Abstract
Background
The purpose of this study was to assess the 1-year clinical, functional, and safety-related outcomes following a switch to olanzapine of at least one typical antipsychotic drug in the previous regimen in the treatment of patients of schizophrenia in Japan.
Methods
Using data from a large 1-year prospective, multicenter, naturalistic study of olanzapine for the treatment of schizophrenia in Japan, patients who were switched from any oral typical antipsychotic to olanzapine were identified. Mixed models for repeated measures, controlling for baseline demographics, were utilized to assess outcomes for clinical and functional measures.
Results
Of the 262 patients who switched from typical antipsychotics to olanzapine, 41% were outpatients and 59% were inpatients. Most of these patients were switched due to poor medication efficacy (71.0%) or medication intolerability (25.6%). Most patients (71.4%) completed the 1-year study. Clinically and statistically significant (P < 0.01) improvements were observed in patient illness severity and health-related quality of life, including improvements in global symptom severity and in positive, negative, depressive, and cognitive symptoms. Over half of the patients (58.3%) demonstrated a treatment response to olanzapine and 47.4% achieved symptom remission. Mean weight gain from baseline to endpoint was 2.31 ± 4.72 kg, with 30.4% of patients experiencing clinically significant weight gain (at least 7% of baseline weight).
Conclusion
During this 1-year naturalistic treatment of schizophrenia patients in Japan, switching from typical antipsychotics to olanzapine resulted in significant improvements in patients’ clinical and functional outcomes. Approximately one-third of patients had clinically significant weight gain. These findings highlight the favorable benefit to risk profile of switching to olanzapine following failure on typical antipsychotics.
Introduction
Antipsychotic medications represent the cornerstone of treatment for schizophrenia and are effective for both reducing acute symptoms and for preventing future relapses.Citation1–Citation5 In usual care settings, antipsychotic treatment is often a dynamic process that involves changing medications,Citation6,Citation7 primarily due to problems with medication efficacy,Citation6,Citation8 but also due to patient preferences, medication intolerability, and nonadherence.
In Japan, typical antipsychotics continue to be used frequently for the treatment of schizophrenia.Citation9–Citation11 However, the use of atypical antipsychotics (eg, risperidone, olanzapine, quetiapine, perospirone, and aripiprazole) is rapidly increasing.Citation11 Olanzapine has been reported to be the second most frequently used atypical antipsychotic in JapanCitation11 and may be a likely choice of medication following failure of a typical antipsychotic for patients with schizophrenia.
Japan has been reported to have the highest level of psychiatric inpatient beds per capita.Citation12 Many patients who are treated in hospitals could possibly be treated as outpatients in the community,Citation12 but community-based psychiatric services to handle this load of patients are not fully developed in Japan.Citation11 Understanding any differences in outcomes for patients with schizophrenia treated in inpatient versus outpatient settings may be of particular importance in Japan.
To understand better the outcomes for patients with schizophrenia who switch from a typical antipsychotic to olanzapine in usual care in Japan, we analyzed data for a subset of patients from a larger observational study.Citation13 The objectives of this analysis were two-fold, ie, to assess clinical, functional, and safety-related outcomes following a switch from a typical antipsychotic to olanzapine in a 1-year naturalistic study of schizophrenia patients in Japan, and to compare treatment outcomes between inpatients and outpatients who were switched from a typical antipsychotic to olanzapine.
Methods
Data source
This post hoc analysis used data from a large (n = 1949) multicenter, naturalistic, 1-year, postmarketing surveillance study in Japan.Citation13 Postmarketing surveillance studies are single-arm studies designed to identify occurrences of serious adverse reactions quickly and are required by the regulatory bodies in Japan as part of the approval process to market a new medication. Primary eligibility for the olanzapine postmarketing surveillance study included a diagnosis of schizophrenia based on Diagnostic and Statistical Manual of the American Psychiatric Association, Fourth Edition (DSM-IV)Citation14 criteria and initiation of treatment with olanzapine. In this naturalistic, observational, and noninterventional study, all treatment decisions were left to the discretion of the treating clinician. The initiation of olanzapine could have been a patient’s first antipsychotic treatment, a switch from another antipsychotic treatment, or augmentation of their current antipsychotic regimen. When patients discontinued olanzapine, they discontinued participation in the study. Study enrollment ran from November 2003 until July 2004. Data were collected at the baseline, 3-month, 6-month, and 12-month visits.
All study procedures were approved by the internal review boards at each of the participating medical facilities. Informed consent was obtained based on the rules at each participating institution.
Almost all of the 1949 patients screened in the parent study met all eligibility criteria (94.9%, 1850 of 1949 patients).Citation13 Most of the eligible patients (67.1%, 1241 of 1850 patients) were not switched from an antipsychotic to olanzapine, but either had olanzapine added to their current antipsychotic regimen or were initiating a new course of antipsychotic treatment with olanzapine. The current analysis was further restricted to the 262 patients who were switched from an oral typical antipsychotic to olanzapine.
Measures
In order to avoid interference with usual-care processes, the procedures in this observational study were designed to capture only a limited amount of information. Invasive or cumbersome measures were not included.
The Clinical Global Impression-Schizophrenia (CGI-SCH) is a clinician-rated measure consisting of five ratings, ie, global severity, positive, negative, cognitive, and depressive symptoms. All ratings are made on an anchored scale ranging from no symptoms (0) to severe symptoms (6).Citation15 The concurrent validity of the CGI-SCH subscales with the corresponding subscales from the more rigorous Positive and Negative Syndrome ScaleCitation16 has been found to range from 0.61 for depressive symptoms to 0.86 for positive symptoms, with the remaining correlation coefficients ranging from 0.75 to 0.80. Interrater reliability has also been found to be moderately high (interclass correlation coefficients ranging from 0.73 to 0.82) for all but the depressive subscale (0.64).Citation15
The European Quality of Life-5 Dimensions (EQ-5D) is a generic measure of health-related quality of life that includes a visual analog scale of overall health (ranging from 0 to 100) and five measures of specific dimensions (level of movement, control of environment, normal activities, pain/discomfort, and anxiety/depression). The ratings on the five dimensions are used to create health states that have been assigned values (utilities) ranging from death (0) to perfect health (1).Citation17 The construct validity of the EQ-5D has been evaluated in a sample of individuals with schizophrenia: utility scores were moderately correlated with the PANSS Total Score (−0.51) and subscales (−0.20 to −0.59), and World Health Organization Quality of Life-Brief Questionnaire Overall Score (0.55) and subscales (0.32 to 0.64).Citation18
The study also collected a broad array of information on treatment, functioning, and adverse events. Information on concomitant medications included drug name, dose, route of administration, start and stop dates, and therapeutic category (antipsychotic, anticholinergic, antidepressant, anxiolytic/hypnotic, mood stabilizer, or other). In addition to the EQ-5D, measures of functioning included employment status (including working for pay) and number of social activities in the past 4 weeks (0, 1, 2, 3, 4, or 5+). The study included specific ratings of the following adverse events: dystonia/akathisia/parkinsonism, tardive dyskinesia, decreased libido, amenorrhea/other menstrual dysfunction, gynecomastia, galactorrhea, and erectile/sexual dysfunction. The presence of any of the following medical complications was assessed at baseline: hypertension, hyperlipidemia, hepatic dysfunction, renal dysfunction, or other. Finally, body weight in kilograms was measured at each visit.
Procedures
This analysis was restricted to individuals who had been treated with typical antipsychotics prior to initiating olanzapine at baseline (n = 262). The treatment regimens for all patients included in this analysis had at least one typical antipsychotic replaced with olanzapine. Patients were classified as either inpatients or outpatients based on their treatment setting at baseline.
Symptomatic response and remission were defined using previously published definitions based on the CGI-SCH. Response was defined as an improvement of 2 points on the CGI-SCH global severity rating at any visit when the baseline rating was between 4 and 6 points, or a 1-point improvement at any visit when the baseline rating was between 1 and 3.Citation19 Symptomatic remission was defined as mild symptoms (a score ≤ 2) on the CGI-SCH positive, negative, cognitive, and global severity scores.Citation20
Patients’ body mass indices were categorized as follows: underweight (<18.5), normal (≥18.5, ≤23), overweight (>23, ≤30), and obese (>30). This categorization was based on the World Health Organization recommendations for Asians.Citation21
Time to all-cause discontinuation has been described as a measure of effectiveness that incorporates both efficacy and tolerability.Citation22 We defined time to all-cause discontinuation as the number of days between the date a patient initiated treatment with olanzapine and the date on which the patient discontinued taking olanzapine.
Statistical methods
At baseline, comparisons between the inpatient and outpatient groups were completed with t-tests for continuous variables and Chi-square tests for categorical variables. Changes over time for continuous outcome variables were assessed using mixed models for repeated measures with baseline covariates for age, sex, duration of illness, and presence of any medical complication. Changes in categorical outcome variables between baseline and postbaseline visits were assessed using McNemar’s test with missing observations imputed. Time to all-cause discontinuation was assessed using survival analysis with log-rank tests. The survival curves were constructed using unadjusted Kaplan–Meier estimates. A sensitivity analysis was completed through use of the primary symptoms measure (CGI-SCH) using the subset of participants who were treated with olanzapine monotherapy throughout the study. This sensitivity analysis allowed for confirmation that the results were not due to use of concomitant antipsychotic medications. SAS software (version 9.1.3; SAS Institute Inc, Cary, NC) was used for all analyses. The level of significance was set at P < 0.05.
Results
Baseline characteristics
The patients had been diagnosed with schizophrenia for an average duration of 19.5 years, were on average 46.9 years of age, and 51.9% were male. The inpatients (59.1%) were older, with a longer history of schizophrenia, and greater severity of symptoms overall than the outpatients (see ). The most common reason for discontinuing the previous typical antipsychotic was insufficient efficacy (71.0% of patients) followed by medication intolerability (25.6%).
Table 1 Comparison of baseline characteristics of outpatients and inpatients who switched from typical antipsychotic to olanzapine
This naturalistic study followed the switching process from at least one typical antipsychotic to olanzapine as instituted by the treating physicians. Olanzapine was initiated immediately after discontinuing the typical antipsychotic(s) for the majority of patients (69.5%). The typical antipsychotic(s) and olanzapine overlapped for nine patients (3.4%). The duration of overlap was less than 15 days for five patients, with the remaining patients having overlap durations of 17, 28, 56, and 83 days. Finally, there was a gap between discontinuation of the typical antipsychotic(s) and the initiation of olanzapine for 27.1% of patients. The gap was less than 7 days in length for all but six patients. Only 53.8% of patients were initiated on olanzapine monotherapy because some patients continued taking other antipsychotics.
Treatment patterns
Most patients completed the 1-year study (71.4%), with 88.2% completing the 3-month visit and 81.7% completing the 6-month visit. Discontinuation rates did not vary significantly between inpatients and outpatients. During the study, the average daily dose for olanzapine was 12.0 ± 6.0 mg, including 9.9 ± 5.5 mg for outpatients and 13.4 ± 5.9 mg for inpatients (P < 0.001). Combination antipsychotic treatment was common: less than half of the patients (36.6%) were treated with olanzapine monotherapy throughout the study, including 41.1% of outpatients and 33.5% of inpatients (P = 0.21). The remaining 63.4% of patients were either treated with antipsychotic polypharmacy for at least one day or discontinued the study. Patients were less likely to be treated with concomitant oral atypical antipsychotics (26.7%; inpatients 26.5%, outpatients 27.1%) than typical antipsychotics (49.2%; inpatients 51.0%, outpatients 46.7%). The average chlorpromazine equivalent doses of concomitant antipsychotics were 264.8 mg/day for inpatients and 166.4 mg/day for outpatients at baseline and 203.6 mg/day for inpatients and 199.0 mg/day for outpatients at the end of the study. With the exception of antidepressants, the inpatients were significantly more likely to be treated with different classes of concomitant medications measured in the study (see ).
Table 2 Concomitant psychotropic medications at final study visit
Effectiveness outcomes
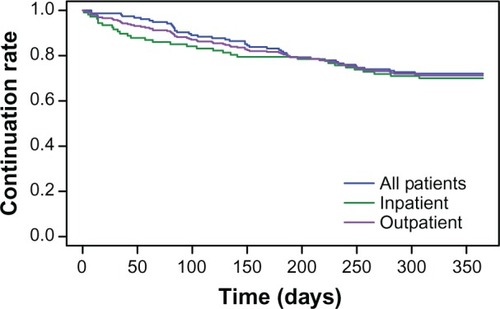
Most patients (71.4%) continued olanzapine treatment for the full 1-year study period. There were no differences between inpatients and outpatients in time to all-cause discontinuation (P = 0.61, see ).
Figure 1 Time to all-cause discontinuation of olanzapine for all patients, inpatients, and outpatients.
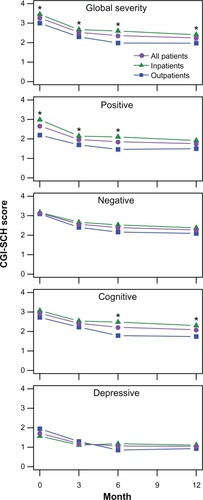
On all of the CGI-SCH subscales, patients improved after initiating treatment with olanzapine (P < 0.001, see ). The differences between inpatients and outpatients were most pronounced for global severity, where the inpatients had statistically significant higher (ie, more symptomatic) scores at each visit (P < 0.05). On the positive subscale, inpatients had significantly higher scores at baseline, 3-month, and 6-month visits. On the cognitive subscale, inpatients had significantly higher scores at the 6-month and 12-month visits. On the positive, cognitive, and depressive subscales, there was a significant time by initial treatment setting interaction (P < 0.05) that appeared to reflect greater change for the outpatients between the 3-month and 6-month visits.
Figure 2 Change in CGI-SCH scores over the 1-year study period.
Abbreviation: CGI-SCH, Clinical Global Impression-Schizophrenia.
The response rate was 58.3% and did not differ significantly between the inpatients (56.8%) and the outpatients (60.9%, P = 0.53). However the remission rate (47.4%) was significantly lower for the inpatients (39.3%) than the outpatients (61.3%, P = 0.002).
Patient functioning improved after initiating treatment with olanzapine. On the EQ-5D visual analog scale, a broad rating of health-related quality of life, patients improved from a score of 50.3 at baseline to 66.9 at the 12-month visit (P < 0.001). There were no significant differences at any time point between inpatients and outpatients on this measure. Similarly, the utility score on the EQ-5D did not differ significantly between inpatients and outpatients at any point in time, but improved from 0.68 at baseline to 0.80 at the 12-month visit for all patients (P < 0.001). Over the 1-year study period, the percent of patients working for pay increased from 7.9% to 12.1% (P = 0.01). The increase in percent of patients working for pay was higher for outpatients (increased from 17.4% to 25.0%, P = 0.05) than inpatients (increased from 2.0% to 4.5%, P = 0.08). The percent of patients engaging in five or more social activities in the previous 4 weeks increased from 17.1% at baseline to 24.6% at the 12-month visit (P = 0.004); for outpatients the change was from 26.1% to 39.1% (P = 0.005), whereas for inpatients the change was from 11.5% to 15.6% (P = 0.20).
Tolerability outcomes
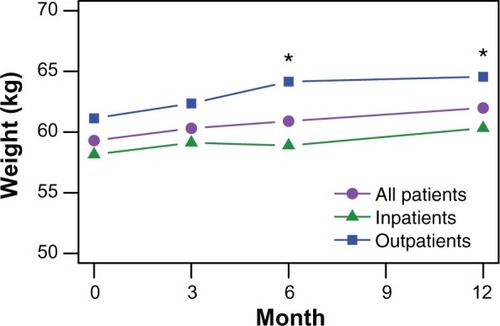
displays the average observed body weight at each visit during the study. Patients who completed the 1-year study gained an average of 2.3 kg (2.4 kg for 64 outpatients and 2.2 kg for 100 inpatients). Overall, one in three patients (30.4% of 227 patients) experienced clinically significant weight gain, defined as a 7% or greater increase over baseline during the study period. In terms of body mass index category, 2.8% of 213 patients decreased a category, 75.6% remained in the same category, and 21.6% increased a category. There were no reported cases of new onset diabetes.
Figure 3 Observed body weight during the 1-year study period.
For the adverse events measured in this study, new onset cases occurred in less than 5% of patients (see ). All five of the patients with new onset dystonia/akathisia/parkinsonism and one of the two patients with new onset tardive dyskinesia were being treated concomitantly with typical antipsychotics.
Table 3 New onset of adverse events
Sensitivity analysis
In order to confirm that the results were not due to use of concomitant antipsychotics, analysis of the CGI-SCH scales was repeated using only participants treated with olanzapine monotherapy throughout the study. The monotherapy subsample consisted of 96 patients, including 44 outpatients and 52 inpatients. In this sensitivity analysis, significant changes from baseline to each post-baseline assessment for inpatients and outpatients on the CGI-SCH global severity and each of the subscales (negative, positive, depressive, and cognitive symptoms) were confirmed (P < 0.01). In addition, differences between inpatients and outpatients on global severity at 6 months, positive symptoms at 3 and 6 months, and cognitive symptoms at 6 and 12 months were confirmed. However, differences between inpatients and outpatients for global severity at baseline, 3 months, and 12 months, and on positive symptoms at baseline and 12 months were no longer significant in this subsample of patients treated only with olanzapine. The results of the sensitivity analysis confirmed significant improvements on all of the subscales of the primary measure of symptoms, but not all of the differences between inpatients and outpatients.
Discussion
Among Japanese inpatients and outpatients with schizophrenia who were treated with medication including typical antipsychotic drugs and who had at least one typical antipsychotic drug switched to olanzapine, the resulting treatment regimen was successful in most cases. Time to all-cause discontinuation can be thought of as a measure of overall effectiveness, incorporating efficacy, safety, and tolerability,Citation22 and most (71.4%) patients continued treatment with olanzapine for the full 1-year study period. In addition, patients showed significant improvements in symptom severity as measured by the global severity, positive, negative, cognitive, and depressive subscales of the CGI-SCH. Patient quality of life and level of functioning also improved, based on improvements in the EQ-5D measures, the percent of patients working for pay, and the percent of patients engaging in social activities. However, approximately one in three patients experienced clinically significant weight gain.
The inpatients appeared to be a unique subgroup of patients. Although both the inpatients and outpatients improved significantly over the study period, the inpatients had a different pattern of response in positive, cognitive, and depressive symptoms that was marked by less improvement than in the outpatients between the 3-month and 6-month visits. In addition to greater symptom response, the outpatients had significantly greater average weight at the 6-month and 12-month visits, which is consistent with past research showing a link between greater treatment response and greater weight gain.Citation23,Citation24 The inpatients were also treated with higher doses, were more likely to be treated with combination antipsychotic therapy, had more severe symptoms throughout the study, were less likely to achieve symptomatic remission, and had lower rates of participation in social activities. Our findings replicate previous research showing that inpatients have more severe symptoms,Citation25,Citation26 tend to be treated with higher doses of antipsychotics,Citation25 and have greater needs for care.Citation26
The results found in this study are similar to those of other typical to atypical switch studies in Japan. One study followed patients who switched from typical antipsychotics to the atypical antipsychotic risperidone and reported significant reductions in symptoms of schizophrenia and significant reductions in the use of anticholinergic medications.Citation27 Another Japanese study followed male patients with schizophrenia who were switched to an atypical antipsychotic (olanzapine, quetiapine, or perospirone) and reported significant improvements in symptoms of schizophrenia, health-related quality of life, reduced use of anticholinergic drugs, and reductions in elevated prolactin levels.Citation28 However, little published research has documented the outcomes in Japan following a switch from typical antipsychotics specifically to olanzapine.
In Europe, a large observational study compared patients who were switched to olanzapine from a variety of antipsychotics (either typical or atypical) with those who were switched away from olanzapine. Patients switched to olanzapine were significantly more likely to respond to treatment, significantly less likely to report extrapyramidal symptoms, and significantly less likely to report loss of libido than patients switched away from olanzapine.Citation29 A large observational study in schizophrenia, that was relatively similar to the current study, followed Asian patients from China, the Philippines, South Korea, and Taiwan who were switched from typical antipsychotics to olanzapine. This similar study found significant improvements in symptoms of schizophrenia, health-related quality of life, and involuntary movements, and a greater weight gain.Citation30 The findings from the current study, which specifically documents the naturalistic outcomes of patients with schizophrenia in the Japanese health care system who were switched from typical antipsychotics to olanzapine are consistent with the findings from studies in other geographic regions.
Although not directly comparable with the switching methodology used in the current study, the findings are also consistent with several head-to-head studies. In naturalistic, noninterventional, observational studies across a variety of geographies, relative to patients with schizophrenia treated with typical antipsychotics, olanzapine-treated patients have remained on treatment longer,Citation31 had greater reductions in CGI-SCH global severity rating,Citation19 greater response rates,Citation31 greater improvements in quality of life,Citation6,Citation32 greater odds of engaging in social activities,Citation19 lower rates of tardive dyskinesia,Citation31 fewer extrapyramidal symptoms,Citation31,Citation32 fewer adverse events related to sexual functioning,Citation31 and greater weight gain.Citation32 In randomized controlled trials, a recent meta-analysis reported that relative to treatment with typical antipsychotics, olanzapine was associated with greater reductions in overall, positive, negative, and depressive symptoms, fewer extrapyramidal symptoms, and greater weight gain.Citation33 Unlike head-to-head randomized clinical trials, when patients fail a given medication in usual care, the next treatment is not chosen at random and appears to reflect the issues resulting in the previous medication’s discontinuation.Citation34 The switching methodology more closely reflects the clinical challenge of finding the right medication for each individual patient. Our results highlight the importance of finding the right medication for the right patient at a given point in time.
Limitations
This observational study was designed to capture treatment outcomes for Japanese patients with schizophrenia in usual clinical care. The primary focus of the olanzapine postmarketing surveillance study was to identify potential safety issues after olanzapine was introduced in Japan. Design considerations favoring external validity were given precedence over those favoring internal validity. Consistent with usual clinical care, treatment was not blinded; therefore, patient and physician expectations may have affected outcomes. The centers that agreed to participate in this olanzapine treatment study may not have been fully representative of all treatment centers in Japan. Because there was no control group in this single-arm study, we cannot be certain that the improvement was due to treatment with olanzapine rather than simply an artifact of time. The results of this study do not provide information about the relative effectiveness of olanzapine versus other antipsychotics, only information about outcomes of olanzapine following a switch from a typical antipsychotic. Finally, antipsychotic polypharmacy was common in this study, which is consistent with other studies of usual care in Japan.Citation9–Citation11 Although the sensitivity analyses using only patients treated with olanzapine monotherapy confirmed the improvements, we cannot be certain that the improvements were not due to other nonantipsychotic medications or psychosocial treatments.
Conclusion
In this 1-year naturalistic study of patients with schizophrenia in Japan, inpatients and outpatients who were switched from typical antipsychotics to olanzapine experienced clinically and statistically significant improvements in their clinical and functional outcomes. One-third of patients had a clinically significant weight gain. Current findings highlight the favorable benefit to risk profile of switching to olanzapine therapy following treatment failure on typical antipsychotics among Japanese patients with schizophrenia.
Disclosure
Technical writing support was provided by Michael Stensland of Agile Outcomes Research Inc, Rochester, MN, and Susan Dennett of Strategic Health Outcomes Inc, Carmel, IN. WY, SF, and NN are full-time employees of Eli Lilly Japan. MT is a contract employee for Eli Lilly Japan. HA-S is a full-time employee of Eli Lilly and Company. WY, SF, NN, MT, and HA-S are all minor stockholders in Eli Lilly and Company. This funded research at the request of Eli Lilly Japan was undertaken under the agreement of the funded research condition of publishing to Article 4 clause 1 of the University of Tokushima School of Medicine.
References
- BuchananRWKreyenbuhlJKellyDLThe 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statementsSchizophr Bull2010361719319955390
- Canadian Psychiatric AssociationClinical practice guidelines. Treatment of schizophreniaCan J Psychiatry20055013 Suppl 17S57S16529334
- FalkaiPWobrockTLiebermanJGlenthojBGattazWFMöllerHJWorld Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia. Part 1: acute treatment of schizophreniaWorld J Biol Psychiatry20056313219116173147
- FalkaiPWobrockTLiebermanJGlenthojBGattazWFMöllerHJWorld Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia. Part 2: long-term treatment of schizophreniaWorld J Biol Psychiatry20067154016509050
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia, second editionAm J Psychiatry2004161Suppl 215615000267
- LiebermanJAStroupTSMcEvoyJPEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med2005353121209122316172203
- ParkSRoss-DegnanDAdamsASSabinJKanavosPSoumeraiSBEffect of switching antipsychotics on antiparkinsonian medication use in schizophrenia: population-based studyBr J Psychiatry200518713714216055824
- Liu-SeifertHAdamsDHKinonBJDiscontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugsBMC Med20053213016375765
- BitterIChouJC-YUngvariGSPrescribing for inpatients with schizophrenia: an international multi-center comparative studyPharmacopsychiatry200336414314912905100
- ChongMYTanCHFujiiSAntipsychotic drug prescription for schizophrenia in East Asia: rationale for changePsychiatry and Clin Neurosci20045816167
- ShinfukuNTanC-HPharmacotherapy for schizophrenic inpatients in East Asia – changes and challengesInt Rev Psychiatry200820546046819012132
- OshimaIMinoYInomataYHow many long-stay schizophrenia patients can be discharged in Japan?Psychiatry Clin Neurosci2007611717717239042
- KuramochiMOnoHNakaharaNHealth outcome survey of olanzapine therapy in patients with schizophrenia: Analytical results of the post-marketing special survey of olanzapineJpn J Clin Psychopharmacol20091217189
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders DSM-IV4th edWashington, DCAmerican Psychiatric Association1994
- HaroJMKamathSAOchoaSThe Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophreniaActa Psychiatr Scand Suppl2003416162312755850
- KaySROplerLAFiszbeinAPositive and Negative Syndrome Scale (PANSS) User’s ManualNorth Tonawanda, NYMulti-Health Systems Inc2000
- The EuroQol GroupEuroQol – a new facility for the measurement of health-related quality of lifeHealth Policy199016319920810109801
- PrietoLSacristánJAHormaecheaJACasadoABadiaXGómezJCPsychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patientsCurr Med Res Opin200420682783515200739
- HaroJMEdgellETNovickDEffectiveness of antipsychotic treatment for schizophrenia: 6-month results of the Pan-European Schizophrenia Outpatient Health Outcomes (SOHO) studyActa Psychiatr Scand2005111322023115701107
- HaroJMNovickDSuarezDAlonsoJLépineJPRatcliffeMRemission and relapse in the outpatient care of schizophrenia: three-year results from the Schizophrenia Outpatient Health Outcomes studyJ Clin Psychopharmacol200626657157817110813
- WHO Expert ConsultationAppropriate body-mass index for Asian populations and its implications for policy and intervention strategiesLancet2004363940315716314726171
- StroupTSMcEvoyJPSwartzMSThe National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol developmentSchizophr Bull2003291153112908658
- Ascher-SvanumHStenslandMZhaoZKinonBJAcute weight gain, gender, and therapeutic response to antipsychotics in the treatment of patients with schizophreniaBMC Psychiatry20055315649317
- Ascher-SvanumHStenslandMDKinonBJTollefsonGDWeight gain as a prognostic indicator of therapeutic improvement during acute treatment of schizophrenia with placebo or active antipsychoticJ Psychopharmacol200519Suppl 611011716280344
- SoniSDMallikAReedPGaskellKDifferences between chronic schizophrenic patients in the hospital and in the communityHosp Community Psychiatry19924312123312381360939
- NakanishiMSetoyaYKodakaMSymptom dimensions and needs of care among patients with schizophrenia in hospital and the communityPsychiatry Clin Neurosci200761549550117875027
- NakanishiSKunugiHMurrayRMNojimaSOgawaTTakahashiTEffects of switching from conventional antipsychotics to risperidone in Japanese patients with chronic schizophreniaPsychiatry Clin Neurosci200660675175717109710
- KanedaYKawamuraIFujiiAOhmoriTImpact of a switch from typical to atypical antipsychotic drugs on quality of life and gonadal hormones in male patients with schizophreniaNeuro Endocrinol Lett2004251–213514015159697
- NovickDHaroJMSuarezDMarques-TeixeiraJNaberDClinical consequences of switching antipsychotic drugs in outpatients with schizophrenia: 36-month results from the European Schizophrenia Outpatient Health Outcomes studyInt Clin Psychopharmacol200823420320818545058
- LuZHuJChenCKEffectiveness and safety of olanzapine in the treatment of schizophrenia among Asian patients switching from conventional antipsychoticsProg Neuropsychopharmacol Biol Psychiatry2007311324016843580
- DossenbachMDyachkovaYPirildarSEffects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12-month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) studyEur Psychiatry200621425125816530390
- MontesJMCiudadAGascónJGómezJCSafety, effectiveness, and quality of life of olanzapine in first-episode schizophrenia: a naturalistic studyProg Neuropsychopharmacol Biol Psychiatry200327466767412787855
- LeuchtSCorvesCArbterDEngelRRLiCDavisJMSecond-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysisLancet20093739657314119058842
- StroupTSLiebermanJAMcEvoyJPResults of phase 3 of the CATIE schizophrenia trialSchizophr Res2009107111219027269