Abstract
This article aimed to review the current prognostic and diagnostic tools used for community-acquired pneumonia (CAP) and highlight those potentially applicable in children with CAP. Several scoring systems have been developed to predict CAP mortality risk and serve as guides for admission into the intensive care unit. Over the years, clinicians have adopted these tools for improving site-of-care decisions because of high mortality rates in the extremes of age. The major scoring systems designed for geriatric patients include the Pneumonia Severity Index and the confusion, uremia, respiratory rate, blood pressure, age >65 years (CURB-65) rule, as well as better predictors of intensive care unit admission, such as the systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH (SMART-COP) score, the Infectious Diseases Society of America/American Thoracic Society guidelines, the criteria developed by España et al as well as the systolic blood pressure, oxygenation, age and respiratory rate (SOAR) criteria. Only the modified predisposition, insult, response and organ dysfunction (PIRO) score has so far been applied to children with CAP. Because none of the tools is without its limitations, there has been a paradigm shift to incorporate biomarkers because they are reliable diagnostic tools and good predictors of disease severity and outcome, irrespective of age group. Despite the initial preponderance of reports on their utility in geriatric CAP, much progress has now been made in demonstrating their usefulness in pediatric CAP.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Community-acquired pneumonia (CAP) refers to pneumonia in a previously healthy patient who acquired the infection in the community, as differentiated from hospital-acquired pneumonia (HAP).Citation1 Ewig however believes CAP does not describe pneumonia acquired in the community per se but represents a concept about a distinct clinical syndrome in distinct community patients.Citation2
It is one of the most common serious infections in childhood, with an estimated incidence of 34–40 cases per 1,000 children in Europe and North America.Citation3–Citation5 Although CAP-related mortality is relatively rare in developed countries, the picture is different in developing countries where pneumonia is one of the major causes of childhood mortality.Citation6 Nevertheless, severe CAP remains the single main cause of mortality from infectious diseases in developed countries.
With respect to etiology, the basic microbial patterns typically consist of Streptococcus pneumoniae, an atypical group, a non-pneumococcal non-atypical group and the Gram-negative group.Citation2 The relative frequency of each group is determined by the severity of pneumonia, age, comorbidity, seasonality and individual risk factors.Citation2 For instance, in developing countries, a causal relationship exists between severe and life-threatening pneumonias and bacterial agents such as S. pneumoniae, Haemophilus influenzae and Staphylococcus aureus.Citation2 A similar trend also occurs in Europe and North America.Citation7 Secondly, age-specific etiologic patterns have been established; Group B streptococcus and Gram-negative enteric bacteria are the most common pathogens in the neonatal age group. Beyond this age bracket, S. pneumoniae remains the most common bacterial agent, while Mycoplasma pneumoniae and Chlamydia pneumoniae (atypical group) are frequently associated with illness in preschool-aged children and are common causative bacteria in older children and adolescents.Citation8,Citation9 In European countries such as Germany, Legionella spp. (especially L. pneumonia and L. pneumophila) have been reported as prominent etiologic agents, especially in adult patients with CAP.Citation10,Citation11 Elsewhere in China, the bacteria have also been noted in the list of common causative pathogens according to a multicenter study,Citation12 while a recent systematic review revealed their contributory role in causing CAP in Asia and the Middle East.Citation13 Despite the need for hospitalization in only a minority of patients, several severity-of-illness tools have been developed, which can help in deciding on admission, especially in the intensive care units (ICUs). In contrast to what is obtainable in geriatric CAP, the absence of a pediatric CAP severity score remains one of the unresolved issues about the disease. In fact, little progress has been made in establishing CAP-specific scores in children. Over the years, clinicians have adopted prognostic scoring systems for improving site-of-care decisions because of high mortality rates in the extremes of age. Precise risk stratification of patients, used currently, aimed at ensuring appropriate site-of-care decisions has its shortcomings. Therefore, the procedure has been improved by the inclusion of several biomarkers in order to differentiate viral from bacterial infections, restrict the duration of antibiotic therapy and function as prognostic indicators.Citation14 With technological advancement, these biomarkers hold great prospects for aiding critical decisions for patients with pneumonia.Citation15 Moreover, the combination of several biomarkers reflecting different pathophysiological pathways can potentially improve the management of CAP in future.Citation16 Interestingly, the prognostic and diagnostic utility of these biomarkers has been demonstrated in both geriatric and pediatric CAP.
This article aims to review these prognostic and diagnostic tools, in addition to highlighting those potentially applicable in children with CAP.
Risk stratification of patients with CAP: the current scoring systems
The main determinants of prognosis in patients with CAP are respiratory failure, sepsis-associated organ dysfunction and unstable comorbidities. Thus, the current risk stratification tools have been fundamentally designed to predict mortality and identify low-risk patients who are potentially suitable for outpatient management.Citation17
This underscores the importance of site-of-care decisions in the management of these patients. Several scoring systems have been developed to predict mortality risk in CAP, and these have been used as guides for patient hospitalization or admission in the ICU.Citation18 The most extensively evaluated scoring systems are the Pneumonia Severity Index (PSI) and the confusion, uremia, respiratory rate, BP, age > 65 years (CURB-65) rule (a modification of the British Thoracic Society [BTS] rule), both of which are basically appropriate in adult patients.
Pneumonia Severity Index
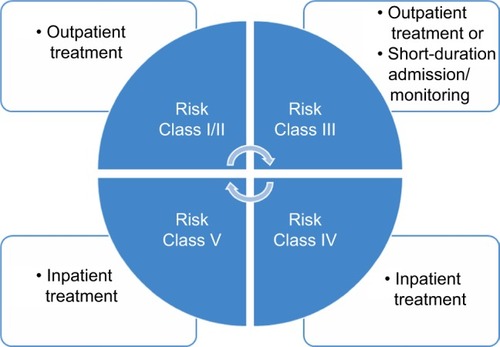
The PSI was introduced to assist in identifying patients with CAP who may either require ambulatory (outpatient) or hospitalized (inpatient) treatment.Citation19 This prognostic tool is based on 20 clinical variables that group the patients into five risk categories (Risk Classes I–V) and could be utilized to predict 30-day survival. Although the PSI performs well as a predictor of mortality, it is time consuming and more complex as it requires multiple clinical and laboratory parameters. Furthermore, the PSI was developed to identify low-mortality-risk patients and may underestimate the severity of CAP.Citation20 For instance, a Risk Class I or II pneumonia patient may require outpatient treatment. A Risk Class III patient (after evaluating other factors such as home environment and follow-up) may need either outpatient or inpatient treatment and surveillance for a short duration, while Risk Class IV and V patients should be hospitalized for treatment ().
Figure 1 Risk stratification of patients with community-acquired pneumonia and treatment algorithm using Pneumonia Severity Index scoring system.
Since PSI may underestimate the severity of illness (which is a limitation), the simpler CURB-65 rule was developed to identify more severely ill patients with ease.
CURB-65 and CRB-65 scoring systems
The CURB-65 parameters consist of the following items from which the acronym was derived: new-onset mental confusion, urea >7 mmol/L, respiratory rate ≥30 breaths/min, systolic blood pressure <90 mmHg or diastolic blood pressure ≤60 mmHg and age ≥65 years.Citation21 It is a 5-point score with three risk categories: low risk (0–1 points), intermediate risk (2 points) and high risk (>3 points). Unlike PSI, this tool is more straightforward and also constitutes an accurate tool for the prediction of 30-day survival.Citation18 The CURB-65 can be further simplified by the omission of the laboratory parameter blood urea nitrogen (BUN), giving rise to only the CRB-65 criteria.
A comparison of the CURB-65 and CRB-65 scoring systems has been made in two studies.Citation22,Citation23 One of the studies reported that both the CURB-65 and the CRB-65 were equally effective in predicting 30-day mortality,Citation22 while in the other study, the investigators noted that both CRB-65 and CURB-65 could predict 30-day survival, even though the CRB-65 tended to underestimate mortality risk: defining 26% of the patients who died as low risk.Citation23 Therefore, it was concluded that the more convenient and readily available CRB-65 should be used in ambulatory patients, while the full CURB-65 rule should be applied to hospitalized patients.Citation23
PSI versus CURB-65
Interestingly, further comparisons between the PSI and the CURB-65 tools have brought to the fore important differences in terms of advantages and limitations. The development and validation of PSI were based on the need to identify low-mortality-risk patients. Thus, this tool can potentially be misleading for site-of-care decisions and can underestimate severity of illness, more especially in young patients who do not have comorbid illnesses.Citation19,Citation24 Conversely, the CURB-65 scoring system may be optimal for identifying high-mortality-risk patients with severe CAP who otherwise might be missed without formal assessment of barely noticeable deviations in key vital signs.Citation21,Citation24 Hence, both scoring systems assess pneumonia and mortality risk from different perspectives, and each is best suited for identifying patients at opposite ends of the CAP-severity spectrum.Citation18
A review of some studies that have compared the PSI and CURB-65 tools in the same population shows that in one report,Citation25 both tools were good at predicting mortality and in identifying low-risk patients. However, the CURB-65 appeared to be more distinctive in defining mortality risk in severe CAP.Citation25 In another study, both the PSI and the CURB-65 were used to evaluate a large number of hospitalized and ambulatory patients with CAP.Citation22 The CURB-65 (and the simpler CRB-65 version) accurately predicted 30-day mortality, the need for mechanical ventilation and perhaps the need for hospitalization.Citation22 Furthermore, the CURB-65 criteria correlated with the time to clinical stability. While the PSI predicted mortality, it was not good at predicting the need for ICU admission. The authors also documented that the CURB-65 tool could not predict the need for ICU admission, although it was observed to be more accurate than the PSI for this site-of-care decision.Citation22
It is therefore important to consider other tools that have more predictive value when deciding the need for ICU admission.
Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) criteria
Some researchersCitation26 evaluated the ATS guidelines,Citation27 which proposed 10 criteria to define severe CAP. It was established that the requirement for ICU was defined by the presence of two of three minor criteria (systolic blood pressure ≤90 mmHg, multilobar disease or the ratio of partial pressure of arterial oxygen to fractional inspired oxygen [PaO2/FiO2 ratio] ≤250) or one of two major criteria (need for mechanical ventilation or septic shock).Citation26 Obviously, this rule had better sensitivity and specificity for defining the requirement for ICU admission than either the PSI or the BTS rule in view of the estimated sensitivity of 69% and specificity of 98%. Based on these findings, the 2001 ATS guidelines for CAP subsequently recommended that severe CAP could be defined on the basis of the presence of these major and minor criteria.Citation27 Against this backdrop, the IDSA/ATS committee released guidelines in 2007, which expanded the criteria for ICU admission to include the presence of at least three of the following nine minor criteria: PaO2/FiO2 ratio <250, respiratory rate >30 breaths/min, confusion, multilobar infiltrates, systolic blood pressure <90 mmHg despite aggressive fluid resuscitation, BUN >20 mg/dL, leukopenia (<4,000 cells/mm3), thrombocytopenia (<100,000 cells/mm3) and hypothermia (<36°C).Citation28 This scoring system nevertheless requires further validation.
Criteria developed by España et al
Another risk stratification tool is the severe CAP model proposed by España et al.Citation29 Having examined records from 1,057 patients, the investigators established that the need for admission into the ICU was defined by the presence of one of two major criteria: arterial pH <7.30 or systolic blood pressure <90 mmHg.Citation29 In the absence of these criteria, severe CAP could also be identified by the presence of two of six of the following minor criteria: confusion, BUN >30 mg/dL, respiratory rate >30 breaths/min, PaO2/FiO2 ratio <250, multilobar infiltrates and age of at least 80 years. When these criteria were fulfilled, the scoring system was 92% sensitive for identifying those with severe disease and was more accurate than the PSI or CURB-65 criteria, although not quite as specific as the CURB-65 rule.Citation29
SMART-COP scoring system
The systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH (SMART-COP) scoring system is another current tool for predicting the need for ICU admission. It was a product of the Australian CAP study that defined the need for intensive respiratory or vasopressor support (IRVS), which was seen as a more objective end point than admission into the ICU.Citation30 The SMART-COP rule (the acronym for systolic blood pressure <90 mmHg, multilobar infiltrates, albumin <3.5 g/dL, respiratory rate elevation [≥25 breaths/min for those age ≤50 years, and ≥30 breaths/min for those age >50 years], Tachycardia [>125 beats/min], confusion, low oxygen [<70 mmHg if age ≤50 years or <60 mmHg if age >50 years] and arterial pH <7.35) performed well for patients under IRVS who were initially admitted into the ICU, as well as for those who were transferred to the ICU after initial admission in the ward. Notably, one study has demonstrated that SMART-COP was superior to the other prognostic scoring tools for predicting the need for IRVS, with a sensitivity of 85% compared to 55% for CURB-65 ≥3 or a PSI class ≥IV.Citation31
Systolic blood pressure, oxygenation, age and respiratory rate (SOAR) criteria
Finally, a specific scoring system for the elderly with the acronym SOAR has also been developed.Citation32 The best predictor of severe CAP and mortality was the presence of two of four SOAR criteria: systolic blood pressure <90 mmHg, PaO2/FiO2 <250, age >65 years and respiratory rate >30 breaths/min. When two criteria were present, the sensitivity for predicting mortality from CAP was 81%, and the specificity was 60%.Citation32 This tool was developed in place of CURB-65 because many geriatric patients have an elevated BUN and confusion, and thus the discriminating value of these parameters is limited in older subjects.Citation17
Modified predisposition, insult, response and organ dysfunction (PIRO) score
In a recent report, some investigators set out to formulate a prognostic scale for estimating mortality, which would be applicable to children with CAP.Citation33 The study enrolled patients younger than 15 years with CAP who were hospitalized over a 10-year period. A point-based scoring system based on the modified PIRO scale used in adults with pneumonia was applied to each hospitalized child, and it comprised the following variables: predisposition (age <6 months, comorbidity), insult (hypoxia [O2 saturation <90%], hypotension [according to age] and bacteremia), response (multilobar or complicated pneumonia) and organ dysfunction (kidney failure, liver failure and acute respiratory distress syndrome). One point was awarded for each criterion that was present (range: 0–10 points). The association between the modified PIRO score and mortality was assessed by stratifying patients into four levels of risk: low (0–2 points), moderate (3–4 points), high (5–6 points) and very high risk (7–10 points). The results showed a significant positive correlation between mortality rates and modified PIRO scores, causing the authors to conclude that the score accurately discriminated the probability of mortality in children hospitalized with CAP and thus could be a reliable tool to select patients for admission to ICU and for adjunctive therapy in clinical trials.Citation33 Although this tool is the modified version of the score initially designed for CAP in adult patients,Citation34 it appears to be the only prognostic scoring system that has been shown to be applicable in pediatric CAP.
Prognostic indicators for complications of CAP
Severe CAP is associated with serious complications, such as respiratory insufficiency, sepsis and mortality. Some studies have highlighted the prognostic indicators of these complications.Citation35–Citation39 In a prospective, randomized study, a group of investigators compared the following: standard treatment plus noninvasive positive pressure ventilation (NPPV) delivered through a face mask; and standard treatment alone in patients with severe CAP complicated by acute respiratory failure.Citation35 Interestingly, the use of NPPV was found to be well tolerated, safe and associated with a significant reduction in both respiratory rate and the need for endotracheal intubation. Thus, it was concluded that in selected patients with acute respiratory failure, NPPV was associated with a significant reduction in the rate of endotracheal intubation and duration of ICU admission.Citation35 On the other hand, other authors reported that routine clinical and biochemical information can be used to predict early respiratory failure in patients with severe CAP.Citation36 For instance, respiratory failure was found to be independently associated with altered mental state, pulmonary arterial hypertension (PaH) <7.35 mmHg and PaO2 <60 mmHg. Furthermore, a history of heart failure was inversely associated with respiratory failure, while patients who failed to respond had a higher 28-day mortality rate and a longer period of hospitalization.Citation36 In one study among adult patients with pneumococcal CAP, sepsis and the CRB-65 scores were used to allocate patients to subgroups with low, intermediate and high risk.Citation37 The authors observed that even though both scores performed equally well in predicting mortality, the prediction of survival in the intermediate-risk group appeared more accurate with the sepsis score.
Notably, as CAP becomes more complicated with severe sepsis and septic shock, blood lactate concentration is increased as a result of anaerobic production (via the Na+/K+-ATPase channel) and a decrease in lactate utilization.Citation38 In another study among critically ill geriatric patients admitted into the ICU with severe CAP, lactate clearance was found to be a useful and inexpensive biomarker, as well as a reliable predictor of patient outcome.Citation39 Thus, it is an important prognostic tool for CAP-related sepsis.
Biomarkers in geriatric and pediatric CAP: the progress so far
An ideal biomarker for CAP should lead to a prompt diagnosis, have a prognostic value and facilitate therapeutic decision-making.Citation16 Biomarkers provide reliable information about host response to infection as well as pathogenic activity within the host, which can serve to support clinical parameters in making decisions.Citation15 Hitherto, biomarkers have been used to differentiate bacterial and viral etiologies in childhood CAP.Citation40,Citation41 Recently, they have emerged as predictors of disease severity and prognosis.Citation42–Citation44
From a pathophysiological perspective, it is possible that the combination of several biomarkers reflecting different aspects of CAP (infection, cardiovascular impairment, sepsis and respiratory failure) can be useful. Hence, the novel biomarkers fall into these major groups: inflammatory markers, namely, procalcitonin (PCT), C-reactive protein (CRP) and white blood cell (WBC) count; cardiovascular markers, such as proadrenomedullin (proADM), proatrial natriuretic peptide (proANP), proarginine vasopressin (copeptin) and C-terminal proendothelin-1 (CT-proET-1); and biomarkers of stress, such as cortisol.Citation45 Adrenomedullin (ADM) has several actions, including immune modulation, vasodilation, direct bactericidal activity and electrolyte homeostasis. Endothelin-1 is a potent vasoconstrictor, and its endothelial synthesis is triggered by hypoxia and pulmonary infections.Citation46 PCT is a specific marker for bacterial infections, while arginine vasopressin plays a role in the regulation of cardiovascular and osmotic homeostasis. Plasma levels of ANP are elevated in patients with chronic heart failure and sepsis and predict impaired left ventricular ejection fraction.Citation47,Citation48
Notably, these biomarkers have not only been established as diagnostic and prognostic tools in adult CAPCitation40–Citation45,Citation49–Citation56 but have also been demonstrated to have wide applicability in pediatric CAP.Citation57–Citation73
In one major prospective study that compared these new biomarkers in predicting short- and long-term all-cause mortality in adult CAP, the investigators found that the cardiovascular biomarkers (proANP, copeptin, CT-proET-1 and proADM) were not only good predictors of short- and long-term outcomes in CAP, but they were also superior to inflammatory markers, and at least comparable to the CRB-65 score, in terms of performance.Citation45 ProADM was noted as the biomarker with the best performance, while the combination of CRB-65 score and proADM was suggested as the best predictor for both short- and long-term mortalities.Citation45
Some recent studies that investigated the utility of serum biomarkers to measure CAP severity and predict prognosis have focused on CRP, PCT and cortisol.Citation49–Citation52 For example, a study of 185 adult patients who had PCT measured within 24 hours of the diagnosis of CAP showed that PCT levels correlated with the PSI score and the development of complications.Citation52 Levels were also elevated in nonsurvivors when compared with that in survivors. Serial measurements of PCT have similarly been used to define prognosis in severe CAP patients.Citation49 Other investigators have reported that non-survivors had significantly higher PCT levels; with serial measurements, survivors had a reduction in PCT levels, while nonsurvivors had a rise by the third day.Citation49
Interestingly, researchers are now beginning to combine the findings of biomarker estimations with the results of the prognostic scoring systems in adult patients with CAP.
In one study that evaluated PCT, CRP and CRB-65 in 1,672 adult patients with the disease, PCT levels on admission were elevated in nonsurvivors, and the receiver operating characteristic (ROC) curve for survival was similar for PCT and CRB-65, but each was better than CRP.Citation53 A reduced PCT level specifically predicted a low risk of mortality independent of CRB-65 category.
In another study of 278 adult patients presenting with CAP to an emergency department in Switzerland, the authors showed that cortisol levels could be used to predict severity of disease and mortality-related outcome.Citation54 Free and total cortisol levels were found to be correlated with severity of CAP as shown by the PSI score; a level of total cortisol >960 nmol/L had a sensitivity of 75% and a specificity of 71.7% for predicting mortality. In a related study, baseline cortisol was noted to be the best predictor of mortality among 72 adult patients with severe CAP who were admitted to the ICU, as mortality was more frequent in those with higher baseline levels.Citation55 When baseline cortisol was used to predict mortality, it was more accurate than CURB-65 using the area under the ROC curve for mortality, but CRP was not a good biomarker for the same purpose. In one systematic review and meta-analysis, the authors nonetheless concluded that despite the fact that biomarkers are able to predict mortality with moderate-to-good accuracy in adult CAP, they have no clear advantage over CAP-specific scores.Citation56 Nevertheless, in a recent review that aimed to determine the role of PCT in predicting CAP severity in children, there appears to be an increase in studies confirming its utility in CAP management in pediatric patients.Citation61 In one of such recently published studies, 119 children aged 1–14 years were admitted for radiographically documented CAP, and baseline PCT, CRP and routine laboratory tests were conducted on admission. The investigators found that PCT was correlated to the main inflammatory markers in children with CAP; CRP, unlike PCT, was able to predict the severity of CAP, confirming its usefulness in the management of the disease.Citation57
The medical literature is currently replete with other reports that indicate the utility of biomarkers as diagnostic, prognostic and discriminatory tools, as well as being a possible therapeutic guide in pediatric CAP.Citation58–Citation73 For instance, as a discriminatory tool, Esposito et alCitation60 investigated the usefulness of biomarkers such as lipocalin-2 (LIP2) and syndecan 4 (SYN 4) in differentiating bacterial from viral etiology in CAP. They estimated the serum levels of these biomarkers together with the WBC counts and CRP levels in 110 children aged <14 years who were hospitalized for radiographically proven CAP. However, their findings showed that the estimation of LIP2 and SYN4 levels did not obviate the need for using clinical and laboratory parameters to define the etiology and severity of pediatric CAP.Citation60
In a retrospective cohort study of 108 pediatric patients divided into four categories of those with bacterial pneumonia or bronchitis and those with nonbacterial pneumonia or bronchitis, Hoshina et alCitation64 compared the WBC and neutrophil counts, as well as the CRP and PCT levels, among the cohorts. The authors were able to demonstrate that PCT was the most useful marker to differentiate bacterial from nonbacterial pneumonia, while neutrophil count was the major contributor to the discrimination of bacterial from nonbacterial bronchitis.Citation64 In contrast, Don et al,Citation69 in their study of 101 children with radiographically proven CAP, reported that WBC count, CRP, PCT and erythrocyte sedimentation rate (ESR), or their combinations, showed a limited discriminatory role during screening between bacterial and viral pediatric CAP. They further noted that if all or most of these markers were raised, bacterial etiology was most likely, but reduced values did not preclude bacterial etiology. Their findings were corroborated by another study,Citation72 which observed that CRP, PCT, WBC and ESR had only limited value in differentiating bacterial from viral pneumonia; none of the combinations of these markers showed sufficient sensitivity and specificity to be used in pediatric CAP. Thus, the evidence is still weak – and there is no consensus yet – on the utility of biomarkers in differentiating the microbial etiology of CAP. In addition, Tumgor et alCitation70 similarly reported that interleukin (IL)-6, IL-8 and CRP levels were elevated in children with CAP and HAP but were not useful in differentiating the various etiologic agents. Remarkably, one published report from a prospective study of 310 children admitted for uncomplicated CAP used a PCT cutoff value to guide antibiotic therapy in these patients and showed that an algorithm-based approach can significantly reduce antibiotic use and antibiotic-related adverse reactions in those with uncomplicated disease.Citation67 However, the authors could not validate the risk of using this approach in severe cases of CAP because the study included mainly children with mild-to-moderate forms of the disease.
Again, several reports show the diagnostic utility of established and emerging biomarkers in pediatric CAP.Citation58,Citation61,Citation62,Citation65,Citation66,Citation68,Citation71,Citation73 In a recently published study, Li et alCitation58 used proteomics to identify 27 potential plasma proteins in children with M. pneumoniae pneumonia (MPP) and found that human apolipoprotein C-I precursor (APOC1) was a potential novel biomarker for the rapid and noninvasive diagnosis of MPP in children, an observation that the authors believed could offer new insights into the pathogenesis and biomarker selection of MPP in pediatric patients.
Alcoba et alCitation62 evaluated the predictive value of proADM and copeptin for complicated CAP (evidenced by bacteremia and empyema) in 88 pediatric patients aged 0–16 years who were seen in an emergency setting. The investigators noted that only proADM appeared to be a reliable predictor for complicated CAP and could thus aid the clinician with decisions such as in-patient care and choice of the route of antibiotic administration. Similarly, other researchers have reported the diagnostic and prognostic utility of this biomarker.Citation66 In a prospective observational study of children with CAP seen at the emergency department, proADM values were analyzed in relation to clinical parameters such as need for oxygen therapy, duration of oxygen therapy, disease complications, pyrexia, antibiotic therapy, admission to ICU and duration of hospitalization; specifically children who had complications such as pleural effusion had higher levels of proADM.Citation66
In another study by Wrotek et al,Citation65 soluble urokinase plasminogen activator receptor (suPAR) level was estimated in 227 children with CAP and in 119 healthy counterparts. The marker, which is an activator of the immune system, was significantly more elevated in children with CAP than in healthy subjects. In addition, suPAR level was significantly higher in children with severe form of the disease compared with those who had nonsevere illness, indicating that its elevation may also reflect the severity of pneumonia. The same authors also demonstrated a positive correlation between serum suPAR and nonspecific inflammatory markers, such as CRP and PCT, in pediatric CAP.Citation63
Elsewhere, in South Korea, a group of researchers reported that serum PCT levels had better diagnostic accuracy for lobar pneumonia compared with the predictive ability of serum CRP level and ESR.Citation68 They also noted a significant correlation of the PCT level with the CRP level, ESR and WBC count, suggesting that serum PCT level was a better marker than CPR and ESR for the diagnosis of lobar pneumonia in pediatric CAP.Citation68 In a Chinese study that investigated the clinical value of the expression of neutrophil surface cluster of differentiation (CD)64 in the diagnosis of CAP in children, the authors observed that the CD64 index and CRP levels were significantly higher in patients with bacterial pneumonia than in their counterparts with viral and mycoplasma pneumonia.Citation73 In addition, the CD64 index was observed to be significantly higher in children with severe pneumonia than in those with milder forms of the disease, while its expression also significantly decreased in those with severe bacterial infection after antibiotic therapy, indicating that its determination contributes to early diagnosis of bacterial CAP.Citation73 Finally, some biomarkers have also been documented as prognostic tools in pediatric CAP in a recent study in India, which investigated the possible role of systemic immune and inflammatory mediators as biomarkers that could predict CAP severity or outcome.Citation59 The researchers observed that IL-6, IL-8, IL-13, interferon-gamma (IFN-γ) and lower C–C motif chemokine ligand 22 (CCL22) levels were significantly more elevated in patients with severe CAP compared with those with mild CAP. Furthermore, based on higher macrophage inflammatory protein-1 alpha (MIP-1α), IL-8, IL-17 or lower CCL22-response pattern at the time of enlistment, children with fatal outcome displayed a markedly different pattern of inflammatory response compared with those grouped with the same disease severity, but with non-life-threatening outcome.Citation59
Conclusion
Most of the prognostic scoring systems currently under use are more applicable to CAP in adult patients. So far, only the modified PIRO score has been applied to pediatric patients. The most evaluated tools – PSI and CURB-65 – are accurate for predicting mortality in adults with CAP but are not direct indicators of disease severity.
Thus, they cannot replace the use of clinical assessment to determine hospital admission or ICU care. At best, these scoring systems are decision support tools that cannot be utilized as the gold standard for site-of-care decisions.Citation18
Since none of the tools is without its limitations, there has been a paradigm shift to incorporate biomarkers in risk stratification because they are good predictors of disease severity and outcome, irrespective of age group. Although the role of biomarkers was initially more established in geriatric CAP, they have now been proven to be useful in the diagnosis of pediatric CAP, as well as in predicting severity and outcome in children. Despite the progress made so far in applying these biomarkers in pediatric CAP, more research is still needed to validate their diagnostic and prognostic utility in the disease.
Acknowledgments
The authors acknowledge the invaluable information obtained from the article by Niederman.Citation18
Disclosure
The authors report no conflicts of interest in this work.
References
- BradleyJSByingtonCLShahSSPediatric Infectious Diseases Society and the Infectious Diseases Society of AmericaThe management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of AmericaClin Infect Dis2011537e2521880587
- EwigSCommunity-acquired pneumonia: definition, epidemiology, and outcomeSemin Respir Infect19991429410210391404
- British Thoracic Society Standards of Care CommitteeBritish Thoracic Society guidelines for the management of community acquired pneumonia in childhoodThorax200257suppl 1i1i2411994552
- MurphyTFHendersonFWClydeWAJrCollierAMDennyFWPneumonia: an eleven-year study in a pediatric practiceAm J Epidemiol1981113112216257108
- JokinenCHeiskanenLJuvonenHIncidence of community-acquired pneumonia in the population of four municipalities in Eastern FinlandAm J Epidemiol199313799779888317455
- ReddSCVreulsRMetsingMMohobanePHPatrickEMoteeteeMClinical signs of pneumonia in children attending a hospital outpatient department in LesothoBull World Health Organ19947211131188131246
- McIntoshKCommunity-acquired pneumonia in childrenN Engl J Med200234642943711832532
- PrincipiNEspositoSMycoplasma pneumoniae and Chlamydia pneumoniae cause lower respiratory tract disease in pediatric patientsCurr Opin Infect Dis20021529530012015465
- Heiskanen-KosmaTKorppiMJokinenCEtiology of childhood pneumonia: serologic results of a prospective, population-based studyPediatr Infect Dis J199817119869919849979
- von BaumHEwigSMarreRCompetence Network for Community Acquired Pneumonia Study GroupCommunity-acquired Legionella pneumonia: new insights from the German Competence Network for community acquired pneumoniaClin Infect Dis20084691356136418419436
- van de GardeEMEndemanHvan HemertRNPrior outpatient antibiotic use as predictor for microbial aetiology of community-acquired pneumonia: hospital-based studyEur J Clin Pharmacol200864440541018060396
- HuangHHZhangYYXiuQYCommunity-acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patientsEur J Clin Microbiol Infect Dis200625636937416767484
- PetoLNadjmBHorbyPThe bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic reviewTrans R Soc Trop Med Hyg2014108632633724781376
- GrossmanRFCommunity-Acquired Pneumonia: Advances in ManagementACCP Pulmonary Medicine Board Review26th edGlenview, ILACCP2009
- FlorinTAAmbroggioLBiomarkers for community-acquired pneumonia in the emergency departmentCurr Infect Dis Rep2014161245125348745
- KrügerSWelteTBiomarkers in community-acquired pneumoniaExpert Rev Respir Med20126220321422455492
- KolditzMEwigSHöffkenGManagement-based risk prediction in community-acquired pneumonia by scores and biomarkersEur Respir J201341497498423018905
- NiedermanMSMaking sense of scoring systems in community acquired pneumoniaRespirology200914332733519353770
- FineMJAubleTEYealyDMA prediction rule to identify low-risk patients with community-acquired pneumoniaN Engl J Med199733642432508995086
- RelloJRodriguezASeverity of illness assessment for managing community acquired pneumoniaIntensive Care Med200733122043204417938882
- LimWSvan der EerdenMMLaingRDefining community acquired pneumonia severity on presentation to hospital: an international derivation and validation studyThorax200358537738212728155
- CapelasteguiAEspañaPPQuintanaJMValidation of a predictive rule for the management of community-acquired pneumoniaEur Respir J20062715115716387948
- BauerTTEwigSMarreRSuttorpNWelteTthe CAPNETZ GroupCRB-65 predicts death from community-acquired pneumoniaJ Intern Med200626019310116789984
- NiedermanMSFeldmanCRichardsGACombining information from prognostic scoring tools for CAP: an American view on how to get the best of both worldsEur Respir J20062791116387929
- AujeskyDAubleTEYealyDMProspective comparison of three validated prediction rules for prognosis in community-acquired pneumoniaAm J Med2005118438439215808136
- EwigSDe RouxABauerTValidation of predictive rules and indices of severity for community acquired pneumoniaThorax200459542142715115872
- NiedermanMSMandellLAAnzuetoAAmerican Thoracic SocietyGuidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and preventionAm J Respir Crit Care Med20011631730175411401897
- MandellLAWunderinkRGAnzuetoAInfectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adultsClin Infect Dis200744suppl 2S27S7217278083
- EspañaPPCapelasteguiAGorordoIDevelopment and validation of a clinical prediction rule for severe community-acquired pneumoniaAm J Respir Crit Care Med2006174111249125616973986
- CharlesPCWolfeRWhitbyMAustralian Community-Acquired Pneumonia Study CollaborationSMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumoniaClin Infect Dis200847337538418558884
- ChalmersJDSinganayagamAHillATPredicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community-acquired pneumoniaClin Infect Dis200847121571157418991510
- British Thoracic SocietyMyintPKKamathAVVowlerSLMaiseyDNHarrisonBDSeverity assessment criteria recommended by the British Thoracic Society (BTS) for community-acquired pneumonia (CAP) and older patients. Should SOAR (systolic blood pressure, oxygenation, age and respiratory rate) criteria be used in older people? A compilation study of two prospective cohortsAge Ageing200635328629116638769
- ArayaSLoveraDZarateCApplication of a Prognostic Scale to estimate the mortality of children hospitalized with community-acquired pneumoniaPediatr Infect Dis J201635436937326629871
- RelloJRodriguezALisboaTGallegoMLujanMWunderinkRPIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumoniaCrit Care Med200937245646219114916
- ConfalonieriMPotenaACarboneGDella PortaRTolleyEAMeduriGUAcute respiratory failure in patients with severe community-acquired pneumonia: a prospective randomized evaluation of noninvasive ventilationAm J Resp Crit Care Med199916051585159110556125
- HoogewerfMOosterheertJJHakEHoepelmanIMBontenMJMPrognostic factors for early clinical failure in patients with severe community-acquired pneumoniaClin Microbiol Infect2006121097110417002609
- SchaafBKruseJRuppJSepsis severity predicts outcome in community-acquired pneumococcal pneumoniaEur Respir J200730351752417537775
- JamesJHLuchetteFAMcCarterFDFischerJELactate is an unreliable indicator of tissue hypoxia in injury or sepsisLancet1999354917750550810465191
- Abd Elaziz MohamedKAbd Elgalil AhmedDPrognostic value of lactate clearance in severe community acquired pneumoniaEgypt J Chest Dis Tuberculosis201463410531058
- MoulinFRaymondJLorrotMProcalcitonin in children admitted to hospital with community acquired pneumoniaArch Dis Child200184433233611259234
- ToikkaPIrjalaKJuvénTSerum procalcitonin, C-reactive protein and interleukin-6 for distinguishing bacterial and viral pneumonia in childrenPediatr Infect Dis J200019759860210917215
- KrügerSEwigSKundeJHartmannOSuttorpNWelteTPro-atrial natriuretic peptide and pro-vasopressin to predict short- and long-term survival in community-acquired pneumoniaThorax20106520821420335288
- KrügerSEwigSMarreRProcalcitonin predicts patients at low risk of death from community-acquired pneumoniaEur Respir J20083134935517959641
- KoivulaIStenMMakelaPPrognosis after community-acquired pneumonia in the elderly: a population-based 12-year follow-up studyArch Intern Med19991591550155510421277
- KrügerSEwigSGiersdorfSHartmannOSuttorpNWelteTCardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumoniaAm J Respir Crit Care Med2010182111426143420639437
- CarpenterTCSchornbergSStenmarkKREndothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxiaAm J Physiol Lung Cell Mol Physiol2005289L1075L108216040626
- MorgenthalerNGStruckJChrist-CrainMBergmannAMüllerBPro-atrial natriuretic peptide is a prognostic marker in sepsis, similar to the APACHE II score: an observational studyCrit Care200391R37R45
- JochbergerSMorgenthalerNGMayrVDCopeptin and arginine vasopressin concentrations in critically ill patientsJ Clin Endocrinol Metab200691114381438616940457
- BoussekeyNLeroyOAlfandariSDevosPGeorgesHGueryBProcalcitonin kinetics in the prognosis of severe community-acquired pneumoniaIntensive Care Med20063246947216477418
- BoussekeyNLeroyOGeorgesHDevosPD’EscrivanTGueryBDiagnostic and prognostic values of admission procalcitonin levels in community-acquired pneumonia in an intensive care unitInfection200533425726316091896
- Christ-CrainMStolzDBingisserRProcalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trialAm J Respir Crit Care Med20061741849316603606
- MasiaMGutierrezFShumCUsefulness of procalcitonin levels in community-acquired pneumonia according to severity indexChest20051282223222916236878
- HuangDTWeissfeldLAKellumJAGenIMS InvestigatorsRisk prediction with procalcitonin and clinical rules in community-acquired pneumoniaAnn Emerg Med200852485818342993
- Christ-CrainMStolzDJutlaSFree and total cortisol levels as predictors of severity and outcome in community-acquired pneumoniaAm J Respir Crit Care Med2007176991392017702966
- SalluhJIFBozzaFASoaresMAdrenal response in severe community-acquired pneumonia: impact on outcomes and disease severityChest200813494795418753464
- ViasusDDel Rio-PertuzGSimonettiAFBiomarkers for predicting short-term mortality in community-acquired pneumonia: a systematic review and meta-analysisJ Infect201672327328226777314
- AgnelloLBelliaCDi GangiMUtility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in childrenClin Biochem2016491–2475026386341
- LiJSunLXuFScreening and identification of APOC1 as a novel potential biomarker for differentiate of Mycoplasma pneumoniae in childrenFront Microbiol20167196128018301
- Saghafian-HedengrenSMathewJLHagelEAssessment of cytokine and chemokine signatures as potential biomarkers of childhood community-acquired pneumonia severity: a nested cohort study in IndiaPediatr Infect Dis J201736110210827956727
- EspositoSBianchiniSGambinoMMeasurement of lipocalin-2 and syndecan-4 levels to differentiate bacterial from viral infection in children with community-acquired pneumoniaBMC Pulm Med201616110327439403
- GiuliaBLuisaAConcettaSBrunaLSChiaraBMarcelloCPro-calcitonin and community-acquired pneumonia (CAP) in childrenClin Chim Acta2015451pt B21521826434548
- AlcobaGManzanoSLacroixLGaletto-LacourAGervaixAPro-adrenomedullin and copeptin in pediatric pneumonia: a prospective diagnostic accuracy studyBMC Infect Dis20151534726286191
- WrotekAJackowskaTPawlikKSoluble urokinase plasminogen activator receptor: an indicator of pneumonia severity in childrenAdv Exp Med Biol20158351725315615
- HoshinaTNanishiEKannoSNishioHKusuharaKHaraTThe utility of biomarkers in differentiating bacterial from non-bacterial lower respiratory tract infection in hospitalized children: difference of the diagnostic performance between acute pneumonia and bronchitisJ Infect Chemother2014201061662025027057
- WrotekAPawlikKJackowskaTSoluble receptor for urokinase plasminogen activator in community-acquired pneumonia in childrenAdv Exp Med Biol201378832933423835994
- Sardà SánchezMHernándezJCHernández-BouSTeruelGCRodríguezJVCubellsCLPro-adrenomedullin usefulness in the management of children with community-acquired pneumonia, a preliminar(y) prospective observational studyBMC Res Notes2012536322818355
- EspositoSTagliabueCPicciolliIProcalcitonin measurements for guiding antibiotic treatment in pediatric pneumoniaRespir Med2011105121939194521959024
- LeeJYHwangSJShimJWClinical significance of serum procalcitonin in patients with community-acquired lobar pneumoniaKorean J Lab Med201030440641320805714
- DonMValentFKorppiMCancianiMDifferentiation of bacterial and viral community-acquired pneumonia in childrenPediatr Int2009511919619371285
- TumgorGCelikUAlabazDAetiological agents, interleukin-6, interleukin-8 and CRP concentrations in children with community- and hospital-acquired pneumoniaAnn Trop Paediatr200626428529117132293
- BallinAOsadchyAKlivitskyADalalILishnerMAge-related leukocyte and cytokine patterns in community-acquired bronchopneumoniaIsr Med Assoc J20068638839016833166
- KorppiMNon-specific host response markers in the differentiation between pneumococcal and viral pneumonia: what is the most accurate combination?Pediatr Int200446554555015491381
- CaiQXuMYValue of neutrophil CD64 in the diagnosis of community acquired pneumonia in children]. [Article in ChineseZhongguo Dang Dai Er Ke Za Zhi2012141181982223146726
