. Serious adverse events were noted in <1% of patients (10/1264) and included bradycardia, hypoglycemia, and hypotension.Citation62
Established guidelines on the initiation and management of infants on propranolol have been established.Citation53 These included cardiology evaluation (if available) and baseline EKG prior to dose initiation. Heart rate and blood pressure are monitored after every dose escalation. Frequent feeding has been recommended to prevent hypoglycemia. The goal dose is usually 2 mg/kg/day in divided doses (twice a day [BID] or thrice a day [TID]).Citation53 While some have questioned the lack of evidence in the safety of using propranolol in preterm infants,Citation8 others have demonstrated that preterm infants can have a good response to and statistically significant response to propranolol versus to term infants.Citation63 Additional investigation is needed to definitively establish a treatment regimen for IH in preterm infants.
PHACE syndrome is not a contraindication to propranolol treatment. PHACE patients were further subdivided into “standard risk” and “high risk” groups, with “standard risk” patients in the same risk stratification as non-PHACE patients.Citation53 Thirty-two patients with PHACE syndrome – some considered high risk – were treated with propranolol, and only 1 patient was reported to have a reversible neurological sequelae – mild hemiparesis that improved during propranolol treatment. Nevertheless, extreme caution should be exercised when treating a PHACE patient with propranolol.Citation64
There have been anecdotal reports of using other types of beta-adrenergic receptor blocker for treatment,Citation65,Citation66 but their superiority to propranolol has not been demonstrated. Topical timolol has gained popularity as a low-risk alternative to treat smaller IHs.Citation67 However, it does not penetrate deeper, bulkier IHs.
Corticosteroids
Prior to the discovery of propranolol, corticosteroids were the first-line medical therapy for problematic IHs. Systemic corticosteroid therapy has traditionally been one of the mainstays of treatment in IH. This novel treatment was discovered after systemic steroid therapy was started for the treatment of thrombocytopenia in a child with a hemangioma to prevent further hemorrhagic episodes. The hemangiomas were noticed to become visibly smaller.Citation68
A study by Pope et alCitation69 evaluated the effectiveness of oral versus high-dose pulsed corticosteroids in the treatment of problematic facial IH. In this study, 20 patients were divided into 2 treatment groups, with the oral group receiving prednisolone 2 mg/kg/day in 2 divided doses for 3 months followed by a taper schedule over 6–9 months to prevent rebound. The high-dose pulsed group received pulses of intravenous (IV) corticosteroids monthly for 3 months. The pulse consisted of methylprednisolone 30 mg/kg per day for 3 days. The patients were evaluated at 3 months and 1 year. At the 3-month mark, the patients treated with oral therapy had an improvement in the visual appearance (using a visual analog scale) of the IH versus the IV therapy (P = 0.002) as well as the 1-year mark with a greater improvement in the visual appearance of the oral treatment groups versus IV therapy (P = 0.005). Interestingly in the study, they evaluated 4 angiogenesis markers, plasma bFGF, VEGF, VCAM-1, and endoglin, and urinary bFGF prior to treatment and every month for the first 3 months in a subset of both study groups. VCAM-1 (P < 0.001) and endoglin (P = 0.03) decreased significantly overtime while the others did not change over the study.Citation69
A recent study by Greene et al treated 25 patients with problematic IH that involved the head and neck with 3.0 mg/kg/day of oral prednisolone for 1 month followed by a 0.5 mL taper every 2–4 weeks until discontinuation when the patient reached 10 months of age. All IHs responded to treatment and 88% showed regression and 12% stabilized in growth.Citation14 The Greene et al study showed a rebound growth rate of 8% that was managed successfully by increasing the dosage of prednisolone by 0.5 mL for 1 month before weaning off steroids again.
The long-term side effects of corticosteroid therapy are well established.Citation70,Citation71 Some of which are gastric ulcers, osteoporosis, increased risk of heart disease, increased risk of infections, easy bruiseability, wound complications, glucose intolerance, and hypertension. A study by Boon et alCitation72 identified 80 infants treated with steroids for IHs. Seventy-one percentage of infants developed Cushingoid facies, 42% had a decrease in growth in their weight curve, 35% fell below their growth curve for height, 29% of parents reported irritability, 21% had gastric irritation, and 6% developed oral and/or perineal yeast infection that necessitated antifungal therapy. However, these effects were reversed after cessation of corticosteroid treatment.Citation72 This study highlights some of the potentially serious short-term complications associated with systemic corticosteroid therapy. The authors concluded that oral steroid therapy at a dose of 2–3 mg/kg/day for 1 month and then tapered seem to be safe for the treatment of problematic hemangiomas.
More recently, a randomized clinical trial directly comparing corticosteroids to propranolol demonstrated similar efficacy for corticosteroids and propranolol. The frequency of adverse events (AEs) was also comparable, although the corticosteroid group had more severe AEs. Rate of rebound growth was also comparable. The authors concluded that both medications had similar response rate but corticosteroids resulted in a faster response time, while propranolol was better tolerated.Citation73
In conclusion, these studies demonstrate that corticosteroids may still have a role in medical management. Propranolol has received FDA approval and should be used as a first-line treatment; if propranolol is contraindicated, corticosteroids should be considered.
Interferon
Interferon is included in this discussion as a historical interest. The exact mechanism for corticosteroid and propranolol efficacy is not completely understood; both were discovered serendipitously. However, as IHs are vascular tumors and thought to have proliferation of abnormal vasculature, interferon was proposed to be used as an antiangiogenic agent. Early success in the treatment of a patient with pulmonary hemangiomatosis with interferon encouraged the use of this medication.Citation74,Citation75 In a 1991 study by Ezekowitz et al,Citation76 13 patients with either life-threatening coagulopathy or sight-threatening hemangiomas were treated with interferon alpha after failing steroid therapy. Regression was achieved in 11 of the 13 patients and 3 out of 4 for steroid resistant coagulopathy with little or no toxicity observed at a dose of 2 million units/m2.Citation76 Interferon alpha therapy was thought to be an improvement over steroids for the treatment of life-threatening hemangiomas.
However, this early enthusiasm was tempered by adverse effects of interferon. Effects of systemic interferon therapy are commonly associated with fever, leukopenia, elevation of serum transaminase levels, as well as some CNS toxicity usually manifested by drowsiness or confusion.Citation77 However, the most serious side effect of interferon was irreversible spastic diplegia, a form of hypertonicity and spasticity predominately in the lower extremities.Citation78 The risk of developing spastic diplegia was 19%, and 3/5 patients developed irreversible spastic diplegia. Due to this severe adverse effect, interferon is not used to treat IHs.
Vincristine
Vincristine has been used to treat HHs when corticosteroids have failed.Citation4,Citation56,Citation79 However, since propranolol has demonstrated efficacy in hepatic IHs as well,Citation80 vincristine has become a second-line therapy for hepatic IHs.
Surgical therapy
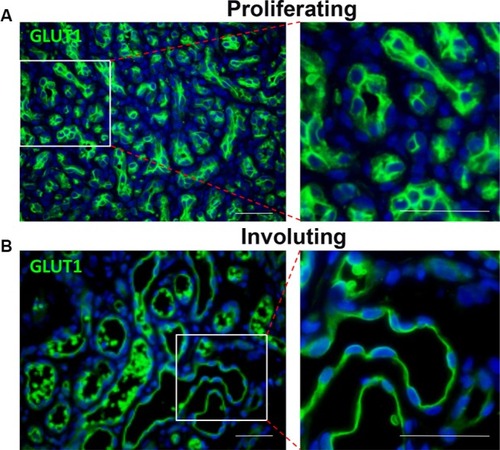
Unlike during the involuting/involuted phase, there are very few indications for surgical intervention during proliferation. One indication is for confirmation of diagnosis. There are other mimickers of IH, and some of them are malignant lesions.Citation25,Citation81 Therefore, if the clinical history, physical examination, and/or imaging studies are not consistent with IH, and there are concerns that the lesion is not an IH, incision or excisional biopsy is indicated to ascertain the diagnosis. IHs have GLUT1+ vessels, and positive GLUT1 staining is diagnostic of IH ().Citation82
Figure 3 Both proliferating (A) and involuting (B) IH endothelial cells express GLUT1 (green).
Abbreviations: IH, infantile hemangioma; GLUT1, glucose transporter 1.
A rapidly proliferating IH can erode the overlying epidermis and cause arterial bleed. This is one of the few absolute indications for surgical resection.Citation15,Citation27 A relative indication for surgical resection during proliferation is ulceration refractory to conservative treatment. An ulceration will ultimately result in a large scar. Until reepithelialization (healing of the ulcer) occurs, the area is tender and at risk for superinfection. If possible, the IH can be resected en bloc. However, if the IH is too large for resection and closure, a partial debulking can be performed, and the rest of the IH can be sutured to each other. The remaining IH can heal and be treated as any non-ulcerated IH.
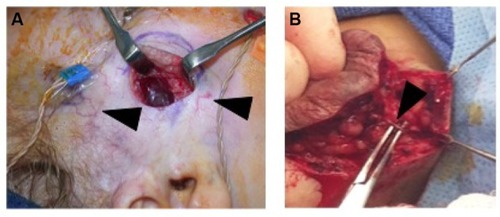
IHs are comprised of a growth of abnormal vasculature; this is surrounded by an avascular fibrous layer. There are multiple feeding vessels that supply the IH, commonly from the deep surface. Therefore, the surgical approach for cutaneous IHs should be to make the skin incision and separate the IH tissues from the surrounding normal tissues at this avascular fibrous plane. This dissection can even be performed with sharp iris scissors. Feeding vessels should be carefully isolated, cauterized, and divided ().Citation83
Figure 4 (A) Intraoperative photo demonstrating that IH can be easily separated from the surrounding soft tissue. Note facial monitoring devices in place. These can be used to assist during facial dissection. Course telangiectasia can be seen as well (arrowheads). (B) Feeding vessels to the IH need to be identified (black arrowhead) and isolated before dividing to minimize blood loss.
Supportive and other adjuvant therapy
As mentioned earlier, other supportive and adjuvant therapies may be indicated. If an airway IH is refractory to interventions, or an airway cannot be securely established, a tracheostomy may be indicated in the most severe cases. In periorbital IHs, an ophthalmologist may prescribe patching or other treatments to correct visual defects. In the case of PHACE syndrome, depending on the associated anomalies, the patient may need consultations and management by cardiology and neurology.Citation38,Citation42 A similar syndrome, PELVIS syndrome, involves hemangiomas in the sacral/perineal area associated with underlying anomalies in the genitourinary systems.Citation84 These patients may need consultations with and management by urologists.
Diffuse HH can be life-threatening. Patients can develop high output cardiac failure from shunting.Citation21,Citation22 In diffuse hepatic IHs, patients may also develop severe and life-threatening hypothyroidism, due to production of type 3 iodothyronine deiodinase in the IH.Citation20,Citation21 Management and replacement of thyroid hormones are essential. In severe cases, liver transplantation may be life-saving.Citation21,Citation22
Treatment modalities: during the involuting/involuted phase
The major morbidities associated with involuting/involuted IH are contour irregularities and disfigurements from a large IH. If there are contour deformities, the only treatment option is surgical debulking and recontouring of affected areas. There is a small role for laser therapy.
Laser
Laser therapy may be indicated to correct persistent coarse telangiectasia without contour irregularities after IH involution. Laser therapy in IH works by inducing injury to the vasculature.Citation85 Lasers only penetrate into the dermis and thus will not have an effect on contour deformity. The main type of laser for color correction is the PDL.Citation17,Citation28,Citation44 It is the first laser specially designed to treat vascular lesions without producing a scar.Citation71 The PDL is characterized by selective destruction of blood vessels by matching the wavelength of light absorbed by hemoglobin.Citation71 The average depth of penetration of PDL is 0.8 mmCitation86 and thus will not affect deeper components of IHs, such as the bulk. The PDL has become the laser of choice for superficial hemangiomas because treatment is relatively simple and complications are rare.Citation44,Citation87
The persistent discoloration from an involuting/involuted IH is usually resected during recontouring procedures. However, on occasion, if surgical debulking is used only to address the abnormal contour, some residual discoloration may remain. The treatment options are to enlarge the area of resection to include all discolored skin (thus leaving a larger permanent scar for the patient) or to resect the minimal area with contour deformity and leave discolored but normal contour IH residuum for laser therapy. This combination approach is preferred by the authors to minimize permanent scarring.
Medical therapy
While propranolol treatment has demonstrated efficacy in treating involuting IHs, there are limited indications to treat IHs beyond the proliferative phase, as the potential benefits to accelerate involution must be weighed against the potential morbidities and inconvenience of using propranolol.Citation88
Surgical therapy
Surgical therapy is the main treatment modality for involuting/involuted IH disfigurement. The goal of the treatment is to remove inelastic, abnormal skin (anetoderma), heal ulceration scar, and to recontour the affected area. It is important to note that the fibrofatty residuum may have already replaced some of the normal subcutaneous tissues. Therefore, complete removal of the IH fibrofatty residuum may not be desirable, as it will leave an atrophic defect.
Another goal is to minimize the size of the scar. In the forehead and cheek, a purse-string closure may be preferable.Citation89,Citation90 The abnormal skin is excised at the base resulting in a circular type of defect. The textbook teaching will require that a 3:1 length-to-width lenticular scar be created to close without skin puckering.Citation89 This will result in a >3× lengthening of the scar. Furthermore, the tension will be bidirection. It may distort surrounding features, such as eyebrows, commissure, or palpebral fissure. A purse-string closure on the other hand will not require further lengthening of the scar. The purse-string will actually contract and minimize the scar. The circumferential and equal distribution of tension will also minimize distortion of surrounding structures. The major drawback is that it will result in a widened, albeit smaller circular or ovoid scar. This can be secondarily revised. The purse-string closure method will result in the smallest possible scar.Citation89,Citation90 It should be avoided in high tension, or otherwise easily closed areas, such as the neck and the scalp. The parents should be counseled that there will be a scar from debulking. However, the resulting scar will be less obvious and more esthetically pleasing than IH deformity. If the IH deformity is so subtle that a scar is not expected to be less conspicuous than the deformity, then surgical intervention is not indicated at all.
A retrospective study on surgical treatment of IH has found that a disproportionate amount of IH requiring surgical debulking occurs on the head and neck, even higher than the normal head and neck distribution of IH occurrence.Citation15 Furthermore, the average size of the deformity is smaller in the head and neck IH, compared to those below the neck, further supporting that head and neck IHs cause disproportionately more disfigurement that require intervention.
Special consideration: lip
IHs on the lip may distort the lip in multiple dimensions: the transverse, cephalad–caudad, as well as the anterior–posterior axes. Therefore, it is not expected that 1 operation will easily and completely restore the normal contour, and a staged approach may be required.Citation91 The goal will be to decrease or normalize 1 or 2 dimensions at each stage. If the IH does not cross the vermilion–cutaneous border, a staged approach can allow debulking and recontouring without extending a scar onto the face. If the vermilion–cutaneous border is crossed, every effort should be made to minimize the scar on the skin if possible,Citation91 and if it is not possible to limit the skin resection only to the abnormal skin from IH disfigurement.
A 1-stage approach can also be used, but this will require extending the scar into the skin.Citation92 As scars are permanent every effort should be made to minimize obvious scarring if possible.
Special considerations: nose
Nasal dorsum
If the nasal skin is stretched and abnormal, they will need to be resected as well and there will be an unavoidable scar. If possible, the incision should be designed to align with the borders of the nasal subunits to make it less conspicuous.
Nasal tip
If the nasal tip distortion is minimal, a closed or an open rhinoplasty approach can be used. The extra fibrofatty residuum is removed. It is imperative to reposition the lower lateral cartilages, as they could be splayed from the IH. Care also should be taken to leave some fibrofatty residuum in place, as the nasal tip normally has some fibrofatty tissues. If all the soft tissues have been aggressively removed, it will result in an abnormal nasal tip with a “pinched” appearance. If the nasal tip skin is stretched and grossly disfigured, then an open rhinoplasty approach with skin resection must be used.Citation93–Citation95 Repositioning of the lower lateral cartilages with an interdomal suture is still necessary. IHs of the alar and other area may need resection and recontouring, and care must be taken to place these incisions along the subunit borders, if possible, as is the case for the nasal dorsum.
Conclusion
Treatment of IHs incorporates a wide range of modalities, from conservative monitoring and reassurance of the parents to urgent surgical intervention for severe morbidities and complications. It is imperative to understand the different growth phases of IHs and what morbidities can be expected at each phase, so that the appropriate treatments may be given at the optimal time.
Acknowledgments
All patients gave consent for their images to be published in this review article; guardian consent was obtained for patients under the age of 18 years.
Disclosure
The authors report no conflicts of interest in this work.
References
- MullikenJBGlowackiJHemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristicsPlast Reconstr Surg19826934124227063565
- FriedenIJHaggstromANDroletBAInfantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7–9, 2005, Bethesda, Maryland, USAPediatr Dermatol200522538340616190987
- SmithCJFFriedlanderSFGumaMKavanaughAChambersCDInfantile hemangiomas: an updated review on risk factors, pathogenesis, and treatmentBirth Defects Res20171091180981528402073
- MullikenJBFishmanSJBurrowsPEVascular anomaliesCurr Probl Surg200037851758410955029
- Hemangioma Investigator GroupHaggstromANDroletBAProspective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristicsJ Pediatr2007150329129417307549
- AmirJMetzkerAKriklerRReisnerSHStrawberry hemangioma in preterm infantsPediatr Dermatol1986343313323774653
- HaggstromANDroletBABaselgaEProspective study of infantile hemangiomas: clinical characteristics predicting complications and treatmentPediatrics2006118388288716950977
- GoelzRPoetsCFIncidence and treatment of infantile haemangioma in preterm infantsArch Dis Child Fetal Neonatal Ed20151001F85F9125352093
- Lopez GutierrezJCAvilaLFSosaGPatronMPlacental anomalies in children with infantile hemangiomaPediatr Dermatol200724435335517845154
- MundenAButschekRTomWLProspective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomaliesBr J Dermatol2014170490791324641194
- BaulandCGSmitJMScheffersSMSimilar risk for hemangiomas after amniocentesis and transabdominal chorionic villus samplingJ Obstet Gynaecol Res201238237137522229643
- BurtonBKSchulzCJAngleBBurdLIAn increased incidence of haemangiomas in infants born following chorionic villus sampling (CVS)Prenat Diagn19951532092147784377
- BaulandCGSmitJMBartelinkLRZondervanHASpauwenPHHemangioma in the newborn: increased incidence after chorionic villus samplingPrenat Diagn2010301091391720824891
- CoutoRAMaclellanRAZurakowskiDGreeneAKInfantile hemangioma: clinical assessment of the involuting phase and implications for managementPlast Reconstr Surg2012130361962422575857
- LeeAHHardyKLGoltsmanDA retrospective study to classify surgical indications for infantile hemangiomasJ Plast Reconstr Aesthet Surg20146791215122124923525
- BaselgaERoeECoulieJRisk factors for degree and type of sequelae after involution of untreated hemangiomas of infancyJAMA Dermatol2016152111239124327540637
- CerratiEWOTMChungHWanerMDiode laser for the treatment of telangiectasias following hemangioma involutionOtolaryngol Head Neck Surg2015152223924325450409
- ChangLCHaggstromANDroletBAHemangioma Investigator GroupGrowth characteristics of infantile hemangiomas: implications for managementPediatrics2008122236036718676554
- CastrénESalminenPGisslerMStefanovicVPitkärantaAKlockarsTRisk factors and morbidity of infantile haemangioma: preterm birth promotes ulcerationActa Paediatr2016105894094527146410
- HuangSATuHMHarneyJWSevere hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomasN Engl J Med2000343318518910900278
- GnarraMBehrGKitajewskiAHistory of the infantile hepatic hemangioma: from imaging to generating a differential diagnosisWorld J Clin Pediatr20165327328027610342
- Christison-LagayERBurrowsPEAlomariAHepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registryJ Pediatr Surg20074216267 discussion 67–6817208542
- JiYChenSXiangBClinical features and management of multifocal hepatic hemangiomas in children: a retrospective studySci Rep201663174427530723
- BarrioVRDroletBATreatment of hemangiomas of infancyDermatol Ther200518215115915953144
- GarzonMCFriedenIJHemangiomas: when to worryPediatr Ann2000291586710941768
- ChamlinSLHaggstromANDroletBAMulticenter prospective study of ulcerated hemangiomasJ Pediatr20071516684689689.e118035154
- ConnellyEAVieraMPriceCWanerMSegmental hemangioma of infancy complicated by life-threatening arterial bleedPediatr Dermatol200926446947219689527
- LiYHuYLiHDengLSuccessful treatment of ulcerated hemangiomas with a dual-wavelength 595- and 1064-nm laser systemJ Dermatolog Treat201627656256727075938
- TingMRosebyRMcAdamCSubglottic infantile haemangioma: a rare but important consideration in young infants presenting with stridorJ Paediatr Child Health201652121111111327567008
- ChunRHMcCormickMEMartinTDroletBAKerschnerJEOffice-based subglottic evaluation in children with risk of subglottic hemangiomaAnn Otol Rhinol Laryngol2016125427327626466859
- OrlowSJIsakoffMSBleiFIncreased risk of symptomatic hemangiomas of the airway in association with cutaneous hemangiomas in a “beard” distributionJ Pediatr199713146436469386676
- SherringtonCASimDKFreezerNJRobertsonCFSubglottic haemangiomaArch Dis Child19977654584599196368
- Spence-ShishidoAAGoodWVBaselgaEFriedenIJHemangiomas and the eyeClin Dermatol201533217018225704937
- CeislerEJSantosLBleiFPeriocular hemangiomas: what every physician should knowPediatr Dermatol20042111914871317
- DroletBADohilMGolombMREarly stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE associationPediatrics2006117395996416510684
- HaggstromANGarzonMCBaselgaERisk for PHACE syndrome in infants with large facial hemangiomasPediatrics20101262e418e42620643720
- HessCPFullertonHJMetryDWCervical and intracranial arterial anomalies in 70 patients with PHACE syndromeAJNR Am J Neuroradiol201031101980198620705698
- HeyerGLMillarWSGhatanSGarzonMCThe neurologic aspects of PHACE: case report and review of the literaturePediatr Neurol200635641942417138012
- SiegelDHTefftKAKellyTStroke in children with posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities (PHACE) syndrome: a systematic review of the literatureStroke20124361672167422442177
- MetryDHeyerGHessCPHACE Syndrome Research ConferenceConsensus statement on diagnostic criteria for PHACE syndromePediatrics200912451447145619858157
- MetryDWHaggstromANDroletBAA prospective study of PHACE syndrome in infantile hemangiomas: demographic features, clinical findings, and complicationsAm J Med Genet A2006140997598616575892
- DijkemaEJLeinerTGrotenhuisHBDiagnosis, imaging and clinical management of aortic coarctationHeart2017103151148115528377475
- JackASChowMMFiorilloLChibukTYagerJYMehtaVBilateral pial synangiosis in a child with PHACE syndromeJ Neurosurg Pediatr2016171707526405843
- ChinnaduraiSSatheNASurawiczTLaser treatment of infantile hemangioma: a systematic reviewLasers Surg Med201648322123326711436
- KesselsJPHamersETOstertagJUSuperficial hemangioma: pulsed dye laser versus wait-and-seeDermatol Surg2013393 pt 141442123279058
- BattaKGoodyearHMMossCWilliamsHCHillerLWatersRRandomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: results of a 1-year analysisLancet2002360933252152712241656
- McCuaigCCCohenLPowellJTherapy of ulcerated hemangiomasJ Cutan Med Surg201317423324223815955
- BruscinoNBonanPCannarozzoGMorettiSLottiTCampolmiPLaser use in infantile hemangiomas, when and howDermatol Ther201225431432122950558
- ReMForteVBerardiCMallardiVRole of endoscopic CO2 laser surgery in the treatment of congenital infantile subglottic hemangioma. Experience in the department of otolaryngology, “Sick Children Hospital”, Toronto, CanadaActa Otorhinolaryngol Ital200323317517914677310
- ClarosAFokouoJVRoquetaCClarosPManagement of subglottic hemangiomas with carbon dioxide laser: our 25-year experience and comparison with the literatureInt J Pediatr Otorhinolaryngol201579122003200726614224
- KackerAAprilMWardRFUse of potassium titanyl phosphate (KTP) laser in management of subglottic hemangiomasInt J Pediatr Otorhinolaryngol2001591152111376814
- FuCHLeeLAFangTJWongKSLiHYEndoscopic Nd:YAG laser therapy of infantile subglottic hemangiomaPediatr Pulmonol2007421899217106903
- DroletBAFrommeltPCChamlinSLInitiation and use of propranolol for infantile hemangioma: report of a consensus conferencePediatrics2013131112814023266923
- Leaute-LabrezeCDumas de la RoqueEHubicheTBoraleviFThamboJBTaiebAPropranolol for severe hemangiomas of infancyN Engl J Med2008358242649265118550886
- SansVDumas de la RoqueEBergeJPropranolol for severe infantile hemangiomas: follow-up reportPediatrics20091243e423e43119706583
- BuckmillerLDyamenahalliURichterGTPropranolol for airway hemangiomas: case report of novel treatmentLaryngoscope2009119102051205419650125
- JephsonCGManunzaFSyedSMillsNAHarperJHartleyBESuccessful treatment of isolated subglottic haemangioma with propranolol aloneInt J Pediatr Otorhinolaryngol200973121821182319796830
- DenoyelleFLeboulangerNEnjolrasOHarrisRRogerGGarabedianENRole of propranolol in the therapeutic strategy of infantile laryngotracheal hemangiomaInt J Pediatr Otorhinolaryngol20097381168117219481268
- FuchsmannCQuintalMCGiguereCPropranolol as first-line treatment of head and neck hemangiomasArch Otolaryngol Head Neck Surg2011137547147821576558
- PeridisSPilgrimGAthanasopoulosIParpounasKA meta-analysis on the effectiveness of propranolol for the treatment of infantile airway haemangiomasInt J Pediatr Otorhinolaryngol201175445546021333364
- HogelingMAdamsSWargonOA randomized controlled trial of propranolol for infantile hemangiomasPediatrics20111282e259e26621788220
- MarquelingALOzaVFriedenIJPuttgenKBPropranolol and infantile hemangiomas four years later: a systematic reviewPediatr Dermatol201330218219123405852
- BrazzelliVGiorginiCBarruscottiSEfficacy of propranolol for cutaneous hemangiomas in premature childrenG Ital Dermatol Venereol2016151548549125692776
- MetryDFriedenIJHessCPropranolol use in PHACE syndrome with cervical and intracranial arterial anomalies: collective experience in 32 infantsPediatr Dermatol2013301718922994362
- BlanchetCNicollasRBigorreMAmedroPMondainMManagement of infantile subglottic hemangioma: acebutolol or propranolol?Int J Pediatr Otorhinolaryngol201074895996120557953
- Abarzua-ArayaANavarrete-DechentCPHeusserFRetamalJZegpi-TruebaMSAtenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled studyJ Am Acad Dermatol20147061045104924656727
- ChakkittakandiyilAPhillipsRFriedenIJTimolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: a retrospective, multicenter, cohort studyPediatr Dermatol2012291283122150436
- ZaremHAEdgertonMTInduced resolution of cavernous hemangiomas following prednisolone therapyPlast Reconstr Surg196739176836018814
- PopeEKrafchikBRMacarthurCOral versus high-dose pulse corticosteroids for problematic infantile hemangiomas: a randomized, controlled trialPediatrics20071196e1239e124717485449
- MoneySThe risks of chronic corticosteroid exposureJ Pain Palliat Care Pharmacother201731216016128375794
- PoetkeMPhilippCBerlienHPFlashlamp-pumped pulsed dye laser for hemangiomas in infancy: treatment of superficial vs mixed hemangiomasArch Dermatol2000136562863210815856
- BoonLMMacDonaldDMMullikenJBComplications of systemic corticosteroid therapy for problematic hemangiomaPlast Reconstr Surg199910461616162310541160
- BaumanNMMcCarterRJGuzzettaPCPropranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: a randomized clinical trialJAMA Otolaryngol Head Neck Surg2014140432333024526257
- WhiteCWSondheimerHMCrouchECWilsonHFanLLTreatment of pulmonary hemangiomatosis with recombinant interferon alfa-2aN Engl J Med198932018119712002710192
- Brouty-BoyeDZetterBRInhibition of cell motility by interferonScience198020844435165186154315
- EzekowitzAMullikenJFolkmanJInterferon alpha therapy of haemangiomas in newborns and infantsBr J Haematol199179suppl 167681931713
- RohatinerAZPriorPBurtonABalkwillFListerTACentral nervous system toxicity of interferonProg Exp Tumor Res1985291972023906762
- BarlowCFPriebeCJMullikenJBSpastic diplegia as a complication of interferon Alfa-2a treatment of hemangiomas of infancyJ Pediatr19981323 pt 15275309544915
- YehIBrucknerALSanchezRJengMRNewellBDFriedenIJDiffuse infantile hepatic hemangiomas: a report of four cases successfully managed with medical therapyPediatr Dermatol201128326727521517953
- MarscianiAPericoliRAlaggioRBrisigottiMVergineGMassive response of severe infantile hepatic hemangioma to propanololPediatr Blood Cancer2009541176
- GarzonMCWeitzNPowellJVascular anomalies: differential diagnosis and mimickersSemin Cutan Med Surg201635317017627607326
- NorthPEWanerMMizerackiAMihmMCJrGLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomasHum Pathol2000311112210665907
- WuJKAschermanJAVascular anomaliesGuyuronBErikssonEPersingJAPlastic Surgery: Indications and Practice11st edChinaSaunders Elsevier2009761777
- GirardCBigorreMGuillotBBessisDPELVIS SyndromeArch Dermatol2006142788488816847205
- TanziELLuptonJRAlsterTSLasers in dermatology: four decades of progressJ Am Acad Dermatol2003491131 quiz 31–3412833005
- AndersonRRParrishJAThe optics of human skinJ Invest Dermatol198177113197252245
- Al BuainianHVerhaegheEDierckxsensLNaeyaertJMEarly treatment of hemangiomas with lasers. A reviewDermatology2003206437037312771489
- ZvulunovAMcCuaigCFriedenIJOral propranolol therapy for infantile hemangiomas beyond the proliferation phase: a multicenter retrospective studyPediatr Dermatol2011282949821362031
- MullikenJBRogersGFMarlerJJCircular excision of hemangioma and purse-string closure: the smallest possible scarPlast Reconstr Surg2002109515441554 discussion 155511932595
- WuJKRohdeCHPurse-string closure of hemangiomas: early results of a follow-up studyAnn Plast Surg200962558158519387166
- ChangCSWongARohdeCHAschermanJAWuJKManagement of lip hemangiomas: minimizing peri-oral scarsJ Plast Reconstr Aesthet Surg201265216316821937296
- LiWYChaudhryOReinischJFGuide to early surgical management of lip hemangiomas based on our experience of 214 casesPlast Reconstr Surg201112851117112421738083
- ArnejaJSChimHDroletBAGosainAKThe Cyrano nose: refinements in surgical technique and treatment approach to hemangiomas of the nasal tipPlast Reconstr Surg201012641291129920885249
- McCarthyJGBorudLJSchreiberJSHemangiomas of the nasal tipPlast Reconstr Surg20021091314011786788
- WarrenSMLongakerMTZideBMThe subunit approach to nasal tip hemangiomasPlast Reconstr Surg20021091253011786787
