Abstract
Autism spectrum disorder (ASD) is a genetically determined neurodevelopmental brain disorder presenting with restricted, repetitive patterns of behaviors, interests, and activities, or persistent deficits in social communication and social interaction. ASD is characterized by many different clinical endophenotypes and is potentially linked with certain comorbidities. According to current recommendations, children with ASD are at risk of having alimentary tract disorders – mainly, they are at a greater risk of general gastrointestinal (GI) concerns, constipation, diarrhea, and abdominal pain. GI symptoms may overlap with ASD core symptoms through different mechanisms. These mechanisms include multilevel pathways in the gut–brain axis contributing to alterations in behavior and cognition. Shared pathogenetic factors and pathophysiological mechanisms possibly linking ASD and GI disturbances, as shown by most recent studies, include intestinal inflammation with or without autoimmunity, immunoglobulin E-mediated and/or cell-mediated GI food allergies as well as gluten-related disorders (celiac disease, wheat allergy, non-celiac gluten sensitivity), visceral hypersensitivity linked with functional abdominal pain, and dysautonomia linked with GI dysmotility and gastroesophageal reflux. Dysregulation of the gut microbiome has also been shown to be involved in modulating GI functions with the ability to affect intestinal permeability, mucosal immune function, and intestinal motility and sensitivity. Metabolic activity of the microbiome and dietary components are currently suspected to be associated with alterations in behavior and cognition also in patients with other neurodegenerative diseases. All the above-listed GI factors may contribute to brain dysfunction and neuroinflammation depending upon an individual patient’s genetic vulnerability. Due to a possible clinical endophenotype presenting as comorbidity of ASD and GI disorders, we propose treating this situation as an “overlap syndrome”. Practical use of the concept of an overlap syndrome of ASD and GI disorders may help in identifying those children with ASD who suffer from an alimentary tract disease. Unexplained worsening of nonverbal behaviors (agitation, anxiety, aggression, self-injury, sleep deprivation) should alert professionals about this possibility. This may shorten the time to diagnosis and treatment commencement, and thereby alleviate both GI and ASD symptoms through reducing pain, stress, or discomfort. Furthermore, this may also protect children against unnecessary dietary experiments and restrictions that have no medical indications. A personalized approach to each patient is necessary. Our understanding of ASDs has come a long way, but further studies and more systematic research are warranted.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with a strong genetic basis and encompasses various conditions with seemingly different phenotypes. ASD is clinically present before the age of 3 years with symptoms concerning mainly two areas which are restricted, repetitive patterns of behaviors, interests, or activities, and persistent deficits in social communication and social interaction.Citation1–Citation3 This “dyad”, implemented in Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 as of 2013, is a reduction of the previous “triad” outlined in DSM-4. Under the new definition of DSM-5, ASD includes what would have been previously assigned as Asperger’s disorder, childhood disintegrative disorder, pervasive developmental disorder not otherwise specified, and autism.Citation4,Citation5 The phenotypic variability of ASD also includes different levels of intellectual development/maturation with dysfunctions of cognition and perception (which are present in 50%–75% of patients) correlating with severity of autistic behavior.Citation4
The incidence of ASD has been rising in the world in recent years. Epidemiological studies in the USA indicate that in the year 1978, 1/10,000 children and adolescents were diagnosed with autism. Ten years later, in 1998, the index was 1/250 children between the ages of 3 years and 10 years, and in 2007, 1/150 children at the age of 8 years. The overall prevalence of ASD in 2010, according to latest data from the Centers for Disease Control and Prevention, was 1/68 children.Citation6–Citation8
Greater incidence is found among boys (4:1).Citation6,Citation7 The reasons for such a significant rise in the prevalence of ASD are not clear.
Our current understanding of the pathogenesis of this condition takes into account genetics along with the environment playing a role as an epigenetic phenotype trigger.Citation9–Citation12 Individualized genetic predispositions aided by trigger factors (infections, toxins, trauma, etc) impair embryogenesis as well as peri- and postnatal development of the child. Maternal genetic factors as well as environmental factors affecting the mother before and during pregnancy also seem to play a role in the pathogenesis of ASD.Citation13 The genetic architecture of autism has proven to be complex and heterogeneous as shown by studies of cytogenetics, linkage, association, whole-genome linkage or association, and whole-genome or exome sequencing.Citation14,Citation15 A complex, multigene model of inheritance implicates the interactions of several genes with most being involved in neurodevelopmental processes (neuronal synaptogenesis, neurotransmitter transformation, neurometabolic conditions, lipid metabolism, and mitochondrial dysfunction).Citation12,Citation16–Citation18 Candidate genes include, among others, MeCP2 (Xq28; Rett syndrome), HOXA-1 (7p15.3) and HOXB-1 (7q21.32; embryonal development of the rhombencephalon), EN2 (7q36.3; developmental regulation of the cerebellum), FOXP-2 (7q31.1; speech disorder), WNT2 (7q31-33; fetal development and social deficits), 5-HTT (17q11.1-q12; hyperserotonemia), GABRA3 (15q11-q13; GABAergic neurotransmission), OXTR 2 (3p26.2; decreased serum oxytocin levels), ASL (22q13.1-q13.2; impairment of purine biosynthesis), and PTEN (macrocephaly, tumor growth).Citation4,Citation16,Citation19–Citation22 Other mono-genetic disorders, such as fragile X syndrome, tuberous sclerosis, Down’s syndrome, phenylketonuria, neurofibromatosis type I, or Angelman syndrome, are found in 10%–15% of patients with ASD.Citation23 Newer and newer genetic testing techniques and technologies are being used to investigate ASD and to develop a screening test which could help identify and diagnose ASD at a much earlier age than what is currently possible.Citation24,Citation25
How autism is viewed has changed greatly since its first description in 1943 by Leo Kanner.Citation26 What was once viewed as an untreatable disease solely concerning the brain is now considered a dysfunction of the central nervous system (CNS) with accompanying disorders of the body in general as well as different organs/systems such as the immunological system or digestive tract.Citation27–Citation29 Genetic polymorphism is associated with variability of the clinical presentation of ASD and with comorbid problems. Comorbidity is common, and >70% of affected individuals have other concurrent conditions such as epilepsy (30%), gastrointestinal (GI) problems (9%–70%), immunodysregulation (38%), or sleep disorders (50%–80%).Citation14 There is no consensus so far concerning how the GI tract is implicated in the pathophysiology of ASD. A search within the Cochrane Library (keywords being autism, gastrointestinal, and diet) resulted in but a few randomized studies. Controlled research studies/trials are evidently needed. This area of medicine gives rise to many questions of which a majority are still waiting for precise and definitive answers.Citation30 This paper addresses the possible links between autism and GI disorders.
Clinical symptoms of digestive tract diseases in children with ASD
Clinical manifestation of digestive tract diseases in children with ASD may differ as compared to children with typical development, and the diagnosis of a GI disorder in children may be more difficult and delayed in time. Subjective symptoms such as pain, discomfort, heartburn, or nausea are very difficult to assess and interpret because of core ASD symptoms such as difficulties in verbal and nonverbal communication as well as an altered perception of pain.Citation30,Citation31
The frequency of GI tract symptoms in children with ASD ranges from 9% to 84% depending on the method (retrospective and prospective studies) compared with 9%–37% for children without ASD.Citation30,Citation32–Citation36 A study by Koves et al observed clinical symptoms from the GI tract in 84.1% of children with ASD compared to 31.2% of children from a control population (where participants studied included siblings).Citation37 However, a study by Black et al, based on a review of hospital documents, showed that GI tract diseases were diagnosed equally frequent in children with ASD and in a control population (9% vs 9%).Citation32 A summary of individual studies using meta-analysis indicates that children with ASD have a greater risk of GI symptoms than those without ASD.Citation31 In a systematic search involving 15 studies and 2,215 children with ASD published in 2014 by McElhanon et al, four clinical variables (general GI concerns, diarrhea, constipation, and abdominal pain) met the six study thresholds for inclusion in the analysis.Citation31 The main results of the meta-analysis showed that children with ASD, in contrast to comparison groups, experienced significantly more general GI symptoms (odds ratio [OR] 4.42), higher rates of diarrhea (OR 3.63), constipation (OR 3.86), and abdominal pain (OR 2.45).
Most common GI symptoms include overproduction of intestinal gasses/flatulence (60%), bloating (38%), abdominal pain (378%), diarrhea (28%), burping/belching (25%), gastroesophageal reflux symptoms (16%), and constipation (10%) ().
Figure 1 GI symptoms (left side) and GI disorders (right side) described in children with autism spectrum disorder.
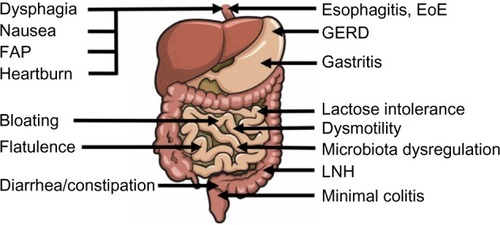
Although diarrhea occurred as the second most common GI symptom in terms of frequency, the definition of diarrhea varied among the included studies, and as such, these results should be viewed with caution. A standardized tool such as the visual Bristol Stool Scale (for a more detailed, consistent examination of possible GI symptoms such as diarrhea and constipation) is recommended to assess GI issues among children with ASD.Citation31
Functional constipation without an underlying pathophysiological cause has been suggested to be present in most individuals with ASD.Citation31 A study by Afzal et al noted moderate-to-severe constipation in 36% of children with ASD compared to 9% in a control population.Citation38 Absent or delayed acquisition of bowel training secondary to difficulty with sensory stimuli, sensory processing, and motor problems may lead to altered GI motility and defecation physiology. Increased intestinal transit time may also result from a high intake of processed food and a lack of fiber-containing fruits and vegetables as a result of severe food selectivity.Citation30,Citation31 Increased intake of refined food products in children with ASD has led to parents reporting worsening of behavioral and GI symptoms. Symptoms such as stomach pains, bloating, or increased flatulence may potentially be explained by the overdosing of carbohydrates or enzyme deficiencies of the mucosal membrane responsible for symptoms of food intolerance. Common examples include lactose intolerance, or intolerance to excess fermentable oligo- and disaccharides, monosaccharides, and polyols (FODMAPs) or lactulose.Citation39 A list of FODMAPS includes fructans, galactans, fructose, and polyols that are contained in several foodstuffs, including wheat, vegetables, and milk derivatives.Citation40 Decreased disaccharidase activity (ie, of lactase, maltase, sucrose glucoamylase, palatinase) associated with functional GI abnormalities was reported in children with autism; however, pancreatic enzymes (amylase, lipase, trypsin, chymotrypsin, carboxypeptidase) did not show a reduction in their activity.Citation15,Citation37,Citation41 According to current guidelines, as a first step toward a diagnosis of lactose intolerance, empiric trials of lactase supplementation or dietary restriction of lactose may be considered, in the proper clinical context, before referral to a gastrologist or allergologist.Citation30 Congenital predispositions (enzyme defects) and acquired conditions (inflammation of the bowel, intestinal microbial imbalance, or antibiotic treatment side effects) are being reported as causes of lactose intolerance in children with autism. Abdominal pain without underlying abnormal laboratory results or diagnostic imaging is classified as functional abdominal pain (FAP). FAP is linked with hyperalgesia, a type of visceral hypersensitivity to pain sensation. Brain dysfunction may be involved in visceral hypersensitivity as shown by changes in cerebral blood flow during visceral stimulation measured using positron emission tomography, functional magnetic resonance imaging, or single-photon emission computed tomography.Citation42
GI problems including feeding problems, dysphagia, nausea, bloating, profound constipation, or diarrhea can reflect functional GI dysmotility (oropharyngeal, esophageal, and bowel dysmotility). These problems as well as FAP can be considered as symptoms of dysautonomia.Citation43 The enteric nervous system, described as a “second brain”, is a part of the autonomic nervous system (ANS) that directly controls the GI system being involved in gut motility, local secretion, and absorption.Citation14 Dysautonomia is the disorder of the ANS associated with several diseases, also with autism, but often misdiagnosed and left untreated.Citation44 Dysautonomia encompasses abnormal reflex adaptive reactions of control mechanisms in the brain and/or peripheral distribution of the sympathetic and parasympathetic nervous systems. It is hypothesized that limbic system control of the ANS is affected, especially damage to the amygdala (eg, by seizures).Citation45 In children with autism, dysautonomia may manifest in many ways: it may be expressed in behaviors such as “hand flapping” or usage of peripheral vision which may be explained as attempts to avoid “unpleasant autonomic sensations” caused by stimulation of visual pathways that disturb sympathetic/parasympathetic homeostasis.
Association between GI disorders and behavior in children with ASD
The presence of GI disorders in children with ASD poses the question about a possible association between GI disorders and severity of ASD symptoms. Behavior characteristics hypothesized to be expressions of GI problems are common in children with ASD. Facial grimacing, teeth gritting, excessive chewing (of food or items) belong to frequently observed facial expressions of GI symptoms in children with autism. Accompanying vocal behaviors such as sobbing, screaming, or delayed echolalia may also be present. Motor behaviors such as the child placing pressure on the stomach with their own hands or objects, including chairs or tables, may be associated with the abdominal area and are commonly reported by parents and/or caregivers. Such behaviors may be collectively described as abdominal behaviors. Facial expressions and abdominal behaviors may be present as separate entities or coexist with general motor behaviors. General motor behaviors typical for this group of children include self injuries, increased repetitive/stereotypic movements, unusual posturing, or tapping/twitching. Stomach pain or abdominal discomfort in children with ASD may be indicative of motoric excitation which in turn may have an effect on the general state of the child expressed as irritability, oppositional behavior, or sleep disturbances.Citation30 Children with abdominal pain are more likely to present with these psychological disorders, anxiety, behavioral problems, or other psychological symptoms. FAP or discomfort in individuals with ASD may be expressed by various vocal and motor behaviors as listed in a consensus report.Citation30
Associations between GI symptoms and ASD symptoms have been analyzed in previous studies. Maenner et al state that there are no associations between the presence of GI problems and two of the hypothesized behaviors characterized in patients with ASD: stereotypic/repetitive behaviors and self-injurious behaviors.Citation46 In a study by Gorrindo et al, GI dysfunction was significantly associated with sleep disorders and food intolerance but not with irritability or aggressiveness.Citation47 In their study, the most common type of GI disorder in children with ASD (85.0%) was functional constipation and was significantly associated with language impairment. Associations between behavioral problems and GI symptoms were also studied among children with varying subtypes of ASD (ie, high-functioning autism, atypical autism with delayed neurodevelopment).Citation33 In 95 children with high-functioning autism and intelligence quotient (IQ) scores >80, a majority (61%) had at least one reported GI symptom. Children with and without GI problems did not differ in autism symptom severity, adaptive behavior, or total internalizing or externalizing problem scores. However, participants with GI problems had significantly higher levels of affective problems.Citation48 According to Mouridsen et al, children diagnosed with atypical autism and with an IQ <70 are at higher risk of having hospital-diagnosed GI problems than those with an IQ >70. Overall, children with atypical autism had about the same frequency of gastric, intestinal, and hepatic diseases as controls.Citation49 Furthermore, Smith et al confirmed previously reported findings of an increased incidence of bowel symptoms in children with autism and other developmental and neurological disorders than in healthy children.Citation50
Sleep problems are integral components of both the GI system and ASD. Extradigestive symptoms of the alimentary tract include difficulty falling sleep and difficulty staying asleep and have been found in 44%–83% of children with ASD.Citation41,Citation51 Horvath and Perman reported disturbed sleep and night-time awakening in 52% of children with ASD who had GI symptoms (vs 7% of age-matched healthy siblings; P<0.001). Children with ASD who had reflux esophagitis exhibited unexplained irritability more frequently (43%) than those who did not (13%).Citation37 These observations lead to the conclusion that monitoring of sleep quality may be useful during evaluation of treatment effectiveness in GI disorders in children with ASD. Sleep deprivation may have significant health consequences and may worsen cognitive function by exerting an inhibitory action on the proliferation of neurons in the CNS in children with ASD. Neuroendocrine imbalances caused by sleep disorders are comparable to consequences of chronic exposure to stress. The effects of long-term sleep deprivation include inhibition of neurogenesis in the dentate gyrus of the hippocampus with dependent increases in circulating levels of adrenal corticosteroids.Citation52 These aspects should not be ignored, and treatment of GI disorders leading to improvement of sleep quality may prove to be beneficial for a child with ASD and may also serve as an indirect argument linking GI disorders and ASD.
Diagnostic evaluation of GI disorders in children with ASD
The diagnostic evaluation of GI disorders with behavioral manifestations in patients with ASD can be very complex.Citation53 Diagnostic evaluations of GI disorders include a medical history, physical examination, laboratory tests, imaging/radiological studies, functional studies, and endoscopy. Many authors agree that aggressive diagnostic procedures should be judiciously applied in children with autism. In light of this, less invasive methods are recommended before any planned hospitalizations (such as a diagnostic trial with a proton pump inhibitor in gastroesophageal reflux disease before resorting to a pH-meter or an osmotic laxative such as polyethylene glycol 3350 in cases involving constipation). A consensus report recommends that the initial diagnostic evaluations of GI symptoms and disorders in individuals with ASD be chosen dependent on the type of symptoms ().Citation53
Table 1 Initial diagnostic procedures for children with autism spectrum disorder depending on clinical GI disorders
Endoscopic and pathological findings in children with ASD
Endoscopy, although not a first-line approach to each child with ASD, may have high diagnostic value and be crucial for therapy in certain individuals.Citation30 Results of upper and lower endoscopy in children with ASD show that all levels of the GI tract, from the esophagus to the colon/rectum, may be affected. Esophagogastroduodenoscopy results reported by Koves et al showed reflux esophagitis in 69%, chronic gastritis in 42%, and chronic duodenitis with Paneth cell hypertrophy in 67% of patients.Citation15 Results of colonoscopy studies showed ileocolitis and subtle mucosal inflammatory infiltrates of the small and large intestines as common histopathological findings in children with ASD and GI symptoms.Citation54,Citation55 A low-grade chronic inflammation of the intestinal mucosa and a GI mucosal molecular profile that overlapped significantly with prodromal phases of inflammatory bowel disease (IBD) have been diagnosed in some children with ASD.Citation56 In comparison to IBD, there were no inflammatory markers present in the stool. Fernell et al, studying two independent indicators of IBD, the concentration of nitric oxide in the rectum and calprotectin levels in the feces, are of the opinion that there is no evidence linking autism with active colitis.Citation57 Although a low-grade chronic inflammation of the intestinal mucosa is suggested in some cases, the existence of a GI abnormality specific solely to persons with ASD (ie, “autistic enterocolitis”) has not been established.Citation30 Similarly, lymphonodular hyperplasia (LNH) on the mucosa of the lower GI tract described in some children with autism is a nonspecific reactive sign, common in other clinical situations in children without autism.Citation57 This condition may be the result of long-term retention of intestinal contents (constipation), local infections, parasitic infestations, and chronic exposure to food allergens but may also occur in healthy children.Citation58 Scandinavian authors described regression of LNH in children with cow’s milk protein allergy after treatment with an elimination diet (milk-free).Citation59 Therefore, LNH found in children with ASD may be considered as a secondary response of the intestinal mucosa to food proteins.
Immune-mediated GI food allergies
GI food allergies are a spectrum of disorders that result from adverse immune responses to dietary antigens. Immunologic GI reactions to dietary proteins are classified as immunoglobulin (Ig) E mediated, non-IgE mediated, or mixed ().Citation60–Citation62
Table 2 Classification of immune-mediated gastrointestinal food allergies
A diagnosis of an immuno-based inflammatory process in the alimentary tract serves as a biological basis to implement a therapeutic elimination diet in accordance with current guidelines for diagnosing food allergy.Citation61–Citation63
The diagnosis of IgE-mediated food allergy is based on a patient’s history, skin prick tests, and/or serum allergen-specific IgE, or if available, on molecular-based allergy diagnostics using purified natural or recombinant allergenic molecules (allergen components) instead of allergen extracts.Citation64 The diagnosis of a non-IgE-mediated allergy characterized by nonspecific symptoms is frequently delayed because of insufficient definitive diagnostic biomarkers; however, atopy patch tests with foods may be useful in same cases. An oral food challenge (OFC) is recommended to establish a definitive diagnosis. Amelioration or resolution of symptoms resulting from an elimination diet and relapse of symptoms during an OFC are now accepted as indications for continuation of diet therapy. OFC results should be monitored and reassessed periodically.
IgE-mediated reactions and mast cell activation could contribute to immune and neuroinflammatory abnormalities in patients with ASD.Citation65 Diet-related intestinal histopathological findings in children with ASD were observed by Ashwood et al.Citation66 The researchers found that children with autism treated with an elimination diet (without gluten or casein) had a significantly smaller number of eosinophils in inflammatory bowel infiltrates compared with a group of children with autism not treated with an elimination diet. Eosinophils in inflammatory infiltrates of the mucosa are a component of allergic esophagitis and allergic gastroenteritis, and IgE-mediated and/or cell-mediated chronic inflammatory disorders.Citation67 Eosinophilic esophagitis has been defined as a clinicopathologic disorder characterized by symptoms related to esophageal dysfunction and pathologically related to eosinophil-predominant inflammation.Citation68 Clinically, the disease can present in children with characteristic symptoms such as dysphagia or food impaction but may also present with various nonspecific symptoms such as abdominal pain, vomiting, reflux-related symptoms, and failure to thrive in young children. An allergic etiology of eosinophilic inflammation has been strongly suggested in a majority of patients with evidence of food or aeroallergen hypersensitivity where treatment included the avoidance of specific foods and airborne allergens.Citation69 Three elimination diets have been developed for patients with eosinophilic esophagitis: amino acid-based formula, targeted elimination diet, and empiric elimination diet.Citation67
There are currently no conclusive studies confirming that food allergy is more prevalent in children with ASD than in the general population, and recommendations aimed for the general population are accepted. However, the prevalence of GI food allergies may be underestimated because they can be difficult to diagnose even based on endoscopic and histopathological findings. Additionally, endoscopy with anesthesia is not recommended to routinely diagnose food allergy in children with ASD. The exceptions include diagnosis of celiac disease (CD; gluten-sensitive enteropathy) for which this diagnostic approach is strongly recommended.
Gluten-related immune- mediated disorders
Gluten-related disorders may present with different clinical outcomes and include autoimmune forms (CD, gluten ataxia, and dermatitis herpetiformis), allergy (wheat allergy [WA]) and non-autoimmune, nonallergic disorders (non-celiac gluten sensitivity [NCGS]).Citation39,Citation70 CD is an immune-mediated enteropathy triggered by the ingestion of gluten-containing grains in genetically susceptible individuals. Tissue transglutaminase 2 (TG2) is the primary autoantigen of CD, and anti-tissue TG2 antibodies are used as a serological marker of CD.Citation40 The frequency of ASD coexisting with CD or other reactions to gluten such as NCGS or WA is unknown. In a study by Koves et al, serological testing for CD turned out negative in 400 patients with ASD.Citation15 However, in a recent study, Buie points out that with the high prevalence of both autism and CD (one in 133 individuals), one should expect that in a certain number of people, these two will coexist simultaneously.Citation71 Similarly, Klein et al also draw attention to endophenotypes of autism which present with CD.Citation72 A study by Lau et al showed increased immune reactivity to gluten (IgG anti-gliadin antibody) in a subset of children with autism with GI symptoms.Citation73 The levels of other CD-specific serologic markers, that is, antibodies to deamidated gliadin peptides and anti-tissue TG2, did not differ between patients and controls. An association between increased anti-gliadin antibodies and the presence of HLA-DQ2 and/or HLA-DQ8 was not observed. The authors concluded that this immune reactivity to gluten was by a mechanism which appeared to be distinct from that in CD.
Gluten immune-mediated disorders differ in serologic profile depending on the target organ. Organ-specific gluten reactions involve immune responses toward other TG isoforms including TG3 (epidermal TG expressed in the skin, linked with dermatitis herpetiformis) and TG6 (expressed in the brain, linked with gluten ataxia).Citation74 It is suggested that TG activity is involved in molecular mechanisms responsible for the pathogenesis of neurodegenerative diseases.Citation75 Increased prevalence of TG6 antibodies was found in sera from schizophrenia patients by Cascella et al.Citation76 The authors emphasize that as TG6 is primarily expressed in the brain, serum TG6 IgA antibodies represent a marker of neuroinflammation.
Given the above-mentioned studies, one may pose the question if the pathophysiology of neuroinflammation in autism may be linked with gluten by a TG6 isoform, especially in those children with evidence of immune dysregulation and abnormalities of intestinal permeability. This issue remains to be resolved.
Gluten-related disorders include gluten sensitivity (NCGS) characterized by intestinal and extra-intestinal symptoms related to the ingestion of gluten-containing food in subjects who are not affected with either CD or WA.Citation40 NCGS presents with typical GI symptoms such as abdominal pain and chronic diarrhea and other extra-intestinal manifestations. Several studies suggested a relationship between NCGS and neuropsychiatric disorders (autism, schizophrenia).Citation40
At present, the knowledge on gluten-related disorders in autism is preliminary, and more research is required to develop criteria for identifying subgroups of children with autism who would benefit from a gluten-free diet.
Hypothesis of intestinal barrier integrity impairment
Leaky gut syndrome as a sign of enteropathy in children with ASD has been discussed by many and confirmed by some authors.Citation77 The intestinal barrier is multi-structured and involved in epithelial cell integrity, epithelial transcellular and paracellular permeability, innate immune response, and mucus production. Mucosal immune cells constitute approximately 70% of the immune cells within the body, and dysfunction in these cells may result in systemic consequences.Citation54 Gut-associated lymphoid tissue is a component of mucosa-associated lymphoid tissue. Decreased mucosal immunity may predispose to neuroimmune abnormalities and increased autoimmune responses, especially in children with a positive family history of autoimmunity.Citation14,Citation78,Citation79 Only one layer of intestinal epithelial cells (epithelium) separate the contents of the intestinal lumen and access to a number of immune cells in the lamina propria of the mucosa and internal environment of the body. Tightness between epithelial cells depends on intricate connections located on the lateral surface of the cell membrane.Citation80 The three main parts of this complex are the following: first, tight junctions which allow for close contact between cell membranes by way of numerous proteins (occludins, claudins, junction adhesion molecules); second, adherens junctions with dynamic properties, thanks to the activity of contracting actin microfilaments; and third, desmosomes – acting as single-point connections with mechanical functions.Citation80 Increased intestinal permeability may be genetically determined as in the case of IBDs but may also result from inflammation or chronic emotional stress.Citation81,Citation82
Increased permeability of the intestinal barrier has been described in 43%–76% of children with ASD both with and without GI symptoms.Citation37,Citation83 The leaky gut hypothesis was investigated by Navarro et al in a randomized double-blind, placebo-controlled study on the effects of gluten and milk on intestinal permeability and behavior in children with ASD over a period of 4 weeks.Citation84 The study, although underpowered to show small differences, did not support an association between dietary gluten/milk, intestinal permeability, and behavioral changes in subjects with ASD. The evidence of abnormal GI permeability in individuals with ASD is still limited, and prospective studies should be performed to determine the role of abnormal permeability in ASDs.Citation30
Preclinical evidence of gut microbiota effects on the brain and the “missing microbiota hypothesis”
Renaissance of the gut microbiota
In 1909, Kendall wrote that the alimentary tract is the perfect incubator providing an appropriate temperature and a steady supply of nutrition for intestinal flora which in turn may affect the general health state of the host.Citation85 This observation is currently undergoing a certain renaissance. Studies with gnotobiotic animals (specifically germ-free [GF] mice) are providing much information on the microbiome’s influence on the host organism. GF mice lacking a microbiome are characterized by a high susceptibility to infection, decreased activity of digestive enzymes, decreased production of cytokines, small variance in Igs, smaller body mass than mice raised in normal conditions, no possibility to induce obesity through a specific diet, and reduced social interaction.Citation86 Although these experimental studies may not be extrapolated to a human population, they provide, however, incentive to further study the microbiota in humans. To date, there is rapidly increasing evidence of host–microbe interaction at virtually all levels of complexity, ranging from direct cell-to-cell communication to extensive systemic signaling involving various organs and organ systems, including the CNS. As such, the discovery that different microbial compositions are associated with alterations in behavior and cognition has significantly contributed to establishing the microbiota–gut–brain axis as an extension of the well-accepted concept of the gut–brain axis.Citation87 The influence of gut and microbiota on human health is notably studied in patients with autoimmune diseases and those diagnosed with neuropsychiatric disorders, including autism.Citation88–Citation91
How does the gut talk to the brain?
The gut and brain are closely informed about each other. Bidirectional gut–brain communication involves different pathways and elements of the gut connectome (enteric neural network). Afferent, gut–brain, signaling involves the enteroendocrine system, cytokines, sensory epithelial cells, and intestinal microbiota ().
Figure 2 Gut–brain axis.
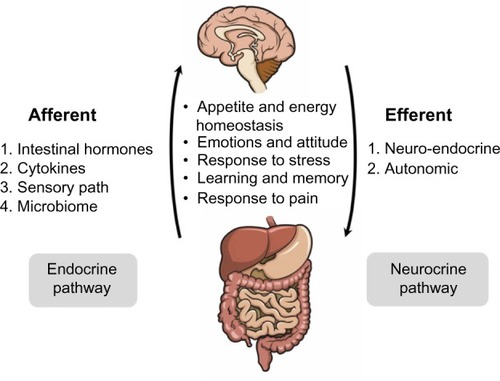
Efferent, brain–gut, signaling includes neuroendocrine and autonomic regulation.Citation86 This gut–brain axis modulates the body’s metabolism and behavior by taking part in the regulation of appetite and energy homeostasis, stress response, emotions and attitude, learning and memory, and response to pain.Citation92
The homology between the gut–blood barrier and blood–brain barrier
The homology between the gut–blood barrier (GBB) and blood–brain barrier (BBB) may play an important role in the multipotent communication between the gut and brain. The BBB, as a selective permeability barrier separating circulating blood from brain extracellular fluid in the CNS, plays an important role in gut–brain communication. Its function is analogous to that of the GBB and also exhibits similar anatomical features such as being formed by brain endothelial cells connected by tight junctions. The homology between the GBB and BBB includes increased permeability resulting from activity of inflammatory mediators (interleukin-6, tumor necrosis factor, etc). Disruption of the integrity of the BBB allows the entrance of leukocytes/lymphocytes and/or neurotoxins into the brain.Citation93,Citation94 Neurotropic viruses as well as bacterial toxins produced in the intestines may reach the CNS via enteroendocrine cells or directly via the vagus nerve which innervates the distal part of the small intestine.Citation95,Citation96
Clinical observations and research directions
Clinical observations revealed significant temporary alleviation of autistic behaviors after antibacterial interventions (treatment by antibiotics such as vancomycin or metronidazole) in children with regressive autism and drew researchers’ attention to the possible role of intestinal microbiota changes in the pathogenesis of ASD.Citation97–Citation99
The human microbiome is dominated by two phyla, the Firmicutes (∼75%) and Bacteroidetes (∼20%), with lesser contributions from Proteobacteria and Actinobacteria. Overgrowth of obligate anaerobic organisms such as Clostridium spp. (Firmicutes), as well as Desulfovibrio spp., and a significant decrease in the Bacteroidetes/Firmicutes ratio were found in children with autism.Citation95,Citation97,Citation100–Citation102 Fermentation end products of dietary fibers, fermentable carbohydrates, and resistant starches, which are not broken down in the upper digestive tract by these anaerobic intestinal microbiota, are short-chain fatty acids (SCFAs) such as propionate, acetate, and butyrate.Citation103 Propionic acid and other SCFAs are of particular interest because of their wide range of effects on a host’s health and ability to cross GBB and BBB.Citation104,Citation105 SCFAs serve as energy substrates for colonocytes, modulate colonic pH, regulate colonic cell proliferation and differentiation, and contribute to hepatic gluconeogenesis and cholesterol synthesis.Citation102
Propionic acid has been found to be elevated in stool samples or urine of children with autism.Citation106–Citation108 Conditions known to be associated with elevated SCFA levels include inherited and acquired conditions such as propionic/methylmalonic acidemia, biotinidase/holocarboxylase deficiency, and mitochondrial disorders.Citation109,Citation110 These conditions present with developmental delay, regression, seizure disorders, as well as GI symptoms.Citation111 A subset of children with autism with elevated SCFA levels may benefit from a low-carbohydrate diet reducing SCFA production. Williams et al indicated a relationship between human intestinal gene expression and bacterial community structure and may provide insights into the pathophysiology of GI disturbances in children with autism.Citation102
A link between microbiota and metabolism may be found in children with autism and hyperoxalemia/hyperoxaluria.Citation112 Disorders of oxalate metabolism may be the result of a genetic background (severe hyperoxalemia and hyperoxaluria), increased GI permeability, or decreased intestinal bacterial strains in the microflora participating in oxalate degradation (ie, Oxylobacter formigenes). Disorders of oxalate metabolism may be suspected in those children with food selectivity who strongly avoid eating foods high in oxalates: fruits (strawberries, berries, apples, black grapes, tangerines, kiwis), grains (millet, oats, wheat), or nuts.
The complex relationship between human intestinal gene expression and bacterial community structure provide insights into molecular mechanisms underlying the pathophysiology of GI disturbances in children with autism.Citation113 Additional studies on this subject were started in many countries such as in the USA in 2007 with The Human Microbiome Project. Today’s knowledge, however, allows for experimental therapeutic use of fecal microbiota transplantation (FMT) (approved by the US Food and Drug Administration in 2013) mainly as a treatment for patients suffering from Clostridium difficile infection (CDI).Citation114 Children diagnosed with ASD and CDI are considered as potential recipients who may benefit from FMT.
Epigenetic role of the microbiota
Children with autism often have a positive history of complications during prenatal and perinatal periods. The beginning of human life is a crucial period of exposition to factors determining the composition of the intestinal microbiota. Imbalance in a child’s intestinal microbiota may be a result of many different factors such as maternal infections during pregnancy, insufficient bacterial colonization due to cesarean section, inadequate nursing with breast milk, infections with pathogenic microbes, antibiotic exposure (during both pre-and postnatal periods), and consumption of refined foods with little fiber (which act as prebiotics).Citation115 An example of postnatal exposure to antibiotics through diet is connected with feeding low doses of antibiotics (termed subtherapeutic antibiotic treatment) to farm animals to increase their rate of growth.Citation116 These factors relating to environment/lifestyle and disease state may lead to a reduced or altered intestinal flora with subsequent possible epigenetic modifications (eg, DNA methylation) and finally changes in the phenotype.Citation20,Citation117 Heterogeneity of ASD expressed as different endophenotypes may reflect a varied genetic basis as well as exposure to different epigenetic factors in the beginning of life.Citation118
Nutritional treatment of children with ASD
The biological activity of the gut ecosystem closely reflects diet quality, which plays a critical function in the establishment, maturation, and maintenance of microbial diversity.Citation90 The microbiome, intestinal epithelial barrier, and dietary intake interact and modulate one another.Citation103 Dietetic interventions may ameliorate clinical symptoms in certain children with autism encouraging parents to test out different diets.Citation119 However, there is still an insufficient number of randomized research studies on efficiency of dietary interventions as seen with searches yielding single results in the Cochrane Database of Systematic Reviews.Citation120 According to a study by Pennesi and Klein, children with ASD treated with an elimination diet may be diet responders and diet nonresponders.Citation119 A common discussion involves the possible therapeutic effects of eliminating gluten or casein from the diet (casein-free, gluten-free [CFGF] diet).Citation73,Citation84,Citation121,Citation122 A blinded study by Elder et al evaluated a cycled 12-week elimination diet (CFGF) with a 12-week casein- and gluten-rich diet in a group of 15 children with autism and found no differences in developmental markers or behaviors.Citation122 Likewise, a more recent study by Navarro et al did not support an association with gluten and/or milk in the diet and autistic behavior.Citation84
On the other hand, Knivsberg et al in a single blind study including ASD children with urinary peptide abnormalities over a period of 1 year observed an improvement in a group of children on a restriction diet (CFGF) as compared to control subjects.Citation123 This draws attention to an opioid phenotype of autism linked with diet and food-derived oligopeptides called exorphins.Citation124,Citation125 Exorphins are derived from casein and/or gluten when proteins are incompletely digested due to decreased activity of the enzyme dipeptidyl peptidase IV.Citation126,Citation127 Beta-casomorphin (from casein) and/or gliadomorphin (from gluten) can cross the epithelial monolayer as confirmed using Caco-2 cell monolayers, a model of human intestinal paracellular absorption.Citation128 Exogenous opioid peptides may have an influence on GI functions, and after passing the gut barrier, beta-casomorphins may affect functions of the immunological system as well as dopaminergic, serotoninergic, and GABAergic systems in the brain regulating opioid receptor development and elicit behavioral effects.Citation129 Decreased perception of pain, constipation, pruritis, stereotypical behavior, a labile affect, decreased social interaction, and disordered development are a potential complex of common symptoms in certain patients with ASD. These signs and symptoms overlap with the biological activity of opioids. This may be associated with high rates of gluten and/or casein exclusion diets. While successful treatment with favorable outcomes (reductions in stereotypical behavior or self-harm, improvement of speech and social interactions) using naltrexone, an opioid receptor antagonist, has been published, the theory still remains to be confirmed in clinical studies.Citation2,Citation124,Citation130,Citation131 Analyses of urinary opioids in children with ASD yield conflicting results.Citation132 Reichelt and Knivsberg confirmed the existence of hyperpeptiduria, while Dettmer et al, using different analytical techniques (solid phase extraction-high performance liquid chromatography-tandem mass spectrometry), did not detect increased amounts of endorphins (deltorphins 1 and 2) or exorphins (gliadomorphins, β-casomorphin) in the urine of 56 children with ASD.Citation133,Citation134
Current evidence of the efficacy of gluten and/or casein exclusion diets is poor; however, an opioid phenotype of autism has not been dismissed. Large-scale, good-quality randomized controlled trials are needed.Citation120 Despite different final results, the authors agree that restrictive diets should be implemented only where a food allergy or food intolerance is detected.
Sensory integration dysfunction and sensory sensitivity result in children with autism commonly having self-restricted diets with avoidance of food of a particular color or consistency, picky eating, or disordered eating behaviors which may lead to secondary problems with a child’s nutritional status.Citation135 Studies investigating nutritional quality in children with autism were concisely gathered in a publication by Coury et al.Citation54 Recommended dietary allowances of energy, carbohydrates, fats, proteins, and micronutrients may not be met, or levels of one nutrient may be in excess compared to others.Citation135 Inadequate intakes of vitamin D, vitamin A, and calcium were more common in children with ASD than in healthy children.Citation136 It is recommended that pediatricians routinely monitor anthropometry as part of the evaluation of children with ASD. Primary care nutritional assessment of each person with an ASD should include 1) weight to height or body mass index, 2) weight to age, 3) height to age, and 4) any marked changes in growth rate (percentiles over time).Citation53 Treatment with an elimination diet cannot increase the risk of nutritional deficiencies. Complementary and alternative medicine (CAM) commonly used in management of children with ASD should meet the same requirements. CAM therapies often initiated by parents before discussing them with their physician may present with possible adverse side effects if implemented without proper monitoring.Citation137 The American Academy of Pediatrics recommends that CAM therapies be discussed in every child with a chronic illness or disability.Citation138
Conclusion
This paper addresses the possible links between ASD and GI disorders, and debated points are summarized in .
Figure 3 Gut to behavior cycle.
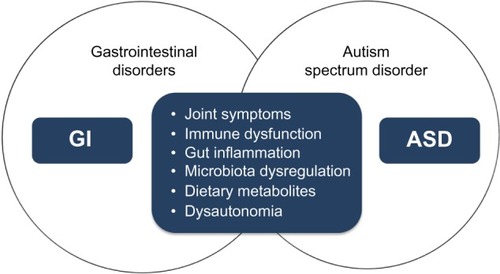
According to current recommendations, children with ASD are at risk of having alimentary tract disorders. GI symptoms may overlap with ASD core symptoms through different mechanisms. Shared pathogenetic factors and pathophysiological mechanisms may possibly link ASD and GI disturbances as shown by most recent studies. ASD is a genetically determined developmental brain disorder; however, immune dysregulation, GI inflammation, malfunction of the ANS, genetic and metabolic activity of the microbiome, and dietary metabolites may contribute to brain dysfunction and neuroinflammation depending upon individual genetic vulnerability. ASD has a heterogeneous phenotype with different subendophenotypes of which some reflect GI abnormalities. GI disorders in children with ASD may vary greatly in their nature and localization. Due to a clinical endophenotype presenting as comorbidity of ASD and GI disorders, we propose treating this situation as an “overlap syndrome” as summarized in .
Figure 4 The concept of the overlap syndrome of GI disorders and ASD.
Abbreviations: GI, gastrointestinal; ASD, autism spectrum disorder.
Practical use of the concept of an overlap syndrome of ASD and GI disorders may help in identifying those children with ASD who suffer from an alimentary tract disease. Unexplained worsening of nonverbal behaviors such as agitation, anxiety, aggression, self-injury, or sleep deprivation should alert caregivers, physicians, and other professionals about this possibility. This may speed up the diagnosis and treatment commencement, and alleviate both GI and ASD symptoms through reducing pain, stress, or discomfort by treating the comorbid disorder. Furthermore, this may also protect children against dietary experiments and restrictions without medical indications.
Our understanding of ASDs has come a long way; however, more work needs to be done in order to determine to what extent are ASD and GI linked with each other. Further studies and more systematic research are warranted.
Acknowledgments
This paper was a part of an invited lecture presented by Jolanta Wasilewska during a seminar at the RIKEN Brain Science Institute Laboratory for Molecular Psychiatry, Hirosawa, Wako, Japan, on August 22, 2014.
Disclosure
The authors declare no conflicts of interest in this work.
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association2000 Text Revision (DSM-IV-TR)
- VolkmarFSiegelMWoodbury-SmithMPractice parameter for the assessment and treatment of children and adolescents with autism spectrum disorderJ Am Acad Child Adolesc Psychiatry201453223725724472258
- LaiMCLombardoMVBaron-CohenSAutismLancet2014383992089691024074734
- MuhleRTrentacosteSVRapinIThe genetics of autismPediatrics20041135e472e48615121991
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edWashington, DCAPA2013
- BradstreetJJSmithSGranpeeshehDEl-DahrJMRossignolDSpironolactone might be a desirable immunologic and hormonal intervention in autism spectrum disordersMed Hypotheses200768597998717150311
- SchneiderTPrzewlockiRBehavioral alterations in rats prenatally exposed to valproic acid: animal model of autismNeuropsychopharmacology2005301808915238991
- The Centers for Disease Control and Prevention (CDC)Autism spectrum disorder prevalence [database on the Internet]2014 Available from: http://www.cdc.gov/media/releases/2014/p0327-autism-spectrum-disorder.html
- MbadiweTMillisRMEpigenetics and autismAutism Res Treat2013201382615624151554
- GoldaniAADownsSRWidjajaFLawtonBHendrenRLBiomarkers in autismFront Psychiatry2014510025161627
- HirabayashiYGotohYEpigenetic control of neural precursor cell fate during developmentNat Rev Neurosci201011637738820485363
- LiuXTakumiTGenomic and genetic aspects of autism spectrum disorderBiochem Biophys Res Commun2014452224425325173933
- MazinaVGerdtsJTrinhSEpigenetics of autism-related impairment: copy number variation and maternal infectionJ Dev Behav Pediatr2015362616725629966
- GoyalDKMiyanJANeuro-immune abnormalities in autism and their relationship with the environment: a variable insult model for autismFront Endocrinol2014529
- KovesKKauszMReserDHorvathKWhat may be the anatomical basis that secretin can improve the mental functions in autism?Regul Pept20021091–316717212409229
- ShimamotoCOhnishiTMaekawaMFunctional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studiesHum Mol Genet2015248240925655139
- AnithaANakamuraKThanseemIBrain region-specific altered expression and association of mitochondria-related genes in autismMol Autism2012311223116158
- CasanovaELCasanovaMFGenetics studies indicate that neural induction and early neuronal maturation are disturbed in autismFront Cell Neurosci2014839725477785
- KorvatskaEVan de WaterJAndersTFGershwinMEGenetic and immunologic considerations in autismNeurobiol Dis20029210712511895365
- BehniaFParetsSEKechichianTFetal DNA methylation of autism spectrum disorders candidate genes: association with spontaneous preterm birthAm J Obstet Gynecol20152124533.e1533.e925687563
- YuTWChahrourMHCoulterMEUsing whole-exome sequencing to identify inherited causes of autismNeuron201377225927323352163
- AbdelrahmanHMSheriefLMAlghobashyAAAssociation of 5-HT2A receptor gene polymorphisms with gastrointestinal disorders in Egyptian children with autistic disorderRes Dev Disabil201436C48549025462508
- ZafeiriouDIVerveriAVargiamiEChildhood autism and associated comorbiditiesBrain Dev200729525727217084999
- HeilKMSchaafCPThe genetics of autism spectrum disorders – a guide for cliniciansCurr Psychiatry Rep201315133423250815
- CarterMTSchererSWAutism spectrum disorder in the genetics clinic: a reviewClin Genet201383539940723425232
- KannerLAutistic disturbances of affective contactNerv Child19432217250
- JyonouchiHAutism spectrum disorders and allergy: observation from a pediatric allergy/immunology clinicExpert Rev Clin Immunol20106339741120441426
- WasilewskaJKaczmarskiMStasiak-BarmutaATobolczykJKowalewskaELow serum IgA and increased expression of CD23 on B lymphocytes in peripheral blood in children with regressive autism aged 3–6 years oldArch Med Sci20128232433122662007
- MostafaGAAl ShehabAFouadNRFrequency of CD4+CD25 high regulatory T cells in the peripheral blood of Egyptian children with autismJ Child Neurol201025332833519713552
- BuieTCampbellDBFuchsGJ3rdEvaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus reportPediatrics2010125Suppl 1S1S1820048083
- McElhanonBOMcCrackenCKarpenSSharpWGGastrointestinal symptoms in autism spectrum disorder: a meta-analysisPediatrics2014133587288324777214
- BlackCKayeJAJickHRelation of childhood gastrointestinal disorders to autism: nested case-control study using data from the UK General Practice Research DatabaseBMJ2002325736141942112193358
- MolloyCAManning-CourtneyPPrevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disordersAutism20037216517112846385
- HorvathKPermanJAAutistic disorder and gastrointestinal diseaseCurr Opin Pediatr200214558358712352252
- KuddoTNelsonKBHow common are gastrointestinal disorders in children with autism?Curr Opin Pediatr200315333934312806268
- LevySESoudersMCIttenbachRFGiarelliEMulbergAEPinto-MartinJARelationship of dietary intake to gastrointestinal symptoms in children with autistic spectrum disordersBiol Psychiatry200761449249717207470
- HorvathKPermanJAAutism and gastrointestinal symptomsCurr Gastroenterol Rep20024325125812010627
- AfzalNMurchSThirrupathyKBergerLFagbemiAHeuschkelRConstipation with acquired megarectum in children with autismPediatrics2003112493994214523189
- FasanoASaponeAZevallosVSchuppanDNonceliac gluten sensitivityGastroenterology20151664805811
- CatassiCBaiJCBonazBNon-celiac gluten sensitivity: the new frontier of gluten related disordersNutrients20135103839385324077239
- HorvathKPapadimitriouJCRabsztynADrachenbergCTildonJTGastrointestinal abnormalities in children with autistic disorderJ Pediatr1999135555956310547242
- Delgado-ArosSCamilleriMVisceral hypersensitivityJ Clin Gastroenterol2005395 Suppl 3S194S203 discussion S1015798485
- AxelrodFBChelimskyGGWeese-MayerDEPediatric autonomic disordersPediatrics2006118130932116818580
- LonsdaleDShambergerRJObrenovichMEDysautonomia in autism spectrum disorder: case reports of a family with review of the literatureAutism Res Treat2011201112979522937241
- HirsteinWIversenPRamachandranVSAutonomic responses of autistic children to people and objectsProc Biol Sci200126814791883188811564343
- MaennerMJArnesonCLLevySEKirbyRSNicholasJSDurkinMSBrief report: association between behavioral features and gastrointestinal problems among children with autism spectrum disorderJ Autism Dev Disord20124271520152522012246
- GorrindoPWilliamsKCLeeEBWalkerLSMcGrewSGLevittPGastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factorsAutism Res20125210110822511450
- MazefskyCASchreiberDROlinoTMMinshewNJThe association between emotional and behavioral problems and gastrointestinal symptoms among children with high-functioning autismAutism201318549350124104507
- MouridsenSEIsagerTRichBDiseases of the gastrointestinal tract in individuals diagnosed as children with atypical autism: a Danish register study based on hospital diagnosesAutism2013171556322987890
- SmithRAFarnworthHWrightBAllgarVAre there more bowel symptoms in children with autism compared to normal children and children with other developmental and neurological disorders? A case control studyAutism200913434335519535465
- SchreckKAWilliamsKSmithAFA comparison of eating behaviors between children with and without autismJ Autism Dev Disord200434443343815449518
- MirescuCPetersJDNoimanLGouldESleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoidsProc Natl Acad Sci U S A200610350191701917517135354
- BuieTFuchsGJ3rdFurutaGTKoorosKLevyJLewisJDRecommendations for evaluation and treatment of common gastrointestinal problems in children with ASDsPediatrics20101125Suppl 1S192920048084
- CouryDLAshwoodPFasanoAGastrointestinal conditions in children with autism spectrum disorder: developing a research agendaPediatrics2012130Suppl 2S160S16823118247
- BrownACMehl-MadronaLAutoimmune and gastrointestinal dysfunctions: does a subset of children with autism reveal a broader connection?Expert Rev Gastroenterol Hepatol20115446547721780894
- WalkerSJFortunatoJGonzalezLGKrigsmanAIdentification of unique gene expression profile in children with regressive autism spectrum disorder (ASD) and ileocolitisPLoS One201383e5805823520485
- FernellEFagerbergULHellstromPMNo evidence for a clear link between active intestinal inflammation and autism based on analyses of faecal calprotectin and rectal nitric oxideActa Paediatr20079671076107917465982
- KokkonenJKarttunenTJLymphonodular hyperplasia on the mucosa of the lower gastrointestinal tract in children: an indication of enhanced immune response?J Pediatr Gastroenterol Nutr2002341424611753163
- TurunenSKarttunenTJKokkonenJLymphoid nodular hyperplasia and cow’s milk hypersensitivity in children with chronic constipationJ Pediatr2004145560661115520758
- SichererSHEigenmannPASampsonHAClinical features of food protein-induced enterocolitis syndromeJ Pediatr199813322142199709708
- Nowak-WegrzynASampsonHAWoodRASichererSHFood protein-induced enterocolitis syndrome caused by solid food proteinsPediatrics20031114 pt 182983512671120
- MuraroAWerfelTHoffmann-SommergruberKEAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergyAllergy20146981008102524909706
- Soares-WeiserKTakwoingiYPanesarSSThe diagnosis of food allergy: a systematic review and meta-analysisAllergy2014691768624329961
- CanonicaGWAnsoteguiIJPawankarRA WAO – ARIA – GA(2)LEN consensus document on molecular-based allergy diagnosticsWorld Allergy Organ J2013611724090398
- CastellaniMLContiCMKempurajDJAutism and immunity: revisited studyInt J Immunopathol Pharmacol2009221151919309548
- AshwoodPAnthonyAPellicerAATorrenteFWalker-SmithJAWakefieldAJIntestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathologyJ Clin Immunol20031123650451715031638
- PapadopoulouAKoletzkoSHeuschkelRManagement guidelines of eosinophilic esophagitis in childhoodJ Pediatr Gastroenterol Nutr201458110711824378521
- DellonESEosinophilic esophagitisGastroenterol Clin North Am201342113315323452635
- Jarocka-CyrtaEWasilewskaJKaczmarskiMGBrief report: eosinophilic esophagitis as a cause of feeding problems in autistic boy. The first reported caseJ Autism Dev Disord2010413372374
- AndersonSEMustACurtinCBandiniLGMeals in our household: reliability and initial validation of a questionnaire to assess child mealtime behaviors and family mealtime environmentsJ Acad Nutr Diet2012112227628422741169
- BuieTThe relationship of autism and glutenClin Ther201335557858323688532
- KleinSSharifi-HannauerPMartinez-AgostoJAMacrocephaly as a clinical indicator of genetic subtypes in autismAutism Res201361515623361946
- LauNMGreenPHTaylorAKMarkers of celiac disease and gluten sensitivity in children with autismPLoS One201386e6615523823064
- StamnaesJDorumSFleckensteinBAeschlimannDSollidLMGluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxiaAmino Acids20103951183119120300788
- IannacconeMTittaFSerretielloEMonfregolaMGentileVPossible physiopathological effects of the transglutaminase activity on the molecular mechanisms responsible for human neurodegenerative diseasesRecent Pat CNS Drug Discov201492768425386917
- CascellaNGSantoraDGregoryPKellyDLFasanoAEatonWWIncreased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patientsSchizophr Bull201339486787122516148
- de MagistrisLFamiliariVPascottoAAlterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relativesJ Pediatr Gastroenterol Nutr201051441842420683204
- AderRCohenNFeltenDLBrain, behavior, and immunityBrain Behav Immun198711163451780
- GoinesPVan de WaterJThe immune system’s role in the biology of autismCurr Opin Neurol201023211111720160651
- LiuZLiNNeuJTight junctions, leaky intestines, and pediatric diseasesActa Paediatr200594438639316092447
- BuhnerSBuningCGenschelJGenetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation?Gut200655334234716000642
- ZareieMJohnson-HenryKJuryJProbiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stressGut200655111553156016638791
- D’EufemiaPCelliMFinocchiaroRAbnormal intestinal permeability in children with autismActa Paediatr1996859107610798888921
- NavarroFPearsonDAFathereeNMansourRHashmiSSRhoadsJMAre ‘leaky gut’ and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders?Nutr Neurosci201518417718524564346
- KendallASome observations on the study of the intestinal bacteriaJ Biol Chem19096499507
- MayerEATillischKGuptaAGut/brain axis and the microbiotaJ Clin Invest2015125392693825689247
- StillingRMDinanTGCryanJFMicrobial genes, brain and behaviour – epigenetic regulation of the gut-brain axisGenes Brain Behav2014131698624286462
- SeveranceEGPrandowszkyECastilioneJYolkenRHGastroenterology issues in schizophrenia: why the gut mattersCurr Psychiatr Rep201517527
- Douglas-EscobarMElliottENeuJEffect of intestinal microbial ecology on the developing brainJAMA Pediatrics2013167437437923400224
- MulleJGSharpWGCubellsJFThe gut microbiome: a new frontier in autism researchCurr Psychiatry Rep201315233723307560
- HeberlingCADhurjatiPSSasserMHypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: links to gut bacteria, oxidative stress, and intestinal permeabilityMed Hypotheses201380326427023273906
- KraneveldADde TheijeCGvan HeeschFThe neuro-immune axis: prospect for novel treatments for mental disordersBasic Clin Pharmacol Toxicol2014114112813624118847
- AngelidouAAsadiSAlysandratosKDKaragkouniAKourembanasSTheoharidesTCPerinatal stress, brain inflammation and risk of autism-review and proposalBMC Pediatr2012128922747567
- TheoharidesTCZhangBNeuro-inflammation, blood-brain barrier, seizures and autismJ Neuroinflammation2011816822129087
- BolteERAutism and Clostridium tetaniMed Hypotheses19985121331449881820
- BohorquezDVShahidRAErdmannANeuroepithelial circuit formed by innervation of sensory enteroendocrine cellsJ Clin Invest2015125278278625555217
- SandlerRHFinegoldSMBolteERShort-term benefit from oral vancomycin treatment of regressive-onset autismJ Child Neurol200015742943510921511
- FinegoldSMDowdSEGontcharovaVPyrosequencing study of fecal microflora of autistic and control childrenAnaerobe201016444445320603222
- CritchfieldJWvan HemertSAshMMulderLAshwoodPThe potential role of probiotics in the management of childhood autism spectrum disordersGastroenterol Res Pract2011201116135822114588
- TomovaAHusarovaVLakatosovaSGastrointestinal microbiota in children with autism in SlovakiaPhysiol Behav201513817918725446201
- FinegoldSMState of the art; microbiology in health and disease. Intestinal bacterial flora in autismAnaerobe201117636736821524713
- EckerCSucklingJDeoniSCBrain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging studyArch Gen Psychiatry201269219520922310506
- GuzmanJRConlinVSJobinCDiet, microbiome, and the intestinal epithelium: an essential triumvirate?Biomed Res Int2013201342514623586037
- KaruriARDobrowskyETannockIFSelective cellular acidification and toxicity of weak organic acids in an acidic microenvironmentBr J Cancer1993686108010878260358
- AdamsJBJohansenLJPowellLDQuigDRubinRAGastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severityBMC Gastroenterol2011112221410934
- MacfabeDFShort-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disordersMicrob Ecol Health Dis201223
- WangLChristophersenCTSorichMJGerberJPAngleyMTConlonMAElevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorderDig Dis Sci20125782096210222535281
- ShawWIncreased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophreniaNutr Neurosci201013313514320423563
- RossignolDAFryeREEvidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autismFront Physiol2014515024795645
- SchreiberJChapmanKASummarMLNeurologic considerations in propionic acidemiaMol Genet Metab20121051101522078457
- Al-OwainMKayaNAl-ShamraniHAutism spectrum disorder in a child with propionic acidemiaJIMD Rep20137636623430497
- KonstantynowiczJPorowskiTZoch-ZwierzWA potential pathogenic role of oxalate in autismEur J Paediatr Neurol201216548549121911305
- CaoXLinPJiangPLiCCharacteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic reviewShanghai Arch Psychiatry201325634235324991177
- AroniadisOCBrandtLJFecal microbiota transplantation: past, present and futureCurr Opin Gastroenterol2013291798423041678
- TohMCAllen-VercoeEThe human gut microbiota with reference to autism spectrum disorder: considering the whole as more than a sum of its partsMicrob Ecol Health Dis2015262630925634609
- BlaserMJFalkowSWhat are the consequences of the disappearing human microbiota?Nature Rev Microbiol200971288789419898491
- GrafodatskayaDChungBSzatmariPWeksbergRAutism spectrum disorders and epigeneticsJ Am Acad Child Adolesc Psychiatry201049879480920643313
- FlashnerBMRussoMEBoileauJELeongDWGallicanoGIEpigenetic factors and autism spectrum disordersNeuromolecular Med201315233935023468062
- PennesiCMKleinLCEffectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: based on parental reportNutr Neurosci2012152859122564339
- AdamsSJBurtonNCutressADevelopment of double blind gluten and casein free test foods for use in an autism dietary trialJ Hum Nutr Diet2008214374
- WhiteleyPShattockPKnivsbergAMGluten- and casein-free dietary intervention for autism spectrum conditionsFront Hum Neu-rosci20126344
- ElderJHShankarMShusterJTheriaqueDBurnsSSherrillLThe gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trialJ Autism Dev Disord200636341342016555138
- KnivsbergAMReicheltKLHoienTNodlandMA randomised, controlled study of dietary intervention in autistic syndromesNutr Neurosci20025425126112168688
- LeboyerMBouvardMPLaunayJMUne hypothese opiacee dans l’autisme infantile? Essais therapeutiques avec la naltrexone [Opiate hypothesis in infantile autism? Therapeutic trials with naltrexone]Encephale199319295102 French8275903
- SahleyTLPankseppJBrain opioids and autism: an updated analysis of possible linkagesJ Autism Dev Disord19871722012163038836
- TrivediMSShahJSAl-MughairySFood-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequencesJ Nutr Biochem201425101011101825018147
- JohnsonBUlbergSShivaleSDonaldsonJMilczarskiBFaraoneSVFibromyalgia, autism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal systemDiscov Med2014189920922025336035
- KlionskyDJAbdallaFCAbeliovichHGuidelines for the use and interpretation of assays for monitoring autophagyAutophagy20128444554422966490
- CieslinskaASienkiewicz-SzlapkaEWasilewskaJInfluence of candidate polymorphisms on the dipeptidyl peptidase IV and mu-opioid receptor genes expression in aspect of the beta-casomorphin-7 modulation functions in autismPeptides201565C611
- LeboyerMBouvardMPLaunayJMBrief report: a double-blind study of naltrexone in infantile autismJ Autism Dev Disord19922223093191345670
- WoodardCGrodenJGoodwinMBodfishJA placebo double-blind pilot study of dextromethorphan for problematic behaviors in children with autismAutism2007111294117175572
- CassHGringrasPMarchJAbsence of urinary opioid peptides in children with autismArch Dis Child200893974575018337276
- ReicheltKLKnivsbergAMCan the pathophysiology of autism be explained by the nature of the discovered urine peptides?Nutr Neurosci200361192812608733
- DettmerKHannaDWhetstonePHansenRHammockBDAutism and urinary exogenous neuropeptides: development of an on-line SPE-HPLC-tandem mass spectrometry method to test the opioid excess theoryAnal Bioanal Chem200738881643165117520243
- KralTVEriksenWTSoudersMCPinto-MartinJAEating behaviors, diet quality, and gastrointestinal symptoms in children with autism spectrum disorders: a brief reviewJ Pediatr Nurs201328654855623531467
- BandiniLGAndersonSECurtinCFood selectivity in children with autism spectrum disorders and typically developing childrenJ Pediatr2010157225926420362301
- AkinsRSAngkustsiriKHansenRLComplementary and alternative medicine in autism: an evidence-based approach to negotiating safe and efficacious interventions with familiesNeurotherapeutics20107330731920643384
- Committee on Children with DisabilitiesAmerican Academy of Pediatrics: counseling families who choose complementary and alternative medicine for their child with chronic illness or disabilityPediatrics2001107359860111230608
