Abstract
Purpose
Formalin-fixed tissue biopsies submitted to the Histopathology Laboratory were explored for possible use in the GeneXpert MTB/Rifampicin assay for EPTB diagnosis, therefore this study aimed to determine the diagnostic utility of the GeneXpert MTB/RIF assay in EPTB detection in formalin-fixed paraffin-embedded tissue biopsies using Ziehl-Neelsen (ZN) and Haematoxylin and Eosin (H&E) staining as a composite reference standard.
Methods
A total of 89 selected archived formalin-fixed paraffin-embedded tissues with histological features of tuberculosis were included in the study. ZN and H&E staining were performed as a composite reference standard, followed by GeneXpert MTB/RIF assay as diagnostic test. Data were analysed using STATA software version 17.0.
Results
Of the 89 specimens enrolled, 55% were male. The Lymph node was the commonest site (64%). 15/89 (16.9%) cases were positive for ZN, 45/89 (50.6%) were positive for H&E and 27/89 (30.3%) were positive for GeneXpert. The overall sensitivity and specificity of GeneXpert were 48.94% (95% CI: 38.55%–59.32%) and 90.48% (95% CI: 84.38%–96.57%), respectively, in lymph node tissues, were 52.94% (95% CI: 39.98%–65.90%) and 82.61% (95% CI: 72.77%–92.45%), respectively, and in Non-Lymph node tissues, were 38.46% (95% CI: 21.61%–55.32%) and 100% (95% CI: 100%–100%), respectively.
Conclusion
The GeneXpert MTB/RIF assay is a potential tool for diagnosing EPTB in FFPE tissues and may be used in the development of an algorithm in the histopathology laboratory.
Keywords:
Introduction
Tuberculosis (TB) is an airborne communicable disease caused by mycobacterium tuberculosis. This usually spreads from one person to another by coughing.Citation1 The disease affects the lungs (pulmonary tuberculosis) but can also affect other sites (extra pulmonary tuberculosis).Citation2 Extra pulmonary tuberculosis (EPTB) represented 15% of the 6.3 million cases of TB globally and 16% of 1.2 million cases of TB in Africa in 2016.Citation3 Children and immunosuppressed patients are at a higher risk of extra pulmonary tuberculosis than the general population.Citation4 In 2018, the prevalence of EPTB among people living with Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS) in Uganda was estimated to be 17%.Citation5 Extra pulmonary tuberculosis mainly affects the lymph nodes, meninges, kidney, spine, abdomen, genitourinary tract, bone, and joints.Citation6,Citation7 Its diagnosis can be challenging because of the presence of a few bacilli in the tissues,Citation8,Citation9 and it can require analysis of fresh or fixed tissue biopsies. Fresh frozen tissues are often not available in clinical practice because resected tissues must be frozen in liquid nitrogen 30–60 min after surgical resection, and the cost of preserving fresh frozen tissue is relatively high because of the maintenance of a constant low temperature. Formalin-fixed paraffin-embedded (FFPE) tissues have several advantages, such as preservation of cellular and architectural morphology, the possibility of storage at room temperature for several years, and easy availability of FFPE blocks, as they are routinely prepared in the pathology departments of most centres.Citation10
Various methods are used to diagnose EPTB, including Ziehl-Neelsen (ZN) staining, mycobacterial culture, histopathology, tuberculin skin test (TST), serological assays, interferon-gamma release assays (IGRAs), and nucleic acid amplification (NAA) tests.Citation11 Culture is the gold standard technique for isolating M. tuberculosis; however, it is time-consuming,Citation12 requires fresh tissue biopsies,Citation7 and has a turnaround time of 4–8 weeks, which makes the technique too slow to aid in treatment decisions and requires skilled technicians.Citation7,Citation13,Citation14
Positive Ziehl-Neelsen (ZN) staining helps to confirm EPTB in a wide spectrum of formalin-fixed paraffin embedded (FFPE) tissues and smears.Citation15 Conventional Smear microscopy is widely used in the diagnosis of EPTB but has a low sensitivity (0%–40%).Citation14 Negative results cannot exclude the presence of TB and cannot differentiate between Mycobacterium tuberculosis and non-Mycobacterium tuberculosis.Citation7,Citation13,Citation16
Histological diagnosis of tuberculosis on Haematoxylin and Eosin (H&E)-stained sections usually relies on the presence of classical necrotizing granulomas with Langhans giant cells and does not distinguish between EPTB and infections from other granulomatous diseases such as sarcoidosis, leprosy, fungal infections, brucellosis, syphilis, and systemic lupus erythematosus.Citation15,Citation17 A negative smear of acid-fast bacilli, lack of granulomas on Haematoxylin and Eosin (H&E) stained section and failure to culture does not exclude EPTB. Diagnostic modalities such as nucleic acid amplification (NAA) have been useful in various forms of EPTB.Citation11,Citation13,Citation18
Nucleic acid amplification tests are diagnostic tests, which detect and amplify genetic material (ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Nucleic acid amplification techniques are rapid and sensitive, which helps in the early diagnosis and management of TB, especially in immunocompromised patients with a history of contact with active tuberculosis patients.Citation18 There are a number of Nucleic Acid Amplification (NAA) methods have been developed, such as polymerase chain reaction (PCR), automated GeneXpert MTB/Rifampicin assay for rapid detection of Mycobacterium tuberculosis (MTB) in clinical specimens of pulmonary and extra-pulmonary tuberculosis cases, which not only provides the advantage of rapid diagnosis but also detects low MTB genomic copies in various specimens.Citation14,Citation18,Citation19
Previous studies have recommended the application of GeneXpert MTB/RIF assay in the detection of EPTB in FFPE tissues, though some had low sensitivity (30%–55%),Citation14,Citation20,Citation21 and others had high sensitivity (60%–90%),Citation12,Citation17,Citation22 using different reference standards. Therefore, the aim of this study is to determine the diagnostic utility of GeneXpert MTB/RIF assay in EPTB detection in formalin-fixed paraffin-embedded tissue biopsies using ZN and H and E staining as a composite reference standard at Mbarara University of Science and Technology Pathology Laboratory in south western Uganda.
Materials and Methods
Study Design
This was a cross-sectional laboratory-based study, conducted from February 2023 to September 2023. The study involved collection of paraffin wax-embedded block tissues from patients diagnosed with EPTB from the archive. The samples were then stored in a tissue block cabin.
Study Site
The study was carried out at the Mbarara University of Science and Technology Pathology Laboratory as well as the Genomics and Translational Laboratory of the same University located in south- western Uganda. They are located in Mbarara city, which is approximately 250 km from the capital city of Kampala. They are managed by pathologists, microbiologists, histologists, laboratory technologists, laboratory technicians, and residents.
It provides clinical services to the Mbarara Regional Referral Hospital and people from the western region of Uganda. It receives samples from all district hospitals in western Uganda, some private facilities, and some parts of neighbouring countries such as Tanzania, Rwanda, and the Democratic Republic of Congo.
Study Population
Archived formalin-fixed paraffin-embedded EPTB tissue specimens with histological diagnostic features of EPTB were obtained at the Mbarara University Histopathology Laboratory, south- western Uganda.
Sample Size Estimation
The sample size was calculated using the Buderer formula to determine the diagnostic accuracy.Citation23 Sensitivity and specificity were calculated using 1.96 Z score corresponding to 95% confidence interval, 5% width of the 95% confidence interval, expected sensitivity and specificity of 84.9% and 92.5%, respectively, from previous study,Citation24 and prevalence of known positives and known negatives of EPTB by composite reference standard of ZN and H&E. This yielded a total of 106 tissue blocks.
Eligibility Criteria
Using medical record system archived formalin-fixed paraffin-embedded tissue samples with diagnostic histological features of extra pulmonary tuberculosis at the study site were selected and included in the study. Poorly processed, damaged, samples without clinical information or demographic data, or with too small amounts of tissue preserved to produce adequate samples for ZN staining, H, E and GeneXpert MTB/RIF assay were excluded from the study.
Sampling Method
A purposive sampling technique was used to select archived samples with histological diagnostic features of EPTB.
Data Collection Tool
A data collection tool was developed to capture clinical information, diagnoses and demographics of the sample. Registers were used to identify details of the samples with a clinical diagnosis of EPTB.
Laboratory Investigations
Tissue Sectioning
Each FFPE tissue block was trimmed and 3 µm thickness for ZN and H&E staining and 10 µm thickness for the GeneXpert assay were cut using a rotary microtome. Two slides were made. One slide was used for ZN staining and the other for H&E staining. The remaining sections (10 µm thickness) were placed in Eppendorf tubes for DNA extraction. Tissue sections for ZN, H, and E staining were floated in 41°C–42°C water baths for 1 minute to remove the folds and wrinkles. The tissue sections were scooped using a slide at a perpendicular angle.
Ziehl-Neelsen Staining
Using laboratory developed manual test, tissue sections were deparaffinised in xylene, rehydrated through graded alcohols in descending order, and then with distilled water. The tissue sections were then stained with the ZN staining technique, which involved strong carbolfuchsin which stained the mycobacteria; acid alcohol, which acted as a decolouriser; and methylene blue, which was a counterstain. This staining technique relies on acid resistance of mycobacteria. Deparaffinization was performed to eliminate paraffin wax in the tissue sections prior to staining. ZN-stained slides were examined, along with the controls for AFBs under light microscope and reported as positive or negative. The AFBs were quantified and recorded as +, ++, and +++.
H&E Staining
The tissue sections were stained with haematoxylin and eosin (H&E), a manual laboratory developed test, and a routine staining technique in the Histopathology Laboratory. Along with the controls, the histological pattern of inflammation in TB was recorded after microscopic examination.
GeneXpert MTB/RIF Assay
DNA extraction was performed at the Genomics and Translational Laboratory, Department of Microbiology, Mbarara University of Science and Technology (MUST) using a specialized kit protocol (Quick-DNATM FFPE, Zymo Research). The tissue sections were deparaffinised using deparaffinization solution, DNA was extracted using proteinase K (Zymo Research) which digested the tissue and dislodged the nucleic acid from the membrane and DNA was purified using Genomic lysis buffer, Genomic wash 1 and 2 and elution buffer.
Following the manufacturer’s instructions and recommendations of GeneXpert (DX system version 6.4, Cepheid, USA), the extracted DNA suspension was mixed with the reagent provided for the assay, and a cartridge containing this mixture was placed in the GeneXpert MTB/RIF machine. All the processes were fully automated. The test simultaneously detected Mycobacterium tuberculosis complex (MTBC) and rifampicin resistance (RIF) within <2h. GeneXpert MTB/RIF results were recorded as detected, not detected, or indeterminate. Proper sample preparation, quality control and quality assurance procedures and validation of GeneXpert MTB/RIF assay on FFPE tissues were performed as recommended by GeneXpert (DX system version 6.4, Cepheid, USA). GeneXpert MTB/RIF assay was validated for use on FFPE tissues using PCR to detect EPTB. Few known ZN and H&E positive and negative tissue cases were subjected to both GeneXpert MTB/RIF assay and PCR assay and results were compared for reliability and accuracy.
Data Management and Analysis
All data sheets were checked for completeness prior to data entry. The information recorded on the data sheets was entered into an Excel spread sheet (Microsoft Office Professional Plus 2010). Appropriate data verification was performed whenever necessary. The dataset was imported into the STATA software (version 17.0; StataCorp LLC, College Station, Texas, United States). Descriptive statistics were used to characterize the population using frequencies, median values, and interquartile ranges (IQRS). Cross tabulation was performed to calculate the diagnostic utility (sensitivity, specificity, negative predictive value, and positive predictive value) of GeneXpert MTB/RIF using the ZN and H&E staining technique as a composite reference standard. Sensitivity, specificity, positive and negative predictive values of GeneXpert assay is reported as proportions with 95% confidence intervals. Receiver operating characteristic curve (ROC) was performed to determine the predictive performance of the GeneXpert assay for EPTB detection.
Results
Demographic Characteristics of Analysed Specimens
Of 106 purposively selected archived tissues with cases of EPTB, 89 were enrolled, and 17 were excluded because they did not fulfil the eligibility criteria, as shown in . Of 89 cases enrolled, 40 (45%) were female and 49 (55%) were male. The female-to-male ratio was 0.8–1. The age of the 89 patients ranged from 1 to 88 years, with a mean of 34.3 years and a median of 30 years (IQR 31). Different tissue types were analysed and classified into ten groups according to the topographic site of the biopsy. Lymph nodes were the most common sites, with 57/89 (64%) specimens, as shown in .
Table 1 Demographic Characteristics of Analysed Specimens
Histopathological Findings on Haematoxylin and Eosin Stain
Necrotizing granulomatous inflammation (classical granuloma) was the predominant histological pattern of inflammation, contributing 45/89 (50.6%) of the specimens. Non-classical epithelioid granulomas were seen in 16/89 (17.9%) cases, and no epithelioid granulomas were observed in 28 /89 (31.5%) cases. Inflammation pattern of classical granuloma was the type of inflammation which was considered as positive for Mycobacterium tuberculosis. The positive rate of H&E staining (classical granuloma) was higher in female 23 (51.1%) than in male 22 (48.9%), in age group of 0–30 years 20 (44.4%), and highest in lymph node tissues 33 (73.3%), as shown in and .
Table 2 Test Results for H&E, ZN and GeneXpert
Table 3 Test Results for ZN, H&E and GeneXpert by Sex, Age and Tissue Types
ZN Findings
ZN staining was positive in 15/89 (16.9%) cases. The lymph node tissues had the highest ZN positivity rate 13 (86.7%). The male had higher ZN positive rate 9 (60%) than in female 6 (40%) and age group of 0–30 years had the highest ZN positivity 7 (46.7%), as shown in and .
GeneXpert Findings
The positivity rate for GeneXpert was 30.3% (27/89). In lymph node tissues, the positivity rate of GeneXpert was 22 (81.5%), whereas in non-lymph node tissues it was 5(18.5%). The bacilli load of GeneXpert was quantified as trace in 21/27 (77.8%) positive cases, very low in 5/27 (18.5%) positive cases, and low in 1/27 (3.7%) positive cases. The Resistance (RR) was indeterminate in 21/27 (77.8%) positive cases. However, 5 of the 27 GeneXpert MTB/RIF-positive cases that showed rifampicin resistance (RR) not detected had a “very low” bacilli load, also rpoB positive and 1 of the 27 rifampicin GeneXpert MTB/RIF-positive cases that showed rifampicin resistance not detected, had a “low” bacilli load and rpoB positive. None of the cases showed a “high” bacilli load and there were no cases of rifampicin resistance. GeneXpert rpoB positive means GeneXpert MTB/RIF assay has detected the rpoB gene, which indicates the presence of rifampicin-resistant Mycobacterium tuberculosis and rpoB is a gene that codes for the beta subunit of RNA polymerase, the enzyme responsible for transcription in Mycobacterium tuberculosis. The positive rate of GeneXpert was higher in females 15 (55.6%) than in males 12 (44.4%), in age group of 0–30 years 16 (59.3%), and highest in lymph node tissues 22 (81.5%), as shown in and .
GeneXpert Findings Using ZN and H&E Histology as Composite Reference Standard
Using the ZN and H&E histology results as the composite reference standard because of their easy availability and low cost, GeneXpert displayed 23 true positives (TP), 38 true negatives (TN), 4 false positives (FP), and 24 false negatives (FN) (). In lymph node tissues, GeneXpert displayed 18 true positives (TP), 19 true negatives (TN), 4 false positives (FP), and 16 false negatives (FN) and in non-lymph node tissues, GeneXpert displayed 5 true positives (TP), 19 true negatives (TN), 0 false positives (FP), and 8 false negatives (FN), as shown in .
Table 4 Overall Contingence Table for All Tissues
Table 5 Contingence Table for Lymph Node and Non Lymph Node Tissues
Diagnostic Utility of GeneXpert MTB/RIF Assay Using ZN, H&E Staining as the Composite Reference Standard
From and , the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of GeneXpert were 48.94% (95% CI: 38.55%–59.32%), 90.48% (95% CI: 84.38%–96.57%), 85.19% (95% CI: 77.80%–92.57%), and 61.29% (95% CI: 51.17%−71.41%), respectively, as shown in . In lymph node tissues, the sensitivity, specificity, PPV, and NPV of GeneXpert were 52.94% (95% CI: 39.98%–65.90%), 82.61% (95% CI: 72.77%–92.45%), 81.82% (95% CI: 71.81%–91.83%), and 54.29% (95% CI: 41.35% - 67.22), respectively, and in non-lymph nodes tissues, the sensitivity, specificity, PPV, and NPV of GeneXpert were 38.46% (95% CI: 21.61%–55.32%), 100% (95% CI: 100%–100%), 100% (95% CI: 100%–100%), and 70.37% (95% CI: 54.55%–86.19%), respectively, as shown in .
Table 6 Diagnostic Utility of GeneXpert Against ZN and H&E as Composite Reference Standard
Table 7 Diagnostic Utility of GeneXpert by Tissue Type Against ZN and H&E as Composite Reference Standard
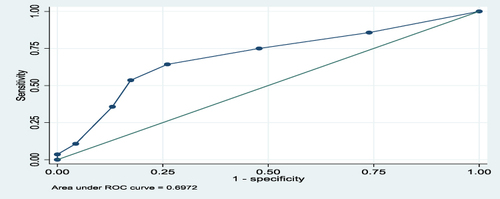
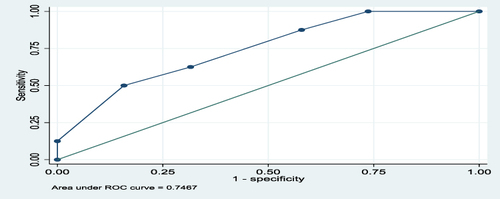
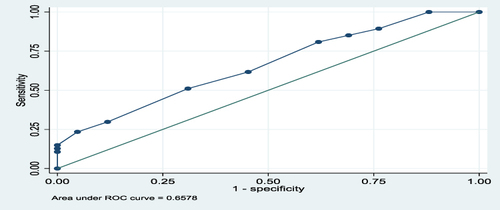
The overall predictive performance of GeneXpert in diagnosis of EPTB in FFPE tissues using a composite reference standard of H, and E, and ZN was 66%, as shown by an area under the ROC curve of 0.6578, as shown in . For lymph node and non-lymph node tissues, the predictive performances of GeneXpert in diagnosis of EPTB using a composite reference standard of H, and E, and ZN were 69.7% and 75%, respectively, as shown by the areas under the ROC curves of 0.697 and 0.747, as shown in and , respectively.
Figure 2 Area under ROC curve for all Tissues.
Discussion
Sensitivity and Specificity of GeneXpert MTB/RIF in Diagnosis of EPTB Assay in Formalin Fixed Embedded Tissues
GeneXpert MTB/RIF showed an overall moderate sensitivity of 48.94% and high specificity of 90.48%. Within lymph node tissues, the sensitivity and specificity of GeneXpert were 52.9% and 82.6%, respectively. In non-lymph node tissues, the sensitivity and specificity of GeneXpert were 38.5% and 100%, respectively. Based on these findings, the diagnostic utility of GeneXpert in lymph node tissues was much better than in non-lymph node tissues. The overall sensitivity and the sensitivity of lymph nodes and non-lymph nodes in our study were relatively good, although low, and the specificities were perfectly good, especially in non-lymph node tissues (100%).
The area under the ROC curve for all tissues (lymph nodes and non-lymph nodes) was 0.6578 and for lymph node tissues was 0.6972 and for non-lymph node tissues was 0.7467. This meant there was 66%, 69.7%, and 75%, respectively, chance of distinguishing between positive and negative classes. The closer the ROC curve is to the upper left corner of the graph, the higher the accuracy of the test because the sensitivity is 1 in the upper left corner and the false positive rate is 0 (specificity = 1).Citation25 Therefore, in this study, the areas under the ROC curves, as mentioned above, showed moderate accuracy (performance) for the test in overall tissues, lymph node, and non-lymph node tissues because they were all above 0.5. The ROC AUC score ranges from 0 to 1, where 0.5 indicates no capacity for class separation (distinguishing between positive and negative classes), and 1 indicates perfect performance.Citation25
Few studies have reported the diagnostic utility of the GeneXpert assay in the diagnosis of EPTB in FFPE Tissues using ZN and H&E as composite reference standards. The findings of this study were almost similar to other studies, and a study conducted in Kenya to demonstrate the potential utility of GeneXpert MTB/RIF which compared the performance of Ziehl-Neelsen staining for the detection of Mycobacterium tuberculosis from FFPE tissues using H&E staining as the gold standard, had a GeneXpert sensitivity of 53.2% and ZN sensitivity of 20.3%.Citation14 In a similar study carried out in Zambia, a low sensitivity and specificity of 30% and 78%, respectively, in lymph node tissues, 35% and 94% in non-lymph node tissues respectively was reported.Citation20 Another similar study reported by Huang, Qin found that the sensitivity and specificity of GeneXpert for FFPE tissues to be 67.3% and 100%, respectively.Citation17
The diagnostic utility of GeneXpert in this study is acceptable, although with low sensitivity, and this low rate of detection could be due to the presence of non-tuberculous mycobacteria.Citation20,Citation26 Histological diagnosis of tuberculosis on haematoxylin and eosin (H&E) stained section usually relies on the presence of classical necrotizing granulomas with Langhans giant cells and does not distinguish between EPTB and infections from other granulomatous diseases such as sarcoidosis, leprosy, fungal infections, brucellosis, syphilis, and systemic lupus erythematous.Citation15,Citation17 In this study, H&E histology yielded positivity of 50.6% (45/89) and GeneXpert yielded 30.3% (27/89) which brings in the possibility of non-tuberculous mycobacteria.
Active TB, in the absence of granulomatous inflammation, could be another reason for the low detection rate.Citation23 In this study, four cases were consistent with reactive lymphadenitis with no granulomas but became GeneXpert positive, which led to false positives thus affecting diagnostic utility.
Another reason could be the heterogeneous distribution of bacilli in the tissue sections, probably because of sampling errors which may lead to false negatives.Citation22 This study, found that some tissues contained bacilli, whereas others did not. In this case, 23 cases, which were H&E positive but GeneXpert negative were reprocessed using different tissue sections and retested with GeneXpert turned out to be positive (three cases). The failure to differentiate classical granulomas from non-classical granulomas on histological sections of the same cases affected the diagnostic utility of GeneXpert. Three cases of non-classical granulomas in this study were positive for GeneXpert.
The pauci-bacillary tissues of EPTB affected the ZN technique, thereby affecting the diagnostic utility of GeneXpert.Citation26 Since ZN technique has low sensitivity, as reported by other studies (10%–30%),Citation14,Citation20 its difficulty for ZN technique to detect few bacilli in tissue sections of EPTB. In this study, ZN technique had the positivity rate of 16.9% (15/89), and most of them were pauci-bacillary.
Improper tissue digestion or cell wall lysis of organism during processing is also another reason, which could have contributed to low detection or low diagnostic utility of GeneXpert as explained in previous studies.Citation27 When the tissue or cell wall of an organism is not well digested by proteinase K or not well lysed with GeneXpert lysing reagent, DNA remains masked which affects the amplification process. In this study, we used rapid digestion (incubation at 55°C for 1–4 hours) to digest the tissue. Some tissues might have remained undigested, which might have affected the amplification process. Standard digestion (incubation at 55°C overnight) would have been better; however, because of time constraints, it was impossible.
The reason for the sensitivity and specificity to be similar to other studies or better than some of the studies mentioned above is unclear, although the possibility of using ZN and H&E as composite reference standards could have contributed. Another reason could be the use of a modified in-house DNA extraction protocol: In some cases (78 cases), tissue DNA was extracted by deparaffinising, digesting tissue using proteinase K enzyme, and later subjected to GeneXpert buffer for debris lysis, and a repeat of cases (30 cases) by subjecting deparaffinised tissue directly to GeneXpert reagent without tissue digestion by enzyme (Proteinase K), all of which gave similar and better results.
Negative Predictive Value and Positive Predictive Value of GeneXpert MTB/RIF Assay in Diagnosis of EPTB in Formalin Fixed Paraffin Embedded Tissues
The GeneXpert assay demonstrated a positive predictive value (PPV) of 85.19% and a negative predictive value (NPV) of 61.29%. In lymph node tissues, GeneXpert assay demonstrated a positive predictive value of 81.8% and a negative predictive value of 54.3%, while as in non-lymph node tissues, it demonstrated a positive predictive value of 100% and a negative predictive value of 70.4%, respectively. In non-lymph node tissues, the PPV (100%) and NPV (70.4%) were perfectly good compared to those PPV (81.8%) and NPV (54.3%) in lymph node tissues. Romdhane, in their study, demonstrated PPV of 100% and NPV of 25% when comparing GeneXpert with microbiology for diagnosis of tuberculous spondylodiscitis in FFPE tissues.Citation28 In another similar study, to determine the performance of GeneXpert Ultra in the diagnosis of tuberculous cervical lymphadenitis in formalin-fixed paraffin-embedded tissues using ZN as gold standard showed a PPV of 100% and NPV of 71%.Citation26 In this study, the PPV was low (85.19%) and NPV (61.3%) was high compared to the above other studies. In non-lymph node tissues, the PPV (100%) and NPV (70.4%) were perfectly good compared with other studies above. This could be due to the use of ZN and/or H&E staining as composite reference standards. This combination increases the probability of a case without a disease not to have a disease or a case with a disease to have a disease. The reason for the low PPV in the overall and lymph node tissues remains unclear.
On top of the above reasons for low detection rate, this study has some other limitations. As this was a retrospective study with no direct contact with patients, we could not obtain some important information on HIV status, mycobacterial culture, which could have guided on result reporting of tests. Lack of resources to perform additional tests such as IHC, PCR was also another limitation.
Conclusion
Based on the findings of this study, the GeneXpert MTB/RIF assay is a potential assay for diagnosing EPTB in FFPE tissues, and may be used as an alternative test for ZN or an “additional test” for the detection of M. tuberculosis in formalin-fixed tissues, especially in cases that are negative for ZN. Therefore, the use of the GeneXpert assay for the diagnosis of EPTB in FFPE tissues and the development of an algorithm is recommended because GeneXpert has shown to be a potential diagnostic assay for the detection of M. tuberculosis in FFPE tissues.
Abbreviations
TB, tuberculosis; AFB, acid fast bacilli; EPTB, extra pulmonary tuberculosis; WHO, World Health Organization; DNA, deoxyribonucleic acid; MTB, Mycobacterium tuberculosis; RIF, rifampicin; ZN, Ziehl-Neelsen; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; H&E, haematoxylin and eosin; PCR, polymerise chain reaction; FFPE, formalin-fixed paraffin embedded; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; NAA, Nucleic Acid Amplification; MUST, Mbarara University of Science and Technology.
Ethical Considerations
The study was approved by the Research Ethics Committee (MUST-2022-400) of the Mbarara University of Science and Technology (MUST) and informed consent waiver was obtained from the Research Ethics Committee (MUST) as the study was using archived tissue samples. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and to ensure confidentiality, the specimens were de-identified by removing their personal information. The study was laboratory-based, with no direct contact with patients.
Disclosure
The authors report no competing interests in this study.
Acknowledgments
Special thanks to the staff of the Histopathology, Genomics, and Translational Laboratories of the Mbarara University of Science and Technology (MUST) for their tremendous support. The Abstract of this paper was submitted to Mbarara University of Science and Technology (MUST) institutional repository.
References
- World Health Organisation. World Health Organisation (WHO) Global tuberculosis report 2014. Geneva WHO; 2014.
- World Health Organization. WHO; Tuberculosis; Available from: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed May 1, 2015.
- World Health Organisation. World Health Organization (WHO) Global Tuberculosis report; 2017.
- WHO, world health organisation (WHO) policy statement on Xpert MTB-RIF 2013; 2013.
- Mohammed H, Assefa N, Mengistie B. Prevalence of Extrapulmonary Tuberculosis Among People Living with HIV/AIDS in Sub-Saharan Africa: A Systemic Review and Meta-Analysis. HIV/AIDS. Auckland, N.Z; 2018:225–237.
- Vadwai V, Boehme C, Nabeta P, et al. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49(7):2540–2545. doi:10.1128/JCM.02319-10
- Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuber Resp Dis. 2015;78(2):47–55. doi:10.4046/trd.2015.78.2.47
- Wamala D, Okee M, Kigozi E, et al. Predominance of Uganda genotype of Mycobacterium tuberculosis isolated from Ugandan patients with tuberculous lymphadenitis. BMC Research Notes. 2015;8(1):398. doi:10.1186/s13104-015-1362-y
- Hoel IM, Syre H, Skarstein I, et al. Xpert MTB/RIF ultra for rapid diagnosis of extrapulmonary tuberculosis in a high-income low-tuberculosis prevalence setting. Sci Rep. 2020;10(1):13959. doi:10.1038/s41598-020-70613-x
- Gao XH, Wei Q, Bi S, et al. Comparison of fresh frozen tissue with formalin-fixed paraffin-embedded tissue for mutation analysis using a multi-gene panel in patients with colorectal cancer. Front Oncol;2020. 10. doi:10.3389/fonc.2020.00010
- Lange C, Mori T. Advances in the diagnosis of tuberculosis. Respirology. 2010;15(2):220–240. doi:10.1111/j.1440-1843.2009.01692.x
- Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, et al. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: a multicenter surveillance. Sci Rep. 2019;9(1):18515. doi:10.1038/s41598-019-55112-y
- Mehta PK, Raj A, Singh N, et al. Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol Med Microbiol. 2012;66(1):20–36. doi:10.1111/j.1574-695X.2012.00987.x
- Njau AN, Gakinya SM, Sayed S, et al. Xpert(®) MTB/RIF assay on formalin-fixed paraffin-embedded tissues in the diagnosis of extrapulmonary tuberculosis. Afr J Lab Med. 2019;8(1):748. doi:10.4102/ajlm.v8i1.748
- Purohit M, Mustafa T. Laboratory Diagnosis of Extra-pulmonary Tuberculosis (EPTB) in Resource-constrained Setting: state of the Art, Challenges and the Need. J Clin Diagn Res. 2015;9(4):EE01–EE6. doi:10.7860/JCDR/2015/12422.5792
- Haldar S, Bose M, Chakrabarti P, et al. Improved laboratory diagnosis of tuberculosis–the Indian experience. Tuberculosis. 2011;91(5):414–426. doi:10.1016/j.tube.2011.06.003
- Huang S, Qin M, Shang Y, et al. Performance of Xpert MTB/RIF in Diagnosis of Lymphatic Tuberculosis from Fresh and Formaldehyde-Fixed and Paraffin Embedded Lymph Nodes. Tuberculosis. 2020;124:101967
- Agrawal M, Bajaj A, Bhatia V, et al. Comparative study of GeneXpert with ZN stain and culture in samples of suspected pulmonary tuberculosis. J Clin Diagn Res. 2016;10(5):DC09. doi:10.7860/JCDR/2016/18837.7755
- Saglam L, Akgun M, Aktas E. Usefulness of induced sputum and fibreoptic bronchoscopy specimens in the diagnosis of pulmonary tuberculosis. J Int Med Res. 2005;33(2):260–265. doi:10.1177/147323000503300215
- Polepole P. Performance of the Xpert MTB/RIF Assay in the Diagnosis of Tuberculosis in Formalin‑fixed, Paraffin‑embedded Tissues. Int J Mycobacteriol. 2017;6:6.
- McMillen T, Usiak SC, Chen LH, et al. Evaluation of the Xpert MTB/RIF Performance on tissues: Potential impact on airborne infection isolation at a tertiary cancer care center. Infect Control Hosp Epidemiol. 2018;39(4):462–466. doi:10.1017/ice.2018.7
- Budvytiene I, Banaei N, Land GA. Simple processing of formalin-fixed paraffin-embedded tissue for accurate testing with the Xpert MTB/RIF assay. J Clin Microbiol. 2020;58(3). doi:10.1128/JCM.01905-19
- Buderer NMF. Statistical methodology: i. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emer Med. 1996;3(9):895–900. doi:10.1111/j.1553-2712.1996.tb03538.x
- Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44(2):435–446. doi:10.1183/09031936.00007814
- Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean j Anesthes. 2022;75(1):25–36. doi:10.4097/kja.21209
- Romdhane E, Arfaoui A, Benabdessalem C, et al. Performance of GeneXpert Ultra in the Diagnosis of Tuberculous Cervical Lymphadenitis in Formalin Fixed Paraffin Embedded Tissues. Tuberculosis. 2020;125:102012
- Barcelos D, Franco MF, Leão SC. Effects of tissue handling and processing steps on PCR for detection of Mycobacterium tuberculosis in formalin-fixed paraffin-embedded samples. Rev Inst Med Trop São Paulo. 2008;50:321–326. doi:10.1590/S0036-46652008000600002
- Romdhane E, Rammeh S, Bouaziz CM, et al. Performances of single tube nested polymerase chain reaction and GeneXpert ultra on Formalin fixed paraffin embedded tissues in the diagnosis of tuberculous spondylodiscitis. Clin Rheumatol. 2021;40(10):4317–4323. doi:10.1007/s10067-021-05782-9