Abstract
The global experience with pediatric Kaposi sarcoma (KS) has evolved immensely since the onset of HIV (human immunodeficiency virus). In this review, current perspectives on childhood KS are discussed in the context of the HIV epidemic in sub-Saharan Africa. Endemic (HIV-unrelated) KS was first described over 50 years ago in central and eastern Africa, regions where human herpesvirus-8, the causative agent of KS, is endemic. With the alarming rise in HIV prevalence over the past few decades, KS has become not only the most common HIV-related malignancy in Africa, but also one of the most common overall childhood cancers throughout the central, eastern, and southern regions of the continent. The unique clinical features of pediatric KS that were described in those early endemic KS reports have been re-affirmed by the contemporary experience with HIV-related KS. These characteristics include a predilection for primary lymph node involvement, significant proportions of patients lacking prototypical cutaneous lesions, and the potential for fulminant disease progression. Other clinical features that distinguish childhood KS from adult disease include disease presentation with severe cytopenias, and the common occurrence of childhood KS without severe CD4 count suppression. Distinct clinical heterogeneity in disease presentation and treatment response have been demonstrated. Long-term complete remission and event-free survival can be achieved—especially in children with lymphadenopathic KS—utilizing treatment with antiretroviral therapy plus mild–moderate chemotherapy regimens that are well tolerated, even in low-income settings. A pediatric-specific staging classification and risk-stratification platform have been retrospectively validated, and may help guide therapeutic strategies. With expansion of the HIV treatment infrastructure throughout Africa, coupled with recent developments in establishing comprehensive pediatric oncology programs, there is great potential for improving outcomes for children with KS. Increased awareness of the unique clinical nuances and collaborative evaluations of pediatric-specific treatment paradigms are required to optimize survival for children with KS.
Introduction
Although four epidemiologic variants of Kaposi sarcoma (KS) have been established, childhood KS primarily occurs as either endemic (HIV-unrelated) or epidemic (HIV-related) disease. Endemic KS was originally described over 50 years ago in eastern and central Africa,Citation1–Citation4 the region where the causative agent for KS, human herpesvirus-8 (HHV-8)/KS-associated herpesvirus (KSHV), is endemic.Citation5,Citation6 Decades later, the onset of the HIV epidemic catapulted KS into becoming one of the most common overall childhood cancers in regions of Africa with high HHV-8 and HIV prevalence rates.Citation7,Citation8 The two other epidemiologic variants of KS are exceedingly rare in children; classic KS primarily occurs in the elderly population of the Mediterranean region, while the occurrence of transplantation-related KS in children is restricted to case reports.Citation9,Citation10 This review will focus on HIV-related pediatric KS in the contextual backdrop of its occurrence in a region where endemic disease was originally described.
The devastation of widespread HIV infection has been most pronounced in sub-Saharan Africa. This imbalance is even more emphatic in children; the 2016 UNAIDS report reveals that 90% of the world’s two million children living with HIV reside in sub-Saharan Africa. The overwhelming prevalence of HIV in a region where HHV-8 is endemic explains the staggering discrepancy in KS incidence in HIV-infected children globally.Citation11 In the pre-antiretroviral therapy (ART) era in the US and Europe, there were merely 14 cases of childhood KS reported over a 20-year span of multiple HIV-cancer registries.Citation12–Citation17 In comparison, several reports describing relatively large cohorts of children and adolescents with KS in sub-Saharan Africa have been published in the last decade.Citation18–Citation24 Ultimately, KS has become one of the most common pediatric malignancies in central, eastern, and southern Africa.Citation8 Increased awareness of its unique clinical characteristics and therapeutic response is vital in the quest to improve overall outcomes for children with cancer throughout the region.
Epidemiology
In the late 19th and early 20th century, KS existed in the literature as a rare malignancy throughout central-eastern Europe and the Mediterranean region.Citation1 The first pediatric case was described in a 5-year-old Italian boy by de Amicis in 1882.Citation82 Case reports that followed included children of Asian, European, Middle-Eastern, and African descent, describing a rare but global disease.Citation1,Citation25,Citation26
By the middle of the 20th century, it became clear that KS clustered in a belt of countries, moving across the equator from central into southern Africa.Citation3,Citation4,Citation27 Even prior to the HIV pandemic, the incidence of KS in Uganda was three times that of Europe, carrying an annual incidence of 3.7 per 100,000 persons.Citation26,Citation28,Citation29 In the region stretching across the Democratic Republic of Congo, western Uganda, and Tanzania, lifetime incidence of KS reached as high as 16 per 1,000.Citation30,Citation31
With the arrival of HIV in the late 20th century, the global incidence of KS increased multiple times over. In Uganda, annual incidence of KS in males increased over 10-fold from 1960 to 1990.Citation26 Pediatric KS increased dramatically in those regions where KS was previously endemic.Citation32 An incidence as high as 59/100,000 has been reported across Botswana, Malawi, Lesotho, Zimbabwe, Mozambique, South Africa, and Zambia.Citation33
Although HIV infection plays a major role, the relative incidence of KS generally corresponds with the seroprevalence of HHV-8, demonstrating significant geographic variation worldwide.Citation30,Citation34–Citation37 This should be expected considering the known role of HHV-8 in KS oncogenesis.Citation38 HHV-8 is typically transmitted by saliva, and, in the case of children, by food and drink-sharing within households.Citation39 In high-prevalence areas, seroprevalence increases with age.Citation35,Citation36,Citation40 In Uganda, HHV-8 is detected in 23% of 3–5 year olds, 32% of children aged 10–13, and 50% of adults over 50.Citation35 HHV-8 prevalence is low in children in areas where pediatric KS is rare, such as the US and northern Europe, where HHV-8 is detected in 1–6% of children.Citation5,Citation41 In comparison, HHV-8 prevalence is intermediate in the Mediterranean region.Citation6 It is important to note that HHV-8 prevalence varies widely across Africa as well, with the highest rates occurring in central and eastern Africa.Citation30,Citation42
Endemic, HIV-negative KS remains prevalent in the regions where it pre-dated HIV. Prevalence of HIV negative patients among pediatric KS cases ranges from 10–14% in Malawi to 22% in Uganda.Citation7,Citation22,Citation24,Citation43,Citation44 Outside of Africa, HIV-unrelated pediatric KS in the form of transplantation-related and classic disease is extremely rare and has been reported in fewer than 50 patients.Citation45
For reasons that are unknown, KS is more common in males than in females.Citation1,Citation26,Citation34 The difference in male versus female incidence is much more pronounced in HIV-negative adults, with a male predominance ranging from 10–20:1.Citation26,Citation34 In HIV negative children, the male-to-female ratio ranges from 2–7:1.Citation1,Citation22,Citation24,Citation44 The ratio in HIV positive children is tighter, though it still favors males by as much as 2.5:1, and may be closer to 1:1 in some settings.Citation18,Citation21–Citation23 The underlying innate risk factor for endemic KS is as yet unknown, but the male predominance suggests a possible sex-linked genetic predisposition.
Precise and accurate estimation of the disease burden of pediatric KS is challenging due to a paucity of population-based studies, few hospital registries in endemic regions, under-reporting, and potential misdiagnosis. In a review of pediatric-specific epidemiological studies of KS in Africa, 15 studies published since 1970 were identified. These studies included population-based estimates of pediatric KS, and the reported incidence varied widely.Citation32 For example, estimates of KS incidence in Kampala, Uganda through the 1990s ranged from 5–160 cases per 100,000 children, and in Lusaka, Zambia from 0.05–75.7 cases per 100,000 children during the 1980s.Citation26,Citation46–Citation48 Methods of patient registration and identification of KS cases vary across studies, and comparisons between them should be made with discretion.
The Kampala Cancer Registry of Uganda, established in 1954, offers valuable insight into the incidence of KS before and throughout the HIV epidemic. The registry reported an annual incidence of pediatric KS of 2.5 per million children from 1960–1971, increasing over 20-fold to 55.8 per million children from 1991–1997.Citation26 There is some evidence to suggest a decrease in pediatric KS incidence since its peak in the early 1990s, a phenomenon seen in children within high-income countries as well as in African adults, all likely secondary to increased utilization of ART.Citation49 In another study from Kampala, investigators reported a fall in pediatric KS incidence from a peak of 62.6 per million in the early 1990s to 44.6 per million in the early 2000s.Citation50 However, as the pediatric HIV treatment and care infrastructure has expanded, and awareness of pediatric KS has increased, trends have also demonstrated increasing rates of new childhood KS diagnoses, despite increased access to ART in Malawi.Citation51 Ultimately, KS is among the top three most-common childhood malignancies overall in numerous countries in central, eastern, and southern Africa, where it carries a disease burden greater than that of childhood acute lymphoblastic leukemia in the US.Citation8,Citation34,Citation43,Citation50,Citation52
Clinical characteristics
From the original reports of endemic KS in Africa, it was clear that the clinical manifestations in children were distinctly different from those seen in adults.Citation3,Citation4 Clinical descriptions of adult KS are characterized by widely recognized features—hyperpigmented skin and oral lesions, woody edema, and visceral disease.Citation53,Citation54 In contrast, the early descriptions of endemic KS in children offered striking photographic representations of KS presenting primarily with massive lymphadenopathy ().Citation3,Citation4 The unique features of endemic KS in children included the predominance of lymph node involvement, rapid progression of disease in the absence of treatment, and a significant percentage of patients presenting without prototypical cutaneous lesions.Citation3,Citation4
Figure 1 Photographic representations of lymphadenopathic Kaposi sarcoma.
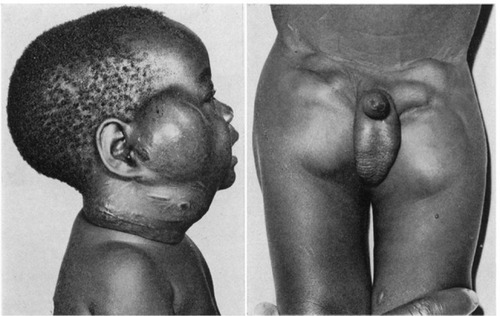
As the contemporary experience with HIV-related pediatric KS in Africa has evolved, it has become increasingly clear that the early descriptions of endemic childhood KS are reproducible decades later. Recently published data from HHV-8 endemic regions with high HIV prevalence demonstrate high rates of lymphadenopathic KS, ranging from 52–93% in pediatric KS cohorts from Uganda, Malawi, and Mozambique ().Citation18,Citation19,Citation21–Citation24 As the awareness of lymphadenopathic KS has increased, it appears that the recognition of its clinical presentation has resulted in even higher proportions of lymph node disease. The three most recent publications have reported lymphadenopathy to be the most common clinical presentation of childhood KS.Citation22–Citation24 It is important to point out that, in the recent cohort from Lilongwe, Malawi, 26% of all patients presented primarily with lymphadenopathy in the absence of prototypical skin, oral, or woody edema manifestations.Citation23 The majority (72%) of the lymphadenopathy-only patients were diagnosed definitively by biopsy, providing compelling evidence reaffirming the early observations of childhood KS made 50 years ago.
Table 1 Comparison of clinical features among pediatric KS cohorts in Africa
The anatomical sites of involvement in pediatric KS have been previously defined and are described briefly here.Citation23 Lymph node KS is characteristically firm and non-tender; involved nodes tend to be bulging, with diameters greater than 2 cm, and often much larger. They are distinctly different from the shoddy, soft, raisin-sized lymph nodes that are typically seen in the setting of an infectious illness, and/or as a manifestation of HIV-associated generalized lymphadenopathy. Hyperpigmented skin lesions are typically raised papular lesions, but flat macules can also occur. Oral mucosal lesions can be flat or nodular, and, although typically found on the palate, can present anywhere in the oral mucosa. Woody edema is characteristically firm and resembles the texture of tree bark. It typically presents in the extremities, but can occur in the inguinal and pubic region as well. Flesh-colored subcutaneous nodules are characteristically not hyperpigmented, and are located distinctly beneath the skin. They are either movable isolated nodules, or clusters of fibrous lesions associated with underlying woody edema. Some patients present with facial edema that is typically soft, peri-orbital, and distinct from woody edema. Other types of lesions that can occur, albeit less frequently, are exophytic or pedunculated masses and hyperpigmented lesions of the eye (conjunctiva).
Visceral disease can present in the lungs or the organs of the abdominal cavity. Because definitive diagnosis is established via bronchoscopy or endoscopy of the gastrointestinal (GI) tract, diagnosing visceral KS can be challenging in low- and middle-income countries where these procedural modalities are lacking.Citation23 Pulmonary KS typically presents either with serosanguineous pleural effusions, or with parenchymal disease that can appear as reticulo-nodular infiltrates on a chest X-ray. Patients may present in overt respiratory distress or be asymptomatic; therefore, staging chest X-rays are important to obtain for all patients diagnosed with KS. A high index of suspicion should be maintained in patients with upper airway obstruction that improves after the initiation of chemotherapy, as this may indicate the presence of upper airway KS lesions. Visceral disease in the abdominal cavity presents either in the form of GI disease (with lower GI bleeding) or as severe ascites. Upper GI KS can present with dysphagia, and, in the absence of endoscopy, a high index of clinical suspicion must be maintained in patients who present with dysphagia that improves after the initiation of chemotherapy.Citation23 Presentation with visceral disease only, in the absence of any other KS lesions, is relatively difficult to establish in low-income countries.
The clinical signs of visceral KS can overlap with and mimic other opportunistic illnesses. For example, pulmonary tuberculosis and pulmonary KS are challenging to differentiate with limitations in diagnostic resources. Similarly, GI bleeding can occur from both infectious illnesses and GI KS. Therefore, empiric treatment with anti-bacterial, anti-tuberculosis, and anti-parasitic agents is often indicated. In such scenarios, the diagnosis of visceral involvement in children with KS can be a process of exclusion, and is often re-affirmed after failure of these empiric anti-microbial treatments plus apparent improvement in symptoms after initiation of chemotherapy.
Generally, the authors place great emphasis on lymphadenopathic KS in children to increase awareness of this less well-appreciated phenomenon, as missed diagnoses commonly occur. Most general clinicians presume that lymphadenopathy in HIV-infected children is related to tuberculosis or other bacterial infection, a clinical manifestation of lymphoma, or represents HIV-associated generalized lymphadenopathy. Consequently, many patients are eventually diagnosed with lymph node KS after considerable delay—after having initiated ART and having failed empiric anti-bacterial and anti-tuberculosis treatment. Therefore, it is important to maintain a high index of suspicion for the diagnosis of KS in HIV-infected children presenting with persistent lymphadenopathy.
It is likewise important to recognize that childhood KS can present in the prototypical fashion that is more commonly recognized in adults i.e., with hyperpigmented skin and oral lesions, woody edema, and visceral disease. Hyperpigmented skin lesions are commonly reported, with rates of cutaneous involvement as high as 82–93% in some cohorts,Citation19,Citation21,Citation24 but as low as 48–59% in others ().Citation18,Citation22,Citation23 Clinical presentation with woody edema tends to occur in older children and adolescents, while lymph node involvement is associated with younger age, especially in the HHV-8 endemic regions of eastern and central Africa.Citation18,Citation21,Citation55 Interestingly, data from South Africa, where childhood HHV-8 infection rates are significantly lower than in eastern Africa,Citation30,Citation42 revealed a much lower percentage (30%) of patients with the lymphadenopathic sub-type, suggesting that regional variation may occur depending on the endemic nature of HHV-8.Citation20
Peripheral blood cytopenias are another unique feature of childhood KS and are relatively common at the time of presentation.Citation21–Citation24 The extent of such cytopenias can be quite severe, with some patients presenting with life-threatening anemia and/or symptomatic thrombocytopenia. Although peripheral blood count analyses have not been systematically reported in all of the pediatric KS cohorts from Africa, data from Lilongwe and Blantyre, Malawi demonstrate the severity of these cytopenias. In the earlier cohort from Lilongwe, 29% of patients presented with hemoglobin <7 g/dL and 30% with platelet count <100×109/L.Citation21 In the more recent cohort, 37% presented with hemoglobin <8 g/dL, while 28% had a platelet count <100×109/L.Citation23 In the two recently published cohorts from Blantyre, the low end of the interquartile range for platelet count was 66 and 76×109/L, respectively, indicating that at least 25% of both cohorts presented with significant thrombocytopenia.Citation22,Citation24 Although anemia has been reported in African adults with KS, it is rare to find descriptions of severe thrombocytopenia.Citation56 Although comprehensive bone marrow evaluations have not been performed in children with KS, the authors theorize that the cytopenias in KS are not due to malignant infiltration of the marrow, but rather secondary to bone marrow suppression from the systemic effects of KS and the underlying HHV-8 infection. This theory is derived from the analogous phenomenon described in two other HHV-8 related malignancies—multicentric Castleman disease (MCD) and the KSHV inflammatory cytokine syndrome (KICS)—and discussed further in the section on HHV-8 virologic considerations.Citation57–Citation59 It is important to point out that these severe cytopenias improved promptly after the initiation of chemotherapy in the recent cohort from Lilongwe, with 88% of patients having achieved resolution of their severe thrombocytopenia within 2 weeks of the first dose of chemotherapy (without the aid of transfusion).Citation23
An additional nuance of childhood KS is that CD4 counts are often not severely suppressed—defying common conceptions of HIV-related disease in adults. A multi-center study from South Africa and the more recent Malawian cohorts have demonstrated median/mean CD4 counts ranging from 354–440 cells/mm3, indicating that the majority of patients developed KS despite not having a severely suppressed CD4 count ().Citation20,Citation22–Citation24 The authors hypothesize that intrinsic compromise of qualitative T-cell function in children vertically-infected with HIV render them vulnerable to the oncologic complications of HHV-8 infection, regardless of their quantitative CD4 count. In contrast, adults living in HHV-8 endemic regions will have likely experienced HHV-8 infection during childhood.Citation60 In theory, these adults would have already developed an effective immune response to HHV-8 during childhood and would require HIV-induced immune dysfunction to enable the potential for secondary reactivation of HHV-8. This would be mechanistically distinct from an HIV-infected child who experiences primary HHV-8 infection with a naïve and impaired immune system, and potentially contributes to the distinctions between pediatric and adult KS.
Treatment outcomes
Reports on the treatment of pediatric KS in sub-Saharan Africa have primarily highlighted outcomes for patients receiving chemotherapy plus ART.Citation18–Citation24,Citation61 While the success of ART alone for adult KS patients with limited disease has been well documented in the US and Europe,Citation62,Citation63 the experience with adult and pediatric KS appears to be different in Africa.Citation64,Citation65 Whether this is due to inherent biological differences of KS in Africa, or the tendency for KS in Africa to present as advanced-stage disease, or both, remains unclear. Nonetheless, the authors have observed that pediatric KS in HHV-8 endemic regions uncommonly presents with limited disease.Citation55 In reviewing the pediatric KS data from sub-Saharan Africa, only three of the seven major published cohorts reported any long-term survivors after treatment with ART alone—in two patients out of 73 from Uganda, one out of 70 from the South African cohort, and seven out of 82 from the combined Malawi/Botswana experience ().Citation18,Citation20,Citation21 So, while success has been reported for children with KS treated with ART alone, it is in strikingly few patients, suggesting that the vast majority of children will require chemotherapy plus ART.
Table 2 Comparison of treatment outcomes among pediatric KS cohorts in Africa
A combination of multiple additional factors further contributes to the rationale that ART alone is insufficient therapy for the majority of children with KS. During the early years of the ART experience in Africa, mortality rates reached approximately 60% ().Citation18,Citation20–Citation22 While the improved outcomes of more recent cohorts (with overall survival in the 60% range) are potentially rooted in multiple factors, it is important to recognize that the development of systematic KS treatment programs providing both ART and chemotherapy has produced improved outcomes, despite including very sick patients.Citation23,Citation24 For example, in a recent pediatric KS cohort from Mbeya, Tanzania, 76% of patients met criteria for World Health Organization-defined severe immunosuppression at diagnosis, and 38% had severe acute malnutrition. Despite the complexity of patients in this cohort of 34 children receiving treatment with ART plus chemotherapy, 71% survived with a median follow-up of 26 months.Citation66
Additionally, in the era of increasing availability of ART in sub-Saharan Africa, more recent cohorts have included high numbers of patients (ranging from 49–77%) diagnosed with KS, despite already being on ART ().Citation22–Citation24,Citation66 While some patients develop KS in the scenario of an immune reconstitution inflammatory syndrome phenomenon,Citation67 the summarized published experience with HIV-related pediatric KS in Africa demonstrates that ART alone rarely provides adequate treatment. Moreover, the authors anecdotal experience in Malawi and Tanzania re-affirms the early observations of children with endemic KS from 50 years ago—that in the absence of chemotherapy, the majority of patients will experience disease progression, that at times can be fulminant.Citation3,Citation4
While various chemotherapy regimens have been utilized in the treatment of pediatric KS in Africa, a universal standard has not been established.Citation61 Liposomal doxorubicin is widely accepted as the standard first-line chemotherapeutic option for the treatment of adult KS in high-income countries; however, due to its prohibitive cost, it is not available in low-income settings in Africa.Citation60,Citation64,Citation65 The chemotherapeutic agents that have been described for the treatment of pediatric KS in Africa include bleomycin, vincristine, standard doxorubicin, etoposide, and paclitaxel—either as monotherapy or in various combinations.Citation18–Citation24 As previously mentioned, the more recent cohorts have reported improved treatment outcomes, but the better survival rates are probably attributable to multiple contributing factors rather than inherent differences in the chemotherapy regimens. Improvements in the overall pediatric HIV treatment infrastructure, supportive care capacity, ancillary services that support the diagnosis and treatment of childhood cancer in Africa, and increased awareness of the clinical presentation of pediatric KS are among the important factors that have contributed to overall improved outcomes. While a comparison of heterogeneous cohorts across Africa and the various treatment outcomes reported may not agree on a single universal treatment approach, there is a common need to identify safe but effective regimens that minimize the treatment toxicity in complex immunosuppressed patients.Citation61
The original chemotherapeutic approach to KS in Malawi was vincristine monotherapy; this decision was based upon the limited availability of chemotherapy, as well as the safety profile of a non-myelosuppressive agent like vincristine in the setting of limited capacity to deliver complex oncology care in HIV-infected patients. However, the early experience with vincristine monotherapy demonstrated its relative inefficacy and resulted in the search for improved chemotherapeutic options, leading to the combination regimen of bleomycin and vincristine (BV).Citation22,Citation68 BV offered a reasonable and practical alternative, being a minimally myelosuppressive regimen that could be administered in an outpatient setting without the extensive supportive care resources that are commonly required for the majority of chemotherapy regimens utilized in pediatric oncology centers in high-income countries. Based upon the favorable experience with BV early on, it became the standard regimen for the pediatric KS treatment programs in Lilongwe, Malawi and Mbeya, Tanzania.Citation21,Citation66
As the pediatric KS experience in Lilongwe and Mbeya evolved, it became apparent that, while BV was safe and produced excellent long-term outcomes in some patients, it was inadequate to prevent KS-related mortality in others.Citation21 A retrospective evaluation of the clinical factors associated with event-free survival (EFS) and overall survival (OS) in 70 pediatric KS patients in Lilongwe determined distinct findings.Citation23 A significant favorable association was observed between lymphadenopathic KS and both improved EFS and OS.Citation23 This contrasted dramatically with children having visceral KS or disseminated skin/oral disease (defined as having ≥20 hyperpigmented skin/oral lesions with widespread distribution), who demonstrated an increased risk for mortality and a failure to achieve EFS.Citation23 Woody edema was associated with increased risk for failure to achieve EFS, but without increased mortality.Citation23 Overall, BV was well tolerated, with 18.6% of patients developing grade 4 neutropenia, and without incidence of grade 3 or 4 thrombocytopenia—an important factor for clinical sites with limited access to platelet transfusions. The overall treatment-related mortality was 7%, which compared favorably to the 10% treatment-related mortality among all pediatric oncology patients in Lilongwe, establishing BV as a relatively safe first-line chemotherapeutic option.Citation23,Citation43
Based upon the spectrum of distinct clinical phenotypes observed in pediatric KS patients, a risk-stratification platform was proposed with the ultimate goal of guiding treatment strategies. The Lilongwe Pediatric KS Staging Classification was derived from the aforementioned analyses of clinical risk factors differentiating four clinical groups with distinct prognostic patterns after treatment with BV plus ART (): Stage 1, mild KS limited to cutaneous and oral manifestations (expected to have favorable survival, potentially without requiring chemotherapy); stage 2, lymphadenopathic KS (2-year EFS 73% and OS 75%); stage 3, woody edema KS (2-year EFS 29% and OS 79%); and stage 4, visceral and/or disseminated skin/oral KS (2-year EFS 0% and OS 12%).Citation55 This classification ultimately seeks to determine risk-stratified therapeutic options—guiding which patients may be treated with ART alone, those who may have a favorable prognosis with BV plus ART, and those who require alternative chemotherapeutic options due to the high risk of treatment failure with BV.Citation55 Ultimately, pediatric KS is similar to essentially all childhood malignancies, demonstrating distinct heterogeneity in clinical features and treatment response, thereby necessitating a risk-stratified treatment approach to individualize therapeutic options based upon expected prognostic criteria.
Figure 2 The modified Lilongwe pediatric Kaposi sarcoma staging classification.
Abbreviation: KS, Kaposi sarcoma.

Virologic considerations
While a comprehensive review of HHV-8 viral pathophysiology is beyond the scope of this paper, one cannot discuss KS without addressing the essential role of the causative agent. For further in-depth discussion of this topic, the reader is referred to recently published expert reviews on HHV-8 and its associated malignancies.Citation60,Citation69,Citation70 HHV-8 was first discovered in KS tissue biopsies in the 1990s,Citation38 and subsequently has also been associated with several lymphoproliferative disorders—primary effusion lymphoma (including the extracavitary variant), MCD, diffuse large B-cell lymphoma arising in the setting of MCD, and germinotrophic lymphoproliferative disorder.Citation71 It is important to note that HHV-8 associated lymphoproliferative disorders can occur concurrently with KS.Citation72 KICS is an additional recently defined disorder that is typically associated with KS or primary effusion lymphoma and is characterized as a hyper-inflammatory state caused by excessive levels of various cytokines, especially interleukin (IL)-6 and IL-10.Citation58,Citation73 The HHV-8 related viral pathophysiology in KICS is analogous to that seen in MCD, and the two conditions share significant clinical and biological overlap.Citation59
A distinguishing factor that is common to both MCD and KICS is the role of lytic phase HHV-8 replication in driving the disease pathophysiology.Citation59 In contrast, adult KS in the US and Europe generally arises in the setting of latent phase HHV-8 infection.Citation74,Citation75 A unique aspect of lytic phase infection is expression of the HHV-8 encoded protein viral IL-6, a homolog to human IL-6.Citation76,Citation77 Elevated levels of both viral and human IL-6 are characteristic of MCD and KICS, and these excessive cytokine levels play an important role in the unique clinical manifestations of both conditions.Citation58,Citation59,Citation73,Citation78 Peripheral blood cytopenias are common in both MCD and KICS, and appear to occur as a result of viral and cytokine-mediated bone marrow suppression.Citation57 Analysis of bone marrow findings in patients with MCD and cytopenias reveal a distinct lack of malignant infiltrates in the marrow, and a lack of evidence for a hemophagocytic process.Citation57 Common findings in the bone marrow evaluations included reactive plasmacytosis and scattered HHV-8 infected mononuclear cells.Citation57
As an HHV-8 associated malignancy with clinical manifestations that commonly include lymphadenopathy and peripheral blood cytopenias, MCD shares clinical overlap with the common findings of lymphadenopathy and cytopenias seen in pediatric KS patients in HHV-8 endemic regions of Africa. Although all of the lymph node biopsies in pediatric KS patients from Lilongwe have revealed only KS, without evidence of MCD,Citation23 the authors hypothesize that it is only a matter of time before increased access to pathology resources in the region will enlighten a broader understanding of the spectrum of HHV-8 associated malignancies in endemic regions. Indeed, a case series of adults with MCD from Lilongwe was recently published, establishing an important precedent to increase awareness of this diagnostic entity.Citation79 Moreover, a study evaluating the HHV-8 viral profile from Malawian adult KS specimens demonstrated the existence of a sub-group of patients who exhibited an extended viral transcription profile, in comparison to adult KS from the US, which is characterized by transcripts restricted to the HHV-8 latency locus.Citation80 Synthesizing these considerations—the overlap in clinical features between pediatric KS and other HHV-8 associated lymphoproliferative disorders that are typically driven by lytic phase infection, plus the established precedence of a distinct viral pathophysiology in adult KS in Africa—the authors hypothesize that the unique features of childhood KS in HHV-8 endemic regions may be rooted in distinct viral pathophysiologic mechanisms.
Future directions
Over the past half-century, childhood KS has become increasingly relevant in sub-Saharan Africa. As one of the most common overall childhood cancers in numerous countries in central, eastern, and southern Africa, there is a definite need for improved therapeutic strategies, especially with expanding pediatric HIV and oncology treatment infrastructure throughout the region.Citation81
Based upon feedback received for the original proposed paradigm for the Lilongwe Pediatric KS Staging Classification, we have recently made a slight modification to the staging system (). In the original classification, stage 1 was intended to include patients with mild cutaneous/oral disease, a group that might be expected to improve with ART alone. This meant that stage 2 included both patients with moderate cutaneous/oral disease, plus those with KS lymphadenopathy (as both groups require chemotherapy plus ART). This caused confusion in interpreting the staging classification, as stage 2 included a broad list of clinical features. Therefore, in an effort to render the staging classification easier to define, the modified classification restricts stage 2 to patients with KS lymphadenopathy. Thus, both patients with mild and moderate cutaneous/oral disease are assigned to stage 1, as stages 1A and 1B, respectively.
In re-analyzing the data based upon this modification, it is apparent that the outcomes reported in the pediatric KS staging classification/risk stratification paper remain unchanged. Of the 37 patients originally identified as stage 2 in the published data, 34 (92%) were assigned to stage 2 because of lymph node involvement; three patients would have been stage 1B using the modified system. In re-analyzing survival outcomes for the modified stage 2 group of patients with KS lymphadenopathy, the 2-year EFS is 74%, similar to the 2-year EFS of 73% in the publication.Citation55 This modified staging classification provides a clearer differentiation of the clinical phenotypes—(1) KS limited to cutaneous/oral involvement, (2) lymphadenopathic KS, (3) woody edema KS, and (4) visceral and/or disseminated KS.
The current therapeutic approach for the pediatric KS treatment programs in Lilongwe, Malawi and Mbeya, Tanzania involves a risk-stratified, response-adapted regimen based upon the pediatric KS staging classification and subsequent response to treatment ().Citation55 As the experience in Malawi has demonstrated, response to treatment is important to monitor.Citation23 This led to a new treatment response definition, termed induction failure, which referred to the failure to achieve at least a 90% reduction in size of all KS lesions (based upon subjective assessment) after the initial induction phase of BV chemotherapy (four cycles given every 2 weeks). Although it is expected for stage 3 woody edema to exhibit a slow response to chemotherapy (83% induction failure rate), for all patients without woody edema, failure to respond robustly to induction BV appears to be a poor prognostic sign and an indication to intensify therapy. Induction failure carried an Odds Ratio of 6.21 (95% Confidence Interval=1.93–19.99, P-value<0.01) for failure to achieve EFS, and an Odds Ratio of 3.02 (95% Confidence Interval=0.95–9.65, P-value=0.06) for risk of death.Citation23 Induction failure potentially represents a concerning prognostic sign that may predict an unfavorable outcome for patients without woody edema. Hence intensification to doxorubicin, bleomycin, and vincristine (ABV)×8 cycles is indicated for stage 1B and stage 2 patients with induction failure, as well as those who experience disease progression or relapse (not due to primary failure of HIV suppression). For those patients who experience worsening of their KS due to failure to control HIV, prompt adjustments in the ART regimen are critical. It is important to point out, though, that woody edema KS (stage 3) is characterized as an indolent, almost chronic condition, with low mortality from KS itself. Therefore, intensification of chemotherapy is not indicated, but rather a focus on improving quality-of-life for patients who typically live with stable chronic edema after completing chemotherapy.
Figure 3 Treatment design schema for a risk-stratified and response-adapted therapeutic approach to pediatric Kaposi sarcoma.
Abbreviations: KS, Kaposi sarcoma; ART, antiretroviral therapy; progressive disease is defined as an increase in the size of existing lesions or the appearance of new lesions; BV, = bleomycin and vincristine; induction BV includes four cycles of BV given every 2 weeks, consolidation BV includes four cycles of BV given every 4 weeks; ABV, doxorubicin, bleomycin, and vincristine, QoL, quality-of-life; complete remission is defined as no clinical evidence of KS lesions; HIV, human immunodeficiency virus.
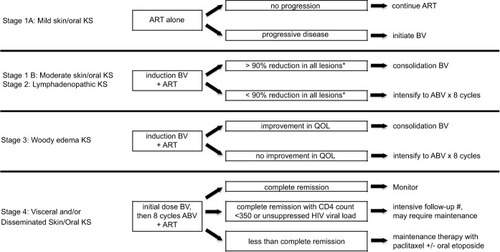
Ultimately, future guidelines that can be adapted across the diverse regions of Africa and worldwide are needed. A prospective evaluation of this risk-stratified, response-adapted approach to therapy in a multi-center international trial is required to determine the optimal therapeutic approach to pediatric KS. In the meantime, the authors advocate for increased awareness of the unique clinical features seen in the pediatric population in an effort to expand access to effective treatment regimens and improve overall outcomes for patients across the region.
Acknowledgments
The authors express gratitude and admiration to the many patients and families battling against the injustice of poverty and the misfortune of severe illness. We thank the many brilliant individuals who have contributed to the development of the pediatric KS treatment programs across the clinical network of sites in Africa set up by the Baylor College of Medicine International Pediatric AIDS Initiative at Texas Children’s Hospital. Especially at the sites in Lilongwe, Malawi and Mbeya, Tanzania, we recognize the expert clinical care and guidance of William Kamiyango, Jimmy Villiera, and Jason Bacha, all of whom made immense contributions to developing the current treatment paradigm. We also gratefully acknowledge the external support of many individuals who have helped guide the clinical programs over the past decade, including Parth Mehta, Jeremy Slone, Carrie Cox, Carrie Kovarik, Michael Scheurer, Carl Allen, Joseph Lubega, Gordon Schutze, Mark Kline, David Poplack, Dirk Dittmer, Asulwisye Kapesa, Phoebe Nyasulu, Avni Bhalakia, Maria Kim, and Saeed Ahmed. We extend sincere gratitude to the many colleagues working at the Baylor Children’s Foundation Clinical Centres of Excellence in Lilongwe, Malawi and Mbeya, Tanzania, the Tingathe Outreach Program, and Kamuzu Central Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- DutzWStoutAPKaposi’s sarcoma in infants and childrenCancer19601368469413818924
- DaviesJNLotheFKaposi’s sarcoma in African childrenActa Unio Int Contra Cancrum19621839439913883834
- SlavinGCameronHMForbesCMitchellRMKaposi’s sarcoma in East African children: a report of 51 casesJ Pathol197010031871995428938
- OlwenyCLKaddumukasaAAtineIOworRMagrathIZieglerJLChildhood Kaposi’s sarcoma: clinical features and therapyBr J Cancer19763355555601276034
- MartroEBulterysMStewartJAComparison of human herpesvirus 8 and Epstein-Barr virus seropositivity among children in areas endemic and non-endemic for Kaposi’s sarcomaJ Med Virol200472112613114635020
- GaoSJKingsleyLLiMKSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcomaNat Med1996289259288705864
- ZieglerJLKatongole-MbiddeEKaposi’s sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infectionInt J Cancer19966522002038567117
- StefanDCPatterns of distribution of childhood cancer in AfricaJ Trop Pediatr201561316517325724211
- CamciogluYPicardCLacosteVHHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiencyJ Pediatr2004144451952315069403
- LeJGanttSAST Infectious Diseases Community of PracticeHuman herpesvirus 6, 7 and 8 in solid organ transplantationAm J Transplant201313Suppl 412813723465006
- Pediatric Aids-Defining Cancer Project Working Group for IeDEA Southern Africa, TApHOD, COHERE in EuroCoordKaposi sarcoma risk in HIV-infected children and adolescents on combination antiretroviral therapy from sub-Saharan Africa, Europe, and AsiaClin Infect Dis20166391245125327578823
- BiggarRJFrischMGoedertJJRisk of cancer in children with AIDS. AIDS-Cancer Match Registry Study GroupJAMA2000284220520910889594
- PollockBHJensonHBLeachCTRisk factors for pediatric human immunodeficiency virus-related malignancyJAMA2003289182393239912746363
- KestHBroglySMcSherryGDashefskyBOleskeJSeageGR3rdMalignancy in perinatally human immunodeficiency virus-infected children in the United StatesPediatr Infect Dis J200524323724215750460
- GranovskyMOMuellerBUNicholsonHSRosenbergPSRabkinCSCancer in human immunodeficiency virus-infected children: a case series from the Children’s Cancer Group and the National Cancer InstituteJ Clin Oncol1998165172917359586885
- CaselliDKlersyCde MartinoMHuman immunodeficiency virus-related cancer in children: incidence and treatment outcome—report of the Italian RegisterJ Clin Oncol200018223854386111078499
- EvansJAGibbDMHollandFJTookeyPAPritchardJAdesAEMalignancies in UK children with HIV infection acquired from mother to child transmissionArch Dis Child19977643303339166025
- GanttSKakuruAWaldAClinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan childrenPediat Blood Cancer2010545670674
- VazPMacassaEJaniITreatment of Kaposi sarcoma in human immunodeficiency virus-1-infected Mozambican children with antiretroviral drugs and chemotherapyPediatr Infect Dis J2011301089189321730886
- StefanDCStonesDKWainwrightLNewtonRKaposi sarcoma in South African childrenPediatr Blood Cancer201156339239621225916
- CoxCMEl-MallawanyNKKabueMClinical characteristics and outcomes of HIV-infected children diagnosed with Kaposi sarcoma in Malawi and BotswanaPediatr Blood Cancer20136081274128023487320
- ChagalukaGStanleyCBandaKKaposi’s sarcoma in children: an open randomised trial of vincristine, oral etoposide and a combination of vincristine and bleomycinEur J Cancer20145081472148124636877
- El-MallawanyNKKamiyangoWSloneJSClinical factors associated with long-term complete remission versus poor response to chemotherapy in HIV-infected children and adolescents with Kaposi sarcoma receiving bleomycin and vincristine: a retrospective observational studyPloS One2016114e015333527082863
- MackenMDaleHMoyoDTriple therapy of vincristine, bleomycin and etoposide for children with Kaposi sarcoma: Results of a study in Malawian childrenPediatr Blood Cancer2018652
- KocsardEKaposi sarcoma in a Chinese boy (aged 16 years) with localisation on the left lower extremity and on the right caruncula lacrimalisDermatologica1949991434818139274
- WabingaHRParkinDMWabwire-MangenFNamboozeSTrends in cancer incidence in Kyadondo County, Uganda, 1960–1997Br J Cancer20008291585159210789729
- KaminerBMurrayJFSarcoma idiopathicum multiplex haemorrhagicum of Kaposi, with special reference to its incidence in the South African Negro, and two case reportsS Afr J Clin Sci19501112515418312
- GrulichAEBeralVSwerdlowAJKaposi’s sarcoma in England and Wales before the AIDS epidemicBr J Cancer1992666113511371457354
- GeddesMFranceschiSBarchielliAKaposi’s sarcoma in Italy before and after the AIDS epidemicBr J Cancer19946923333368297730
- DollardSCButlerLMJonesAMSubstantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi’s sarcoma belt”Int J Cancer2010127102395240120143397
- Cook-MozaffariPNewtonRBeralVBurkittDPThe geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemicBr J Cancer19987811152115289836488
- ReesCAKeatingEMLukolyoHMapping the epidemiology of Kaposi sarcoma and non-Hodgkin lymphoma among children in sub-Saharan Africa: a reviewPediatr Blood Cancer20166381325133127082516
- RohnerEValeriFMaskewMIncidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in Southern Africa: a prospective multicohort studyJ Acquir Immune Defic Syndr201467554755425393941
- ParkinDMSitasFChirenjeMSteinLAbrattRWabingaHPart I: Cancer in indigenous Africans—burden, distribution, and trendsLancet Oncol20089768369218598933
- ButlerLMWereWABalinandiSHuman herpesvirus 8 infection in children and adults in a population-based study in rural UgandaJ Infect Dis2011203562563421273188
- PfeifferRMWheelerWAMbisaGGeographic heterogeneity of prevalence of the human herpesvirus 8 in sub-Saharan Africa: clues about etiologyAnn Epidemiol2010201295896321074111
- DedicoatMNewtonRReview of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcomaBr J Cancer20038811312556950
- ChangYCesarmanEPessinMSIdentification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcomaScience19942665192186518697997879
- CrabtreeKLWojcickiJMMinhasVKankasaCMitchellCWoodCAssociation of household food- and drink-sharing practices with human herpesvirus 8 seroconversion in a cohort of Zambian childrenJ Infect Dis2017216784284928961804
- WakehamKWebbELSebinaIRisk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in UgandaJ Acquir Immune Defic Syndr201363222823323403859
- AndersonLALiYGraubardBIHuman herpesvirus 8 serop-revalence among children and adolescents in the United StatesPediatr Infect Dis J200827766166418536622
- ButlerLMDorseyGHladikWKaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmissionJ Infect Dis2009200343043819534596
- El-MallawanyNKWasswaPMteteIIdentifying opportunities to bridge disparity gaps in curing childhood cancer in Malawi: malignancies with excellent curative potential account for the majority of diagnosesPediatr Hematol Oncol201734526127429190181
- Mittermayer-VassalloKBandaKMolyneuxEMKaposi sarcoma in HIV-seronegative children presenting to the paediatric oncology ward in The Queen Elizabeth Central Hospital, Blantyre, Malawi during 2002–2014Trop Doct201646313814226620689
- JacksonCCDicksonMASadjadiMKaposi sarcoma of childhood: inborn or acquired immunodeficiency to oncogenic HHV-8Pediatr Blood Cancer201663339239726469702
- MbulaiteyeSMKatabiraETWabingaHSpectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match StudyInt J Cancer2006118498599016106415
- ChintuCAthaleUHPatilPSChildhood cancers in Zambia before and after the HIV epidemicArch Dis Child1995732100104 discussion 104–1057574850
- AthaleUHPatilPSChintuCElemBInfluence of HIV epidemic on the incidence of Kaposi’s sarcoma in Zambian childrenJ Acquir Immune Defic Syndr Hum Retrovirol199581961008548353
- Dryden-PetersonSMedhinHKebabonye-PusoentsiMCancer incidence following expansion of HIV treatment in BotswanaPloS One2015108e013560226267867
- ParkinDMNamboozeSWabwire-MangenFWabingaHRChanging cancer incidence in Kampala, Uganda, 1991–2006Int J Cancer201012651187119519688826
- El-MallawanyNKVillieraJKamiyangoWIncreasing numbers of new Kaposi sarcoma diagnoses in HIV-infected children and adolescents despite the wide availability of antiretroviral therapy in MalawiClin Infect Dis201764681881928077522
- SiegelDAHenleySJLiJPollackLAVan DyneEAWhiteARates and trends of pediatric acute lymphoblastic leukemia - United States, 2001–2014MMWR Morb Mortal Wkly Rep2017663695095428910269
- AntmanKChangYKaposi’s sarcomaN Engl J Med2000342141027103810749966
- HenggeURRuzickaTTyringSKUpdate on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapyLancet Infect Dis20022528129212062994
- El-MallawanyNKKamiyangoWVillieraJProposal of a risk-stratification platform to address distinct clinical features of pediatric Kaposi sarcoma in Lilongwe, MalawiJ Glob Oncol2017JGO170005429272148
- HerceMEKalangaNWroeEBExcellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural MalawiJ Int AIDS Soc2015181992926028156
- VenkataramanGUldrickTSAlemanKBone marrow findings in HIV-positive patients with Kaposi sarcoma herpesvirus-associated multicentric Castleman diseaseAm J Clin Pathol2013139565166123596117
- UldrickTSWangVO’MahonyDAn interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman diseaseClin Infect Dis201051335035820583924
- PolizzottoMNUldrickTSHuDYarchoanRClinical manifestations of Kaposi sarcoma herpesvirus lytic activation: multicentric Castleman disease (KSHV-MCD) and the KSHV inflammatory cytokine syndromeFront Microbiol201237322403576
- BhutaniMPolizzottoMNUldrickTSYarchoanRKaposi sarcoma-associated herpesvirus-associated malignancies: epidemiology, pathogenesis, and advances in treatmentSemin Oncol201542222324625843728
- AnglemyerAAgrawalAKRutherfordGWTreatment of Kaposi sarcoma in children with HIV-1 infectionCochrane Database Syst Rev20141CD009826
- BowerMWeirJFrancisNThe effect of HAART in 254 consecutive patients with AIDS-related Kaposi’s sarcomaAIDS200923131701170619550283
- BowerMDalla PriaACoyleCProspective stage-stratified approach to AIDS-related Kaposi’s sarcomaJ Clin Oncol201432540941424378415
- KrownSETreatment strategies for Kaposi sarcoma in sub-Saharan Africa: challenges and opportunitiesCurr Opin Oncol201123546346821681092
- KrownSEBorokMZCampbellTBStage-stratified approach to AIDS-related Kaposi’s sarcoma: implications for resource-limited environmentsJ Clin Oncol201432232512251325002726
- CampbellLBachaJSloneJSEl-MallawanyNKMwitaLMehtaPSCharacteristics and outcomes of pediatric Kaposi sarcoma patients in the southern highlands zone of TanzaniaPaper presented at: 10th International Conference on Cancer in Africa (AORTIC)November 20, 2015Marrakech, Morocco
- LetangELewisJJBowerMImmune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UKAIDS201327101603161323462220
- MwafongoAARosenbergNENg’ambiWTreatment outcomes of AIDS-associated Kaposi’s sarcoma under a routine antiretroviral therapy program in Lilongwe, Malawi: bleomycin/vincristine compared to vincristine monotherapyPloS One201493e9102024632813
- GoncalvesPHZiegelbauerJUldrickTSYarchoanRKaposi sarcoma herpesvirus-associated cancers and related diseasesCurr Opin HIV AIDS2017121475627662501
- DittmerDPDamaniaBKaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapyJ Clin Invest201612693165317527584730
- ChadburnASaidJGratzingerDHHV8/KSHV-positive lymphoproliferative disorders and the spectrum of plasmablastic and plasma cell neoplasms: 2015 SH/EAHP workshop report-part 3Am J Clin Pathol2017147217118728395104
- MylonaEEBaraboutisIGLekakisLJGeorgiouOPapastamopoulosVSkoutelisAMulticentric Castleman’s disease in HIV infection: a systematic review of the literatureAIDS Rev2008101253518385778
- PolizzottoMNUldrickTSWyvillKMClinical features and outcomes of patients with symptomatic Kaposi sarcoma herpesvirus (KSHV)-associated inflammation: prospective characterization of KSHV inflammatory cytokine syndrome (KICS)Clin Infect Dis201662673073826658701
- GoncalvesPHUldrickTSYarchoanRHIV-associated Kaposi sarcoma and related diseasesAIDS201731141903191628609402
- KaplanLDHuman herpesvirus-8: Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphomaHematology Am Soc Hematol Educ Program2013201310310824319170
- SakakibaraSTosatoGViral interleukin-6: role in Kaposi’s sarcoma-associated herpesvirus: associated malignanciesJ Interferon Cytokine Res2011311179180121767154
- MoorePSBoshoffCWeissRAChangYMolecular mimicry of human cytokine and cytokine response pathway genes by KSHVScience19962745293173917448939871
- PolizzottoMNUldrickTSWangVHuman and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman diseaseBlood2013122264189419824174627
- GopalSFedoriwYMontgomeryNDMulticentric Castleman’s disease in MalawiLancet20143849948115825241721
- HosseinipourMCSweetKMXiongJViral profiling identifies multiple subtypes of Kaposi’s sarcomaMBio201455e016330171425249280
- MillerHSloneJSRaabeEEl-MallawanyNKMehtaPPhelpsBRLessons from pediatric HIV: a case for curative intent in pediatric cancer in LMICsPediatrics20171404
- de AmicisTStudio clinico ed anatomo-patologico su dudici nuove osservazioni di dermo-polimelano-sarcoma idiopaticoTipografia A. Trani. [Clinical and anatomy-pathological study on new dermatological observations of idiopathic dermo-polimelano-sarcoma. A. Trani typography]Naples, Italy1882 Italian
