Abstract
Background
Allergic diseases represent a frequent and increasing condition affecting children. A screening questionnaire allowing an easy identification of children with symptoms of allergic diseases may improve management and clinical outcome. The aim of this study was to develop and validate an easy-to-use screening questionnaire to detect children requiring further allergological evaluations.
Methods
A 10-item questionnaire, evaluating the presence and the history of the most frequent allergic conditions affecting children, including allergic asthma, allergic rhinitis and conjunctivitis, food allergy, and atopic dermatitis, was developed and administered to 214 parents of children from 5 to 10 years of age (163 with allergic disease and 51 healthy, nonallergic children). Validation was performed by Pearson’s correlation between the clinical diagnosis and the responses to the questionnaire. Internal consistency was computed by Cronbach’s alpha correlation coefficient. Sensitivity and specificity of the novel questionnaire were assessed by the receiver operating characteristic (ROC) curve.
Results
Validation analysis of the new children atopy (ChAt) questionnaire showed good internal consistency with a Cronbach’s alpha of 0.757. Responses to the items evaluating the presence of individual allergic conditions significantly correlated with the clinical diagnosis (p<0.001). The ROC curve showed an area of 0.956 and identified a cutoff value >2 of the ChAt questionnaire total score for detection of allergy (sensitivity =0.92 and specificity =0.902).
Conclusion
The novel ChAt questionnaire represents a simple tool able to detect the presence of all major allergic diseases in a pediatric population allowing an early identification of allergic multimorbidity and potentially facilitating clinical management.
Introduction
Allergic diseases represent a leading cause of chronic illness in children, with up to 30% of pediatric population affected by some form of atopic disease.Citation1–Citation3 Moreover, the prevalence of allergy seems to have dramatically increased over the past 25 years, particularly in Western industrialized countries.Citation4,Citation5 Among the Italian children, prevalence of allergic disorders varies from 20% for rhinitis to 10% for eczema/dermatitis, 9% for asthma, and 8% for food allergy.Citation6–Citation8
Since allergic inflammation involves different target tissues (conjunctiva, nose, lung, and skin) causing allergic conjunctivitis (AC), rhinitis (AR), asthma symptoms, and atopic eczema/dermatitis, evaluation of the prevalence of allergic diseases requires that any form of allergic response should be identified and addressed.Citation9 In fact, atopic conditions are often comorbid: 20%–40% of patients with AR are reported to have asthma, and 30%–90% of patients with asthma have AR.Citation10,Citation11 In addition, it has been proposed that early diagnosis and treatment should improve clinical outcome of allergy in children by controlling the progression toward the most severe forms, such as allergic asthma.Citation4 Therefore, screening instruments, allowing an early and simple identification of children with suspected allergic conditions, may improve patients’ health and clinical outcomes.Citation12,Citation13 Questionnaires are useful screening tools with the advantages of being noninvasive, low cost, and easy to administer and provide quick results. Citation13–Citation15 Allergic screening questionnaires for school children have been developed for the detection of individual allergic conditions to improve early identification and management of allergic diseases.Citation12,Citation16–Citation18 Most of them are focused primarily on detection and/or prediction strategies of asthma, with comorbid allergic diseases, including rhinitis and conjunctivitis, used to increase the specificity of allergic diseases detection.Citation17–Citation22 In this study, to identify the children with symptoms of all the major allergic disease types, we developed and validated a novel, simple, and easy-to-use children atopy (ChAt) questionnaire that addresses the primary allergic conditions.
Methods
Children between 5 and 10 years of age were recruited consecutively by the Pediatric Allergology and Immunology Unit, Policlinico Tor Vergata, University of Rome Tor Vergata, Italy, for the development and initial validation phases of the new screening questionnaire for the detection of allergic diseases in children (ChAt questionnaire).
The study was conducted in accordance with the principles embodied in the Declaration of Helsinki of 1965 (as revised in Brazil 2013). All parents of the participants provided written informed consent, and patient anonymity was preserved using methods approved by the Local Ethics Committee of the University of Rome Tor Vergata.
Diagnosis of allergic diseases including AC, AR, allergic asthma, atopic dermatitis, and food allergy was based on clinical history, objective examination, and allergological exams (including skin prick tests and specific Immunoglobu-lin (Ig) E detection).
ChAt screening questionnaire development
The development of ChAt screening questionnaire included the following steps.Citation23,Citation24
Item generation. The aim of this phase was to identify the items and conditions to be included in the screening questionnaire. A preliminary list of items was developed on the basis of the following sources:
An international literature review was performed through a computerized search in the Medline database to identify existing screening questionnaires for allergic diseases in children by using the following keywords/search terms: atopy*, allergy*, asthma*, allergic conjunctivitis*, allergic rhinoconjunctivitis* atopic dermatitis*, food* and allergy, children questionnaire and screening questionnaire.
A panel of four specialists (VM, LC, MS, AL) were required to indicate the most frequent allergic conditions affecting children and how to identify them through simple questions for administration to the parents.
Item reduction. A qualitative selection was performed from the preliminary list of items by eliminating those which were redundant, difficult to understand, or ambiguous. The resulting screening questionnaire included 10 items aimed at identifying a history of allergic diseases and the presence of specific symptoms of allergy: 1) previous diagnosis of allergic diseases, 2) previous use of antial-lergic drugs, 3) presence of symptoms of AC, 4) presence of symptoms of AR, 5) presence of symptoms of asthma, 6) presence of symptoms of atopic dermatitis, 7) history of severe allergic conditions such as anaphylaxis, 8) perennial and/or seasonal presentation of allergic symptoms, 9) family history of allergic diseases, and 10) history of food allergy including elimination diet ().
Table 1 ChAt questionnaire items and score values
Cognitive debriefing interviews with 10 parents of allergic children were performed to verify that the items were clear and easy to understand. The screening questionnaire was administered to the patient population for initial validation.
Validation of the new ChAt questionnaire
Two-hundred and fourteen parents of children between 5 and 10 years of age completed the screening questionnaire. One-hundred and sixty-three children were diagnosed with one or more allergic diseases by allergological exam, and in 51 children allergic diseases were excluded. This group was utilized as the healthy control group for the validation of the ChAt questionnaire. The aim of the validation phase was to evaluate the following psychometric properties of the new instrument:
Validity: using the Pearson correlation coefficient, we evaluated the relationship between the clinical diagnosis and the responses to the questionnaire.Citation13
Internal consistency: a measure of the homogeneity of a scale was computed using the Cronbach’s alpha correlation coefficient, and test–retest reliability, was evaluated by using intraclass correlation coefficient (ICC). Measurements with reliability of 0.70 or more have been recommended for the purpose of comparing groups.Citation14
Discriminant validity was evaluated by comparing the scores of allergic patients. Sensitivity and specificity of the new ChAt questionnaire were assessed by the receiver operating characteristic (ROC) curve in the entire population included in this study. The type and number of allergic diseases affecting the children were related to the ChAt total score by Sperman’s rho test.
The statistically significant cutoff value was set at p<0.05. The entire statistical analysis was conducted by using SPSS software version 18.0.
Results
Parents of one-hundred sixty-three allergic (N=163, mean age 7.2±1.1 years, 63 girls and 100 boys) and non allergic (N=51, mean age 7±0.9 years, 19 girls and 32 boys) children (mean age 7.2±1 years, 132 boys and 82 girls) completed the screening questionnaire.
Among the allergic children, 97 (59.5%) patients had a clinical diagnosis of AR, 51 (31%) of AC, 57 (35%) of asthma, 68 (42%) of atopic dermatitis, and 11 (7%) of food allergy. Eighty-nine of the 163 (55%) allergic patients were diagnosed with more than one allergic condition (59 had been diagnosed with 2 different allergic diseases, 27 with 3, and 3 with 4; ).
Table 2 Clinical characteristics of allergic population included in the study
The completion of the questionnaire by parents of allergic children included in the study is summarized in .
Figure 1 Percentage of positive responses to the ChAt questionnaire items.
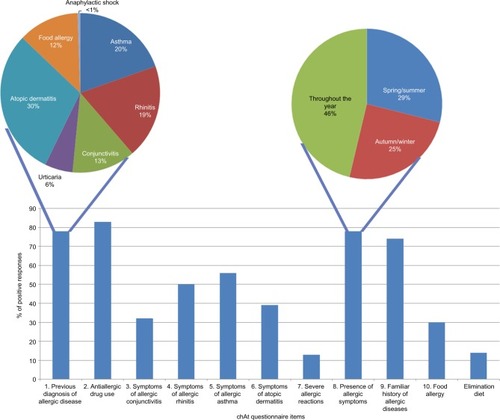
Validation analyses were undertaken considering the group of allergic patients (N=163).
Validity. Item and scale scores were oriented so that No= 0 and Yes =1 (); the sum of scores ranges between 0 and 10, and the higher the score, the higher probability to have an allergic disease.
Internal consistency. The screening questionnaire demonstrated satisfactory Cronbach’s alpha value of 0.757 and ICC of 0.84.
A strong correlation was observed between the clinical diagnosis and the responses to the questionnaire. Specifically, the following items showed strong correlation:
Item 3 – clinical diagnosis of AC vs symptoms of AC (p<0.001, R=0.330)
Item 4 – clinical diagnosis of AR vs symptoms of AR (p<0.0001, R=0.467)
Item 5 – clinical diagnosis of allergic asthma vs symptoms of allergic asthma (p<0.0001, R=0.527)
Item 6 – clinical diagnosis of atopic dermatitis vs symptoms of atopic dermatitis (p<0.0001, R=0.483)
Item 9 – familiar history of allergic disease collected by physician vs positive response to familiar history in the questionnaire (p<0.0001, R=0.368)
Item 10 – presence of food allergy diagnosed by allergolo-gist vs positive response to food allergy with elimination diet in the questionnaire (p<0.0001, R=0.370)
The ROC curve was calculated including the entire population of allergic and nonallergic children. Results showed an area of 0.956 and suggested that a cutoff score of >2 was able to accurately identify the allergic population with a sensitivity value of 0.920 and specificity of 0.902 ().
Figure 2 Receiver operating characteristic (ROC) curve of ChAt questionnaire scores for identification of the presence of allergic diseases.
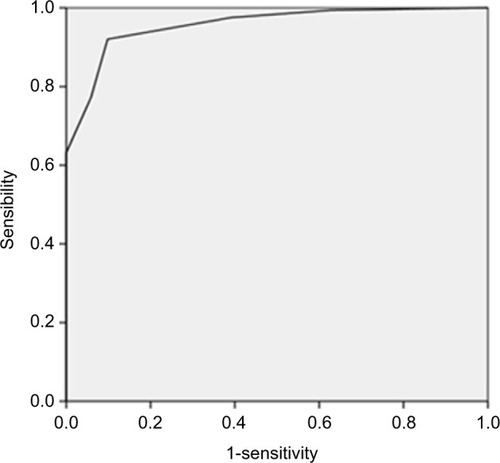
Patients with a diagnosis of allergy showed an average ChAt questionnaire score of 5.2±2, while nonallergic patients showed an average of 1.2±1.2, confirming that the cutoff total score >2 is able to identify the allergic population. In addition, the number of allergic diseases affecting the children was significantly related to the ChAt total score (p<0.001, R=0.695).
ChAt questionnaires with total score >2 identified a population with the possible presence of allergy which requires further clinical investigation for allergic disease, with a sensitivity of 92% (95% CI: 86.75% to 95.69%) and a specificity of 90% (95% CI: 78.59% to 96.74%), a positive likelihood ratio (LR) of 9.39 (95% CI: 4.08 to 21.61) and negative LR of 0.09 (95% CI: 0.05 to 0.15).
Discussion
Atopic diseases represent a substantial burden on the health care system, and great attention should be paid to identify children with allergic diseases in a timely manner and to provide prompt treatment and management of allergic diseases.Citation25 Several screening tools, including screening questionnaires and prediction rules, have been developed to detect specific allergic conditions and the risk of progression toward more severe allergic conditions such as asthma.Citation17,Citation18,Citation24 Since a simple and ready-to-use tool for the identification of children with allergic symptoms including all the most common symptoms of allergic diseases is not currently available, in this study we developed and validated a new 10-item screening questionnaire to identify children with allergic symptoms requiring further clinical evaluation. This questionnaire represents a useful tool for the detection of children with suspicion of allergy in epidemiological, screening studies or in general practice.
Most studies commonly use questionnaires to collect epidemiological and clinical data. Moreover, self-administration of questionnaires avoids interviewer bias.Citation17,Citation18,Citation26,Citation27 On the other hand, the main issue in the validation process of self-administered questionnaires is the capability to understand the items and to appropriately answer them. To facilitate this process, the ChAt questionnaire has been developed with well-defined, objective, and easily understandable questions for parent self-administration. In addition, respondents were selected and screened from the same geographical area (Rome, Italy).Citation28
The ChAt questionnaire includes an item exploring the previous diagnosis of allergic conditions, with specification of the previous diagnosis, and an item about the previous use of antiallergic drugs to aid in the identification of allergic children. The other five items explore the presence of the most frequent symptoms associated with AC, AR, asthma, atopic dermatitis, and food allergy including elimination diet. The responses to these items showed a significant correlation with the clinical diagnosis of the specific allergic condition made by the allergologist, suggesting the ability of the items to identify the presence of the symptoms of specific disease. An additional three items evaluate the history of severe allergic reactions such as anaphylaxis, seasonal and/or perennial symptoms’ presentation, and familial history of allergy, adding important information for the identification of allergic children.
The ChAt score was calculated by the sum of the “YES” responses to the 10 items resulting in a total score ranging from 0–10, with the higher total score indicating a higher probability of allergy. The ROC curve analysis showed that a ChAt questionnaire total score >2 was able to identify children having allergy with high levels of sensitivity (92%) and specificity (90%).
Therefore, this study describes the development and preliminary validation of a simple, standardized, 10-item screening questionnaire that is able to identify atopic children who should be evaluated for the presence of allergic diseases and allergic comorbidities. The ChAt questionnaire shows the limitations characteristic of the use of questionnaires for disease screening, which includes the need to demonstrate the validity in different settings, in terms of sample population, social conditions, and geographic distribution, and to standardize the clinical diagnosis of the specific allergic conditions to be used as “gold standard.”Citation12–Citation15,Citation17–Citation19,Citation28 An epidemiological study is currently ongoing in a large population of schoolchildren to confirm the validity of the ChAt questionnaire at identifying children with allergic symptoms and allowing an early diagnosis of “unknown” cases.
Assessing allergic diseases at a community level using simple questionnaires, such as ChAt, may be a first step to change patient behaviors and to target educational initiatives aimed at improving the allergic disease outcomes. In fact, not only is poor disease control a negative prognostic factor for allergic disease progression, but it may also be associated with low continuity of care due to a poor doctor–patient relationship, negative attitudes, and concerns about the use of medication, and little understanding of the disease.Citation10,Citation29 The consequences of such a scenario are detrimental for atopic children, their families, and the health care system in general.
To conclude, the ChAt questionnaire represents a useful tool to identify children with suspicion of multiple atopic conditions and, in turn, to substantially contribute to further enhance the treatment and clinical management of allergic diseases. In addition, the ChAt questionnaire may facilitate the collection of data on school children population, which could be useful to save time and serve as useful resource in large epidemiological studies.
Acknowledgments
This study was supported by Italian Ministry of Health grant for Young Researchers (GR-2009-1595946).
Disclosure
The authors report no conflicts of interest in this work.
References
- DownsSHMarksGBSporikRContinued increase in the prevalence of asthma and atopyArch Dis Child200184202311124778
- SkonerDPAllergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosisJ Allergy Clin Immunol20011081 SupplS2S811449200
- QuerciaOIncorvaiaCPuccinelliPPrevalence of allergic disorders in Italy: the Cotignola population studyEur Ann Allergy Clin Immunol201244151122519126
- AsherMMontefortSBjörksténBISAAC Phase Three Study GroupWorldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveysLancet2006368953773374316935684
- Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAACThe International Study of Asthma and Allergies in Childhood (ISAAC) Steering CommitteeLancet19983519111122512329643741
- GalassiCDe SarioMBiggeriAChanges in prevalence of asthma and allergies among children and adolescents in Italy: 1994–2002Pediatrics2006117344216396858
- BrescianiniSBrunettoBIacovacciPPrevalence of self-perceived allergic diseases and risk factors in Italian adolescentsPediatr Allergy Immunol20092057858418710432
- TozziAEArmenioLBernardiniRPediatric allergy and immunology in ItalyPediatr Allergy Immunol201122326727621457333
- MuraroAClarkABeyerKThe management of the allergic child at school: EAACI/GA2LEN Task Force on the allergic child at schoolAllergy201065668168920345502
- PolsDHJWartnaJBvan AlphenEIInterrelationships between atopic disorders in children: a meta-analysis based on ISAAC questionnairesPLoS ONE2015107e013186926135565
- GuptaRSheikhAStrachanDPAndersonHRBurden of allergic disease in the UK: secondary analyses of national databasesClin Exp Allergy20043452052615080802
- GalimbertiMPassalacquaGIncorvaiaCCatching allergy by a simple questionnaireWorld Allergy Organ J201511811626140076
- Scientific Advisory Committee of the Medical Outcomes TrustAssessing health status and quality-of-life instruments: attributes and review criteriaQual Life Res20021119320512074258
- FayersPHaysRDRevickiDAReliability and validity, including responsivenessFayersPHaysRDAssessing Quality of Life in Clinical Trials: Methods and Practice2nd edNew YorkOxford University Press20052539
- Garcia de Yebenes ProusMARodriguez SalvanesFCarmona OrtellsLValidación de cuestionarios. [Validation of questionnaires]Reumatol Clin20095171177 Spanish21794604
- Castro-RodriguezJAThe Asthma Predictive Index: a very useful tool for predicting asthma in young childrenJ Allergy Clin Immunol201012621221620624655
- PescatoreAMDogaruCMDuembgenLA simple asthma prediction tool for preschool children with wheeze or coughJ Allergy Clin Immunol2014133111111823891353
- RedlineSLarkinEKKercsmarCBergerMSiminoffLADevelopment and validation of school-based asthma and allergy screening instruments for parents and studentsAnn Allergy Asthma Immunol200390551652812775133
- RedlineSGruchallaRSWolfRLDevelopment and validation of school-based asthma and allergy screening questionnaires in a 4-city studySchool Nurse News20042151214
- JonesABowenMScreening for childhood asthma using an exercise testBr J Gen Pract1994443801271318204321
- BauerEJLurieNYehCGrantENScreening for asthma in an inner-city elementary school in Minneapolis, MinnesotaJ Sch Health199969121610098113
- SavenijeOEMKerkhofMKoppelmanGHPostmaDSPredicting who will have asthma at school age among preschool children Review ArticleJ Allergy Clin Immunol201213032533122704537
- BoniniMBraidoFBaiardiniIAQUA: Allergy Questionnaire for Athletes. Development and validationMed Sci Sports Exerc20094151034104119346984
- SacchettiMBaiardiniILambiaseADevelopment and testing of quality of life in children with Vernal Keratoconjunctivitis questionnaireAm J Ophthalmol200714455756317693381
- DucharmeFMTseSMChauhanBDiagnosis, management, and prognosis of preschool wheezeLancet20143831593160424792856
- MurphyKRZeigerRSKosinskiMTest for respiratory and asthma control in kids (TRACK): a caregiver-completed questionnaire for preschool-aged childrenJ Allergy Clin Immunol200912383383919348922
- SatoKSatoYNagaoMDevelopment and validation of asthma questionnaire for assessing and achieving best control in preschool-age childrenPediatr Allergy Immunol201627330731226659837
- SavitzDAInterpreting Epidemiologic Evidence: Strategy for Study Design and AnalysisOxford, UKOxford University Press2003
- SorianoJBRabeKFVermeirePAPredictors of poor asthma control in European adultsJ Asthma200340780381314626337
