Abstract
Background
Although based on a single system, each laboratory should have their own quality management system, in Ethiopia, quality management systems in medical laboratories were introduced in 2009 with the aim of improving the quality of services.
Objective
This review was designed to evaluate the status of quality management practice and challenges among medical laboratories in Ethiopia.
Methods
A systematic qualitative review of the literature was made by searching the international electronic bibliographic database of PubMed (NML), web of science (TS), google scholar, African journals online (AJOL) and Cochrane Library.
Results
Thirty-six full-text articles, which were published between 2010 and 2022, were included in this review. In this review, 33 of 36 (91.7%) studies showed that status of quality management practice in Ethiopian medical laboratories was limited. As a result, the quality of medical laboratories was inadequate. The main challenges were problems associated with laboratory professionals (35/36=97.2%), inadequate support from management bodies (21/36=58.3%), limited on-job training access (8 /36=22.2%) and high workload (5/36=13.8%).
Conclusion
The status of quality management practice among medical laboratories in Ethiopia is limited. The main quality compromising factors were problems associated with laboratory professionals, inadequate support from management bodies, high workload, and limited on-job training access. Therefore, all responsible stakeholders should focus on ensuring Quality Management Systems and the system should be applied in all Medical laboratories. Only this will ensure the improvement of quality within medical laboratories across Ethiopia.
Introduction
Quality in medical diagnostics defined as the reliability, accuracy, and timeliness of laboratory test results.Citation1 In medical laboratory practice, quality needs to be viewed as “systems thinking”, which is used in other business practices. Systemic thinking is a comprehensive analytical approach to understand how different elements interact within a system or structure for monitoring of quality in medical laboratories. Therefore, to ensure efficiency, effectiveness and accuracy of services provided to the customer, a quality system that monitors these areas is required.Citation2
Inadequate quality of medical laboratory services results in producing wrong information, unnecessary expenditures, suffering and misery in human lives.Citation3 For example, over-treatment of antibiotics for inappropriate clinical conditions leads to the development of drug-resistant microorganisms.Citation4
Quality laboratory testing greatly affect the affordability and quality of patient care. Any errors or defects within a medical laboratory influences patient care and can also incur added costs.Citation5 As a result, nowadays, quality is given a priority in many health care system.Citation6
Quality laboratory services need the practice of a quality management which focuses on applying twelve quality essentials; personnel, organization, purchasing and inventory, equipment, process control, documents and records, information management, occurrence management, assessment, facility and safety, process improvement, and customer services.Citation1 Laboratory quality management is a continuous improvement process that measures processes from a client satisfaction point-of-view.Citation7 Implementing total quality management in a healthcare laboratory need to incorporate quality planning and quality improvement with laboratory quality assurance to provide full quality management system.Citation8
In Ethiopia, quality management system in medical laboratories was implemented since 2009 for the aim of improving quality of services. National Laboratory Strategic Plan was set in 2010 to strengthen laboratory quality systems and laboratory accreditation. As a result, the so called Strengthening Laboratory Management Toward Accreditation (SLMTA) programme was launched in 45 medical laboratories.Citation9 This review was designed to evaluate the status of quality management practice and challenges facing medical laboratories in Ethiopia.
Methods and Materials
Study Design and Setting
Systematic qualitative literature review was made in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelineCitation10 to evaluate the quality management practice status and challenges among medical laboratories in Ethiopia.
Search Strategy
Comprehensive updated published studies from 2010 to 2022 were identified by searching from the international electronic bibliographic database of PubMed (NML), web of science (TS), google scholar, African journals online (AJOL) and Cochrane Library using EndNote X7 application software. Both primary studies and review articles were manually searched with several keywords like “quality”, “medical laboratory”, “quality management system”, “implementation”, and ‘challenges ‘in different combinations. The database searches were performed in the English language without research design restriction in June 2022.
Selection Criteria
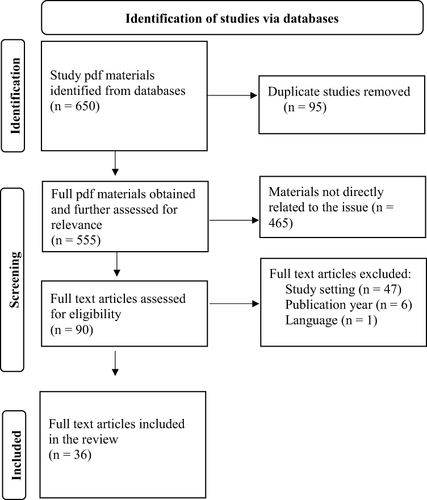
We included studies that addressed the practice of the quality management, service quality, and challenges facing medical laboratories in Ethiopia. Studies were considered eligible if they were published in the English language with the full-text format at peer-reviewed journals and conducted in Ethiopian settings. Based on these criteria, the selection of studies was performed independently by the two authors (BM and HB). Differences were resolved by discussion and consensus. Studies potentially eligible for inclusion in the review were initially screened by title abstract review and/or title and then critical reviewing of full-text studies was made. Finally, from the 650 identified studies, 36 published full-text articles were considered for the synthesis of this review ().
Data Extraction and Analysis
Data extraction form was prepared in Excel sheet by including first author’s name, publication year, objective of the study, study setting, study group, and results (). An analysis of full-text articles was conducted to identify substantial information relevant to the quality management practice status, service quality, and challenges among medical laboratories in Ethiopia.
Table 1 Data Summary for Medical Laboratories Quality Management and Challenges in Ethiopia
Results
This systematic review was conducted on published studies, which were conducted in different health facilities of Addis Ababa, South region, Amhara region, Oromia region, and Tigray region of Ethiopia. Thirty-six studies published between 2010 and 2022 were included in this review. The findings of this review were sorted into two main categories, namely service quality and challenges among medical laboratories.
Service Quality of Medical Laboratories
Even though all laboratory professionals (n=184) were informed about quality management, only about 138 (79%) were engaged in practicing it. These laboratories also had poor or very poor performance in a quality management system with five quality indicators; control of documents, control of records, setting policies and preparation of manuals, setting of processes, procedures, and communication.Citation11 Similarly, medical laboratories statuses towards the AFRO-WHO accreditation showed that only a laboratory from 30 laboratories achieved 156 (62%) scores, which is the minimum score required for WHO accreditation.Citation12 In addition, from laboratories of health center enrolled (n=89), 71 (79.8%) achieved zero stars, only 6 (6.7%) achieved star one, 9 (10.1%) achieved star two, and only 3 (3.4%) achieved star three.Citation13 However, from those laboratories which were implementing the system (n=45), 42 (93%) laboratories showed overall service improvements.Citation14 For example, a laboratory improved from the baseline score (78 points) in 2012 by achieving 198 scores (3 stars) in 2013 and 249 scores (5 stars) in 2014.Citation15 In addition, as one tool of quality management, SLMTA implementation increased the status of 20 laboratories from 29 laboratories from star zero to star one and above.Citation16 Most quality officers and managers of laboratories viewed the SLMTA program as being the most important step in the improvement process of service quality. Nevertheless, from the analysis of 17 CEOs of the hospital, only 10 (59%) understood the requirements, the importance and the outcomes of the SLMTA program, while the others seven CEOs (41%) were uncertain.Citation17
In this review, the service quality of medical laboratories was assessed with five quality indicators; namely frequency of laboratory errors, proficiency test performance, specimen rejection rate, turnaround time, and customer satisfaction.
Frequency of Laboratory Errors
In frequency of laboratory errors, six papersCitation18–23 were looked at specific major technical laboratory errors. Shiferaw et al,Citation18 showed that 47 of 201 (23.4%) laboratories in the study had major false positive and false negative errors in diagnosing tuberculosis. Similarly, Mekonen et alCitation19 showed that 20 of 55 (36.4%) health center laboratories had false positive and false negative results in diagnosing tuberculosis. In addition, Desalegn et alCitation20 demonstrated that only 96 of 135 (68.9%) peripheral laboratories read positive and negative AFB slides correctly. Measuring with some indicators like smear size, specimen quality, smear thickness, smear evenness and smear cleanliness, Weldemhret et alCitation21 showed that the performance of sputum smear quality were 68%, 61, 64%, 62 and 66% respectively. On the other hand, Tadesse et al studyCitation22 showed that 742 of 2606 (28.5%) hematology laboratory specimens in the study had different technical errors. One study conducted, Teka and KibatuCitation23 showed that 65% of the 213-control measurement values were outside of the allowable errors limits in clinical chemistry laboratory.
Proficiency Test Performance
In proficiency test performance, one paper was looked at for evaluating the performance of laboratory professionals in tuberculosis microscopy. Mengistu et alCitation24 showed that only 11 of 81 (13.6%) laboratory professionals reported all positive and negative tuberculosis smear panel slides correctly. The others 70 of 81 (86.4%) laboratory professionals reported at least one false positive or false negative result in tuberculosis microscopy.
Specimen Rejection Rate
In specimen rejection, two articles were looked at for evaluating service quality of medical laboratories. Habtamu Molla et alCitation25 showed that 116 of 8063 (1.4%) laboratory specimens were rejected due to different technical errors. Similarly, Shiferaw et alCitation26 noted that 221 of 42,923 (0.5%) laboratory samples were rejected.
Turnaround Time (TAT)
In turnaround time, two articles were looked at to evaluate service quality of medical laboratories. Gebreyes et alCitation27 showed that only 41 of 253 (16.2%) clinical chemistry test results were released within the target TAT. Shiferaw and YismawCitation28 also showed that 68.1% of the EID for HIV, 53.8% of the TB genexpert tests and 76.5% of the viral load had delayed turnaround time compared with the standard.
Customer Satisfaction
In customer satisfaction, five articles were looked at to evaluate service quality of medical laboratories. Studies showed that different customers of laboratories had different level of satisfaction. For example, Desalegn et alCitation29 noted that 240 of 422 (56.9%) pregnant mothers were satisfied with Focused Antenatal Care (FANC) laboratory service. A study conducted on satisfaction level of patients (n=502), 73.5% of patients were found to be satisfied.Citation30 In another national survey analysis, the satisfaction level of physicians (medical doctors) in medical laboratory services was 55%.Citation31 In one study, the clinician’s (medical doctors, nurses and health officers) satisfaction level with clinical laboratory services was 72.8%.Citation32 However, the pooled client satisfaction level with medical laboratory services was 66%.Citation33
Quality Challenges
In this review, the quality challenges were demonstrated in three categories, which includes problems associated with laboratory professionals, inadequate support from management bodies and others.
Problems Associated with Laboratory Professionals
The main problems were improper provision of information,Citation30,Citation33,Citation34 lack of commitment,Citation11,Citation12,Citation17,Citation22,Citation35,Citation36 poor professional skill,Citation15,Citation18,Citation19,Citation21,Citation23,Citation24,Citation32,Citation37,Citation38 poor communication,Citation31–33,Citation35 poor internal quality control practiceCitation19,Citation23,Citation29,Citation35,Citation36,Citation39,Citation40 and inadequate utilization of laboratory documents.Citation13,Citation14,Citation31,Citation32,Citation35,Citation40,Citation41
Inadequate Support from Management Bodies
In this category, quality challenges were limited budget allocation for laboratory,Citation15–17,Citation35,Citation37,Citation38,Citation41 poor laboratory infrastructure,Citation11,Citation13,Citation16,Citation30,Citation42 limited supply of reagents and equipment’s,Citation16,Citation18,Citation20,Citation23,Citation28,Citation29,Citation36,Citation41–44 poor recognition and rewards for laboratory professionals.Citation45
Others
The other quality challenges were limited on job training accessCitation9,Citation14,Citation17,Citation26,Citation35,Citation36,Citation41,Citation43 and high workload.Citation27,Citation28,Citation38,Citation41,Citation42
Discussion
Practicing quality management is possible in medical laboratories of resource-limited countries.Citation46 However, it was found to be weak implementation status.Citation47 Our systematic review of 36 studies on implementation of quality management system showed that even though there was good beginning in most medical laboratories, still QMS was not achieved as expected in each laboratory.Citation11–17
Practicing the system of quality management facilitated the attainment of target quality indicators, and led to high client satisfaction.Citation48 An interrupted practice of quality systems may cause a services quality decline and hence poor accreditation achievement.Citation49 According to this review, service quality among Ethiopian medical laboratories was limited. This fact was shown with different quality indicators like frequency of laboratory errors, proficiency test performance, specimen rejection rate, turnaround time and customer satisfaction level. The error rate in medical laboratory diagnostics is about 0.3% according to accurate and recent information retrieved from scientific literature.Citation50 However, the error rate in most medical laboratories of Ethiopia was higher than 0.3%.Citation18–23 Even though the variety of proficiency testing was limited, AFB proficiency test performance in most Ethiopian medical laboratories was good when we compared to the standard passing score.Citation51 As a standard, specimen rejection rate in medical laboratories should be below 0.3%.Citation52 When we evaluated the service quality of medical laboratories with the standard specimen rejection rate, most Ethiopian medical laboratories had poor quality services.Citation25,Citation26 In addition, this review noted that most laboratory tests had delayed TAT.Citation27 As good performance laboratory indicator, at least 90% of the tests need to be released within the target turnaround time.Citation28 Customer satisfaction also considered as laboratory service quality indicator and serves as an important improvement process tool using benchmark satisfaction level of 80% and above.Citation53 The overall customer satisfaction level in most Ethiopian medical laboratories was below this benchmark satisfaction level.Citation29–33
Accessing quality laboratory services is a challenge in low-resource countries.Citation54 According to this review, the main challenges of laboratory service were problems associated with laboratory professionals,Citation11,Citation13,Citation15,Citation19 inadequate support from management bodies,Citation11,Citation15,Citation16,Citation45 high workloadCitation27 and limited on job training access.Citation9 A similar study in Nigerian medical laboratories showed that poor infrastructure, financial limitations, insufficient capacity building, lack of consumables and equipment’s, and motivated and dedicated laboratory personnel have been the main service quality challenges.Citation55 Another study at hospitals and institutions of Sri Lanka showed that the main challenges of quality services were lack of knowledge on ISO standards and limited training access for laboratory professionals.Citation56
Conclusion
The status of quality management practice among medical laboratories in Ethiopia is limited. The main quality compromising factors were problems associated with laboratory professionals, inadequate support from management bodies, high workload, and limited on-job training access. Therefore, all responsible stakeholders should focus on ensuring Quality Management Systems and the system should be applied in all Medical laboratories. Only this will ensure the improvement of quality within medical laboratories across Ethiopia.
Recommendation
This review strongly recommends that regional health bureaus, Ethiopian public health institute, Ethiopian Ministry of Health, and other stakeholders should focus on strengthening and implementation of quality management among medical laboratories to ensure the overall quality of healthcare system.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health Organization. Laboratory Quality Management System: Handbook. World Health Organization; 2011.
- Berte LM, Nevalainen DE. Quality management for the laboratory. Lab Med. 1996;27(4):232–235. doi:10.1093/labmed/27.4.232
- Carlson RO, Amirahmadi F, Hernandez JS. A primer on the cost of quality for improvement of laboratory and pathology specimen processes. Am J Clin Pathol. 2012;138(3):347–354. doi:10.1309/AJCPSMQYAF6X1HUT
- Joint W. CDC Conference on Health Laboratory Quality Systems. Geneva: World Health Organization; 2008.
- Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem. 2010;47(2):101–110. doi:10.1258/acb.2009.009222
- Saurav Patra M, Mukherjee B, Das AK. Pre-analytical errors in the clinical laboratory and how to minimize them. Int J Bioassays. 2013;2(3):551–553.
- Simpson K, Kaluzny A, McLaughlin C. Total quality and the management of laboratories. Clin Lab Manage Rev. 1991;5(6):448–9, 52.
- Westgard J, Barry P, Tomar R. Implementing total quality management (TQM) in health-care laboratories. Clin Lab Manage Rev. 1991;5(5):353–5, 8.
- Sisay A, Gurmessa A, Liknew W. Factors affecting implementation of laboratory quality management system in Addis Ababa public health laboratories, Addis Ababa, Ethiopia. J Trop Dis Public Health. 2019;8:343.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi:10.1186/2046-4053-4-1
- Girma M, Deress T, Adane K. Laboratory Quality Management System and Quality Indicators Implementation Status as Perceived by Laboratory Professionals in Preparation for the Accreditation Process from Selected Government Hospitals of Ethiopia. Clin Lab. 2020;66:4. doi:10.7754/Clin.Lab.2019.190718
- Mesfin EA, Taye B, Belay G, Ashenafi A. The status of medical laboratory towards of AFRO-WHO accreditation process in government and private health facilities in Addis Ababa, Ethiopia. Pan Af Med J. 2015;22:1. doi:10.11604/pamj.2015.22.136.7187
- Mulleta D, Jaleta F, Banti H, et al. The Impact of Laboratory Quality Management System Implementation on Quality Laboratory Service Delivery in Health Center Laboratories of Oromia Region, Ethiopia. Pathol Lab Med Int. 2021;13:7. doi:10.2147/PLMI.S314656
- Hiwotu TM, Ayana G, Mulugeta A, et al. Laboratory system strengthening and quality improvement in Ethiopia. Af j lab med. 2016;5(2):1–6.
- Getahun MS, Yemanebrhane N, Desalegn DM, et al. Medical laboratory accreditation in a resource-limited district health centre laboratory, Addis Ababa, Ethiopia. Af J Lab Med. 2019;8(1):1–5.
- Sisay A, Mindaye T, Tesfaye A, Abera E, Desale A. Assessing the outcome of Strengthening Laboratory Management Towards Accreditation (SLMTA) on laboratory quality management system in city government of Addis Ababa, Ethiopia. Pan Afr Med J. 2015;1:20.
- Lulie AD, Hiwotu TM, Mulugeta A, et al. Perceptions and attitudes toward SLMTA amongst laboratory and hospital professionals in Ethiopia. Af J Lab Med. 2016;5(2):1–6.
- Shiferaw MB, Hailu HA, Fola AA, et al. Tuberculosis laboratory diagnosis quality assurance among public health facilities in West Amhara Region, Ethiopia. PLoS One. 2015;10(9):e0138488. doi:10.1371/journal.pone.0138488
- Mekonen A, Ayele Y, Berhan Y, Woldeyohannes D, Erku W, Sisay S. Factors which contributed for low quality sputum smears for the detection of acid fast bacilli (AFB) at selected health centers in Ethiopia: a quality control perspective. PLoS One. 2018;13(6):e0198947. doi:10.1371/journal.pone.0198947
- Desalegn DM, Kitila KT, Balcha HM, et al. Misdiagnosis of pulmonary tuberculosis and associated factors in peripheral laboratories: a retrospective study, Addis Ababa, Ethiopia. BMC Res Notes. 2018;11(1):1–7. doi:10.1186/s13104-018-3414-6
- Weldemhret L, Hailu A, Gebremedhn G, et al. Blinded rechecking of sputum smear microscopy performance in public health facilities in Tigray region, Northern Ethiopia: retrospective cross sectional study. PLoS One. 2020;15(10):e0239342. doi:10.1371/journal.pone.0239342
- Tadesse H, Desta K, Kinde S, Hassen F, Gize A. Errors in the Hematology Laboratory at St Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia BMC Research Notes. Int J Med. 2018;11(1):1–5.
- Teka A, Kibatu G. Quality of liver and kidney function tests among public medical laboratories in western region of Amhara national regional state of Ethiopia. Ethiop J Health Sci. 2012;22(1):19–26.
- Mengistu H, Abebe M, Gezahegn B, Tadesse A, Yismake W, Moges D. Performance evaluation of laboratory professionals on tuberculosis microscopy at Hawassa Town, Southern Ethiopia. Af J Microbiol Res. 2015;9(16):1132–1138. doi:10.5897/AJMR2015.7402
- Habtamu Molla T, Aster T, Fatuma H. Frequency of Specimen Rejection and Associated Factors St. Paul’s Hospital Millennium Medical College, Addis Ababa Ethiopia; 2015.
- Shiferaw MB, Yismaw G, Getachew H. Specimen rejections among referred specimens through referral network to the Amhara Public Health Institute for laboratory testing, Bahir Dar, Ethiopia. BMC Res Notes. 2018;11(1):1–6. doi:10.1186/s13104-018-3891-7
- Gebreyes M, Sisay A, Tegen D, Asnake A, Wolde M. Evaluation of Laboratory Performance, Associated Factors and Staff Awareness Towards Achieving Turnaround Time in Tertiary Hospitals, Ethiopia. Ethiop J Health Sci. 2020;30:5. doi:10.4314/ejhs.v30i1.2
- Shiferaw MB, Yismaw G. Magnitude of delayed turnaround time of laboratory results in Amhara Public Health Institute, Bahir Dar, Ethiopia. BMC Health Serv Res. 2019;19(1):1–6. doi:10.1186/s12913-019-4077-2
- Desalegn DM, Abay S, Abebe A, et al. Quality of focused antenatal care laboratory services provided at public health facilities in Addis Ababa, Ethiopia. Qual Prim Care. 2017;26(3):81–89.
- Abebe DD, Hassen SL, Temesgen MM. Patients’ satisfaction and quality of clinical laboratory services provision at public health facilities in northeast Ethiopia. medRxiv. 2022.
- Hailu HA, Yalew A, Desale A, et al. Physicians’ satisfaction with clinical laboratory services at public hospitals in Ethiopia: a national survey. PLoS One. 2020;15(4):e0232178. doi:10.1371/journal.pone.0232178
- Abebe DD, Temesgen MM, Abozin AT. Clinicians’ perceived quality of laboratory services provided at public hospitals and primary health centres in northeast Ethiopia. 2022.
- Deress T, Million Y, Belachew T, Girma M. Customer satisfaction with clinical laboratory services provided by the Ethiopian health facilities: a systematic review and meta-analysis. 2020.
- Bogale AL, Baye AY. Customer Satisfaction Survey as Quality Indicator on Laboratory Medicine Accreditation: the Case of EPHI National HIV Molecular Reference Laboratory. 2021.
- Asemahagn M. Assessing the quality of tuberculosis laboratory services in selected public and private health facilities in Western Amhara, Ethiopia. J Med Diagn Meth. 2014;3(158):2. doi:10.4172/2168-9784.1000158
- Dabaro D. Factors affecting tuberculosis case detection in Kersa district, south west Ethiopia. J Clin Tuberculosis Other Mycobacterial Dis. 2017;9:1–4. doi:10.1016/j.jctube.2017.08.003
- Getachew A, Cheneke W, Asres Y, Bekele S, Kebede E. Assessment of coverage and quality of selected clinical chemistry tests among medical Laboratories of Health Facilities in Jimma zone, South West Ethiopia. J Trop Med. 2019;2019.
- Mesfin EA, Taye B, Belay G, Ashenafi A, Girma V. Factors affecting quality of laboratory services in public and private health facilities in Addis Ababa, Ethiopia. Ejifcc. 2017;28(3):205.
- Abebaw Y, Kebede A, Eshetu K, et al. Quality assurance practices in tuberculosis diagnostic health facilities in Ethiopia. PLoS One. 2022;17(6):e0269601. doi:10.1371/journal.pone.0269601
- Weldu Y, Gebru H, Kahsay G, Teweldemedhn G, Hagos Y, Kahsay A. Standard operating procedure utilization for tuberculosis microscopy in Mekelle City, North Ethiopia. Am J Clin Pathol. 2017;147(1):83–88. doi:10.1093/ajcp/aqw196
- Fenta DA, Ali MM. Factors affecting quality of laboratory result during ordering, handling, and testing of the patient’s specimen at hawassa university college of medicine and health science comprehensive specialized hospital. J Multidiscip Healthc. 2020;13:809. doi:10.2147/JMDH.S264671
- Hailegiorgis B, Girma S, Melaku Z, et al. Laboratory malaria diagnostic capacity in health facilities in five administrative zones of Oromia Regional State, Ethiopia. Trop Med Int Health. 2010;15(12):1449–1457. doi:10.1111/j.1365-3156.2010.02646.x
- Desale A, Taye B, Belay G, Nigatu A. Assessment of laboratory logistics management information system practice for HIV/AIDS and tuberculosis laboratory commodities in selected public health facilities in Addis Ababa, Ethiopia. Pan Af Med J. 2013;15:1. doi:10.11604/pamj.2013.15.46.1969
- Shumbej T, Menu S, Gebru T, et al. Essential in-vitro laboratory diagnostic services provision in accordance with the WHO standards in Guragae zone primary health care unit level, South Ethiopia. Trop Dis Travel Med Vaccines. 2020;6(1):1–7. doi:10.1186/s40794-020-0101-0
- Dellie E, Andargie Biks G, Asrade G, Gebremedhin T. Intentions to leave and associated factors among laboratory professionals working at Amhara National Regional State public hospitals, Ethiopia: an institution-based cross-sectional study. BMC Res Notes. 2019;12(1):1–7. doi:10.1186/s13104-019-4688-z
- Tamil Selvi M, Implementing AS. QMS and accreditation standards in medical diagnostics for quality of services. Med Lab J. 2019;13(1):37–41. doi:10.29252/mlj.13.1.37
- Schroeder LF, Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141(6):791–795. doi:10.1309/AJCPQ5KTKAGSSCFN
- Musau S, McCarthy K, Okumu A, et al. Experience in implementing a quality management system in a tuberculosis laboratory, Kisumu, Kenya. Int J Tuberculosis Lung Dis. 2015;19(6):693–695. doi:10.5588/ijtld.14.0886
- Tamil SM, Srinivas A. Evaluation of quality management systems implementation in medical diagnostic laboratories benchmarked for accreditation. J Med Lab Diagnosis. 2015;6(5):27–35. doi:10.5897/JMLD2015.0104
- Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007;53(7):1338–1342. doi:10.1373/clinchem.2007.088344
- Aziz MA. External quality assessment for AFB smear microscopy. 2002.
- Dale JC, Novis DA. Outpatient phlebotomy success and reasons for specimen rejection: a Q-Probes study. Arch Pathol Lab Med. 2002;126(4):416–419.
- Bharadwaj S. Customer satisfaction leads to sustainable competitive advantage: with special reference to the Lalimou eco-tourism camp in Nameri national park. Int J Res Humanities Arts Literature. 2018;6(7):29–38.
- Mekonen T, de Dieu Iragena J, Albert H, Kao K, Erni D, Onyebujoh PC. Implementation of quality management systems and progress towards accreditation of National Tuberculosis Reference Laboratories in Africa. Af J Lab Med. 2017;6(2):1–8.
- Nwaokorie F, Ojo E. Overview of the implementation of quality management system in Nigerian medical laboratories. Univ Lagos J Basic Med Sci. 2021;6:548.
- De Silva S, Gunarathna HT, Rajakulasooriya R, Thambavita D. Assessment of knowledge and practices related to technical requirements recommended by ISO 15189: 2012 standards among medical laboratory technology staff in tertiary care hospitals and institutions in Western Province, Sri Lanka. 2021.