Abstract
Purpose
Facial basal cell carcinoma (BCC) poses significant challenges due to its potential for local destruction and impact on quality of life (QoL). Continuous research is necessary to identify novel factors influencing the quality of life within this demographic across diverse cultural settings. The aims of this study were to translate, culturally adapt, and validate the Lithuanian version of Skin Cancer Index, subsequently utilizing this questionnaire in the pilot phase of the study to achieve the following: (1) identify the differences in short- and long-term QoL, (2) establish empirical correlations between SCI scores and aesthetic facial regions, evaluate the potential differences between age, gender, and tumor size groups.
Patients and Methods
A prospective longitudinal study was conducted with 100 consecutive patients. The SCI was translated into Lithuanian language, with a rigorous assessment of its psychometric properties to confirm validity. Alongside hypothesis testing, a detailed analysis of variables was conducted. Statistical techniques, including t-tests and ANOVA, were employed to compare scores across demographic and clinical groups, with effect size calculations for further interpretation.
Results
Our findings demonstrate that the Lithuanian SCI successfully fulfills the criteria established by the COSMIN checklist. Surgical treatment for facial BCC notably enhances QoL, particularly evident six months post-surgery. Analysis of SCI scores identified demographic and clinical factors associated with lower QoL, including female gender, treatment with skin plasty, and tumor sites in aesthetically sensitive areas like the cheek, nose, and eyelid.
Conclusion
The Lithuanian version of the SCI is a reliable and valid tool for assessing QoL in facial BCC patients. Our findings underscore the global relevance of understanding the multifactorial influences on QoL in BCC patients. Early diagnosis, less invasive treatment approaches, and tailored post-operative care are crucial in minimizing the psychological, social, and appearance-related burdens of facial BCC.
Introduction
Non-melanoma skin cancer (NMSC) continues to be the most common neoplasm, with the majority of NMSC cases attributed to facial basal cell carcinoma (BCC).Citation1,Citation2 The frequency of this widespread malignancy has significantly risen over the recent decadesCitation3–5 with a projected continued increase until 2040.Citation6,Citation7 Understanding predictors of treatment success is crucial for improving clinical outcomes worldwide.
Despite its rare metastatic potential,Citation8 BCC can cause substantial local destruction, leading to disfigurement and potentially impacting large areas of soft tissue, cartilage, and bone. Not only does it result in premature morbidity and mortality,Citation9 but also greatly affects the Quality of Life (QoL).Citation10,Citation11 Our previous investigation into QoL studies of individuals, affected by head and neck BCC, revealed a significant research gap in analyzing the multifaceted relationships between QoL and factors such as tumor size, specific facial regions, and surgery types.Citation12
While the distribution of BCC in particular facial regions has been previously described,Citation13,Citation14 it has never been associated with Health-Related Quality of Life (HRQoL). It is plausible to suggest that the prevalence of aesthetically sensitive face areas may have a more pronounced impact on QoL than other locations. While the lesion itself is likely to cause significant distress, the need for facial surgery due to malignancy poses a different level of unease. Surgical treatment can range from minimally invasive proceduresCitation15 to major tumor excisions, requiring extensive reconstructions.Citation16 Nevertheless, it is crucial to take into consideration the largest group of patients treated in skin cancer departments - those undergoing conventional excisionCitation17 with options such as primary closure, local flap, or full-skin graft reconstruction. The interventions entail a different level of discomfort and affect various life aspects, potentially leading to certain alterations in QoL.
The Skin Cancer Index (SCI) is the first specific patient-reported outcome measure (PROM) evaluating the QoL in patients with cervicofacial NMSC.Citation18 In contrast to the widely used Dermatology Life Quality Index (DLQI) or FACE-Q questionnaire, the SCI capturing the emotional and appearance-related domains has been reported to exhibit the highest level of support for its efficacy, sensitivity, and applicability.Citation19–21 To date, SCI has been translated and validated from its original (English)Citation22 into Portuguese,Citation23 Brazilian Portuguese,Citation24 Italian,Citation25 and SpanishCitation23 languages. To use the assessment tool on Lithuanian patients, it is crucial to examine different aspects of the scale’s validity within the Lithuanian patient sample.
The aims of this prospective longitudinal pilot study were to (1) translate, culturally adapt, and validate the Lithuanian version of SCI, (2) identify the differences in short- and long-term QoL, (3) establish empirical correlations between SCI scores and aesthetic facial regions, and evaluate the potential differences between age, gender, and tumor size groups.
Patients and Methods
Procedures and Ethics Statement
The permission to translate and validate the SCI in the Lithuanian language was granted by the scale developers in 2022. The study was carried out under the Lithuanian Bioethics Committee Approval (Approval No. 2022/11-1476-943). In alignment with the Declaration of Helsinki, all study participants provided written informed consent.
Data was collected from 23rd November 2022, to 16th October 2023 at the Vilnius University Hospital Santaros Klinikos Centre of Dermatology (VUH).
Patients
Adhering to the suggested sample size for robust PROM statistical analysis,Citation26,Citation27 we enrolled a total of 100 consecutive patients in the study. Participants were included based on the following criteria:
| ● | Age ≥18 years. | ||||
| ● | Individuals presenting for surgical treatment of clinically suspected or histologically confirmed facial BCC. | ||||
| ● | Ability to comprehend Lithuanian language. | ||||
Participants with substantial cognitive impairment or a limited understanding of the Lithuanian language were excluded from the study.
Data on patient socio-demographic and clinical details were collected. Demographic variables comprised age, gender, marital status, education, place of residence, employment status, and frequency of interactions with family members. Clinical aspects involved the largest tumor diameter, its precise location, and surgery type. Tumors were categorized into specific regions based on their localization using the Facial Aesthetic unit Classification proposed by TT Fattahi.Citation28 Patients were further classified into three distinct groups based on the type of surgery: (E) excision, (P) skin plasty reconstruction by local flaps, and (T) skin graft transplantation. The surgeries were performed following a standardized protocol as day care procedures by a plastic and reconstructive surgeon with and extensive experience in the field.
Administered Outcome Measures
Skin Cancer Index (SCI)
SCI is a skin-cancer-specific QoL measurement instrument with a focus on emotional, social, and appearance aspects. It consists of 15 Likert scale questions, with scores ranging from 1 (very much – indicating a significant impact on quality of life) to 5 (not at all – suggesting no impact on quality of life). The total ranges from 15 to 75 points, with a higher score indicating a better quality of life.
Dermatology Life Quality Index (DLQI)
DLQI is designed to assess the impact of a skin condition on a patient’s life over the past week. Comprising 10 questions, respondents rate their experiences on a scale of 0 to 3 (0 indicating no impact, 1 for a slight impact, 2 for a significant impact, and 3 for a substantial impact). The cumulative score, ranging from 0 to 30, provides an overall measure of the impact of the skin problem on the QoL. The higher total score signifies a greater effect of the dermatological disease on the patient’s life.
The World Health Organization- Five Well-Being Index (WHO-5)
The WHO-5 questionnaire consists of 5 Likert scale questions that capture the respondent’s subjective experience of well-being over the preceding two weeks. The total score ranges from 0 to 100, with a higher indicating a better sense of well-being. The WHO-5 does not encompass all elements of QoL. However, it is often used as a screening tool providing valuable insights into overall quality of life, especially in terms of mental and emotional aspects.
PROM Administration
The paper-based or digital SCI, DLQI, and WHO-5 questionnaires were completed by 100 patients at different time points: (1) the day of surgery, (2) 4 weeks, and (3) 6 months post-operatively. A subgroup of 50 participants additionally filled out the SCI a week before their second appointment.
Translation and Cultural Adaptation
The translation and cultural adaptation process of SCI followed the guidelines outlined by the ISPOR Task Force for Translation and Cultural Adaptation (ISPOR TCA)Citation29 and the COSMIN Study Design checklist.Citation26,Citation27 Initially, the scale was translated from its original English version into Lithuanian by several members of the VUH, fluent in both Lithuanian and English. The forward translation was also conducted by an experienced plastic surgeon. The preliminary samples were then reviewed by a Lithuanian team of 5 resident doctors, 5 nurses, and 5 dermatovenereologists. After a comprehensive examination and discussion, a consensus was reached, resulting in the most suitable version of the forward translation. Backward translation into the English language was performed by two dermatologists who were not familiar with the original version. Minor language adjustments were made after comparing the two versions with the original.
The cognitive debriefing process was carried out on a subgroup of 15 patients presenting to VUH with NMSC. The patients were given the SCI scales and asked about any words that might be hard to comprehend, be susceptible to misinterpretation, or have the potential to be offensive. Additionally, the questions were evaluated regarding their relevance to each subscale. Following a thorough review and refinement based on the cognitive debriefing feedback, a consensus was achieved, leading to the final Lithuanian version of SCI.
Statistical Analysis
The statistical analysis was performed using R Statistical Software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) and MedCalc Software Ltd (Ostend, Belgium; https://www.medcalc.org; 2024). The existence of floor/ceiling effects was acknowledged when >15% of subjects scored at the lowest or highest extremes. A p<0.05 was considered as statistically significant.
Internal Consistency
Cronbach’s alpha coefficient was calculated to assess the internal consistency among items on the Emotional, Social, and Appearance subscales at 3 time points. Coefficient values between 0.70 and 0.95 were considered to be adequate.Citation30,Citation31
Structural Validity
Due to the scale being based on a reflective model, the three-factor confirmatory factor analysis (CFA) was performed. The thresholds for the good CFA fit were as follows: Comparative Fit Index (CFI) >0.90 adequate and >0.95 good; Tucker Lewis Index (TLI) (>0.90 adequate and >0.95 good; Root Mean Square Error of Approximation (RMSEA) <0.08; Standardized Root Mean Squared Residual (SRMR) <0.08, and chi squared (χ2)/degrees of freedom (df) with the desired range of 2–5.Citation32
Criterion Validity
Spearman’s rank correlation coefficient (ρ) was calculated between each subscale and total scores. The coefficient values were considered as follows: very strong 0.80–1, strong 0.6–0.799, medium 0.4–0.599, weak 0.2–0.399, very weak 0–0.199.
Construct Validity
To assess convergent validity, Spearman’s rank correlation coefficient was analyzed between SCI-I, DLQI-I, and WHO-5-I scales. The hypotheses were established a priori:
Positive correlation between SCI-I emotional subscale and WHO-5-I.
Negative correlation between SCI-I emotional subscale and DLQI-I.
Positive correlation between SCI-I social subscale and WHO-5-I.
Negative correlation between SCI-I social subscale and DLQI-I.
Positive correlation between SCI-I appearance subscale and WHO-5-I.
Negative correlation between SCI-I appearance subscale and DLQI-I.
Convergent construct validity was considered appropriate when at least 75% of the expected correlations with other related measures were confirmed.Citation33
Measurement Error and Reliability
The questionnaire was filled out twice by a subgroup of 50 patients in an interval of 5–7 days. Patients were given either paper or digital SCI questionnaires with identical instructions during both the initial and second administrations of the scale. The initial completion of the SCI took place at home, while the second occurred in the hospital—this being the sole point of distinction. The subgroup was additionally questioned about the possible factors that could influence the change of answers in the interim period.
Test–retest reliability (TRR) was assessed by calculating the Intraclass Correlation Coefficient (ICC, two-way mixed-effects model, absolute agreement, 95% CI). ICC values of >0.70 were considered acceptable.Citation33
The standard deviation of differences (SDdif) was calculated to evaluate the dispersion of the differences between test and retest (TR) scores. A smaller SDdif was considered suggestive of good agreement between TR scores. The standard error of measurement (SEM) was calculated with the following formula: SEM = SDdif√(1-ICC). The formula used to determine the smallest detectable change in an individual (SDCind) is expressed as follows: SDCind = 1.96 × √2 × SEM. The smallest detectable change measurable in a group of people (SDCgroup) was calculated by dividing SDCind by √n, where n represents the sample size.Citation33 The mean of the differences between test–retest scores was computed by the mean difference score (MD). Limits of Agreement (LoA) were calculated by the following formula: MD ± 1.96 * SDdif.
Sensitivity to Change and Responsiveness
The sensitivity to change was assessed by calculating the effect size (ES), consecutively interpreting the results using Cohen’s standard values: 0.2-<0.5 low, 0.5–0.8 moderate, and ≥0.8 large effect size. Additionally, the standardized response mean (SRM) was calculated between the 1st and 2nd, 1st and 3rd, as well as 2nd and 3rd visits. P values of <0.05 were considered significant. It was hypothesized that the SCI will show a significant increase in scores from pre- to post-intervention, indicating its responsiveness to intervention-induced variations. The null hypothesis assumed no significant difference, while the alternative hypothesis predicted a meaningful change in scores post-intervention, affirming the questionnaire’s sensitivity to the effects of the intervention.
The questionnaire’s responsiveness was evaluated by conducting statistical comparisons, including t-tests and analysis of variance (ANOVA) followed by post hoc tests, to compare scores across various groups.
Segment Analysis and SCI Score Differences Across Anatomic Units
Possible SCI score differences by age, gender, tumor size, aesthetic facial units, and surgery groups (E, P, T) at 1st, 2nd, and 3rd visits were analyzed. The Student’s t-test was used for dichotomous variables and Analysis of Variance for categorical variables with three or more groups. Where ANOVA was statistically significant, post hoc tests were used to find which groups had reliably different means. Additionally, Cohen’s effect size was calculated to quantify the magnitude of group differences.
Results
One hundred consecutive patients were included in the study. The questionnaires were completed by all study participants at 3 time points. A subgroup of 50 underwent the re-assessment of the reliability evaluation for SCI. A very small percentage of missing values (0.003%) was noted. They were replaced by applying the Mode Imputation method. The Floor and ceiling effects were negative in SCI and WHO-5 questionnaires, with 28 patients reaching floor effect for DLQI. Demographic and clinical information is presented in and .
Table 1 Patient Sociodemographic Characteristics
Table 2 Patient Clinical Characteristics
Translation and Cultural Adaptation
The development of the Lithuanian Skin Cancer Index involved forward translation, backward translation, and a cognitive debriefing process. These procedures collectively ensured linguistic accuracy and cultural appropriateness, ultimately confirming the face and content validity of the scale.
Internal Consistency
The analysis yielded satisfactory Cronbach’s alpha values for each subscale at the 1st, 2nd, and 3rd time points (). The results demonstrate a strong internal coherence among the questionnaire items, affirming the reliability of the instrument for assessing the intended variables. It reinforces the trustworthiness of the collected data for subsequent analyses and interpretation in our study.
Table 3 Internal Consistency of the SCI
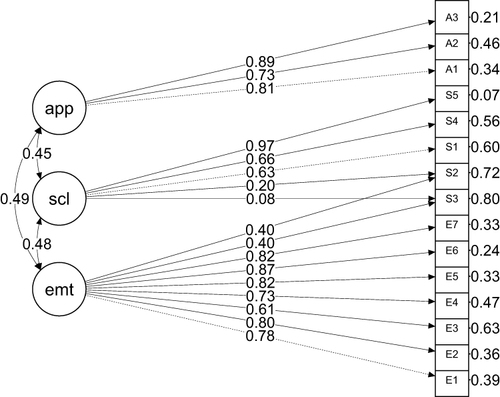
Structural Validity
Based on modification indices (MI), modifications were performed to the original model to achieve better performance. Two cross-loadings were suggested by MI (Q2 and Q3 to emotional subscale) due to χ2 increasing by more than ten units. Notably, items Q2 and Q3, originally part of the Social subscale, were suggested to cross-load in the Emotional subscale. This modification aligns with the theoretical perspective, given that both items (“Felt concerned that your skin cancer may worry friends or family?“ for Q2 and ”Worried about the length of time before you can go out in public?” for Q3) could reasonably pertain to either the Emotional or Social subscale ( and ).
Table 4 Fit Results of the Competing Models Tested (n=100)
Criterion Validity
The Spearman correlations between the SCI-I subscales and their total score are displayed in . The values for the total score range from 0.59 to 0.933 indicating medium to very strong positive association. Notably, the correlation between the Appearance subscale and the total score is relatively lower, a phenomenon likely stemming from the limited number of items in the Appearance subscale (3 out of 15). Nevertheless, the correlation coefficient for Emotional and Social subscales is highly significant and indicates medium coherence among these factors.
Table 5 Correlations Between the SCI – I Subscales and the Total Score
Construct Validity
represents the Spearman correlation coefficients between the SCI-I and WHO-5-I. The results reveal a positive score correlation, ranging from very weak to weak. Highlighting the questionnaire’s particular attention to social aspects, the strongest correlation was observed between the WHO-5-1, 2 and the Social subscale of the SCI-I.
Table 6 Spearman Correlation Coefficients Between SCI – I and WHO-5-I Scores
Spearman correlations between SCI-I and DLQI-I values are displayed in . The analysis indicates that there is a negative correlation between SCI and DLQI, characterized as very weak to medium. The most significant negative correlation is found between DLQI4 and SCI Emotional and Social subscales. Notably, the DLQI shows the strongest correlation with the SCI Social subscale, underscoring the questionnaire’s focus on social aspects.
Table 7 Spearman Correlation Coefficients Between SCI – I and DLQI Scores
The convergent construct validity is supported by the fact that 75% of the hypotheses were confirmed as true.
Measurement Error and Reliability
The test–retest reliability of the questionnaire resulted in an ICC of 0.83 (95% CI = 0.716–0.899) for the overall score (). All ICC values for Emotional, Social, and Appearance subscales are higher than 0.8, suggesting high consistency and reliability between different observers or measurement occasions.
Table 8 Test–Retest Reliability
The SEM for the overall score is 1.77 points, indicating a 68% probability that the true score for a patient falls within a range of −1.77 to +1.77 points relative to the observed score. With a 95% probability, this range expands to −3.54 to +3.54 points. For individual subscales, the SEM values range from 0.316 to 1.22, reflecting the precision with which the true scores of patients in these specific domains can be estimated based on their observed scores. The SDCind for the overall score is 4.906 points. This implies that an individual’s overall quality of life score would need to change by at least 4.906 points before it could be confidently considered as a true change rather than a result of measurement error. Additionally, the SDCgroup value is established at 0.694 for the overall score, providing a benchmark for the minimum change that can be considered significant at a group level.
Sensitivity to Change and Responsiveness
The mean scores of SCI at 1st, 2nd and 3rd visits are presented .
Table 9 Mean SCI Scores at the 1st, 2nd and 3rd Visits
The moderate to large responsiveness levels were observed for 1st vs 3rd as well as for 2nd vs 3rd visits, with the SRM values surpassing 0.5. It was detected that responsiveness fell into “low” category when analyzing the means of 1st vs 2nd visit (SRM <0.5).
Meaningful Changes Over Time
Statistically significant differences were observed in the SCI total and subscale scores across the 1st, 2nd, and 3rd visits (p<0.001). Notably, between the 1st and 3rd visits (p<0.001, mean difference +8.83 points), as well as between the 2nd and 3rd visits (p<0.001, mean difference +6.47 points). However, the only statistically significant difference between the 1st and 2nd visits was found to be in Emotional domain (p=0.044, mean difference +2.02) ( and ).
Table 10 Standardized response mean (SRM)
The results indicate that there is significant improvement in Emotional domain 1 month after surgery compared to baseline scores. Furthermore, the statistically significant improvement in SCI Total and all subscale scores was detected 6 months after surgery, emphasizing the profound impact of the intervention.
Segment Analysis and SCI Score Differences Across Anatomic Units
To guarantee the feasibility of using statistical tests in the pilot phase, the analysis of score distributions across anatomical units excluded finer subunits due to the insufficient number of cases. Further division to subunits was reserved for descriptive statistics and future studies on this topic. Therefore, the mental unit comprising only one participant was excluded from the statistical analysis. The segment analysis with SCI score association to anatomic units at the 1st, 2nd, and 3rd visits is presented in and .
Table 11 Segment Analysis and SCI Score Differences Across Anatomic Units at the 1st Visit
Table 12 Segment Analysis and SCI Score Differences Across Anatomic Units at the 2nd Visit
Table 13 Segment Analysis and SCI Score Differences Across Anatomic Units at the 3rd Visit
SCI Differences Depending on Tumor Location
Across the anatomic units, there were no statistically significant differences observed in SCI Total/Emotional/Social/Appearance scores at 1st, 2nd, and 3rd visits.
SCI Score Differences Between Men and Women
Noteworthy differences were detected in the SCI scores between men and women at all 3 time points, with the exception being SCI Social subscale at 2nd and 3rd visits. Men provided statistically significantly higher SCI scores, indicating better quality of life overall, especially in emotional and appearance aspects both pre- and post-interventionally.
Score Differences Between Men and Women by Anatomic Units
Upper lip unit group, consisting only of 1 woman participant was excluded from the analysis.
Considering the SCI score disparities between genders across anatomical units, three areas were found to exhibit statistically significant differences. Men showed higher SCI Total/Social/Appearance scores before surgery when their tumor was in either the cheek (p<0.05) or eyelid unit (p<0.05). This trend persisted post-surgery (visit 2nd), particularly in the cheek region (p<0.05). Six months after intervention, men with primary tumors in the nose unit evaluated their QoL in emotional domain statistically significantly better than women (p<0.01).
The anatomical tumor location did not appear to influence the scores between men and women on the SCI-I-Emotional, SCI II-Emotional/Social, and SCI III-Total/Social/Appearance subscales.
SCI Score Differences Between E, P, T Groups
The results indicate that significant differences between the SCI scores of E, P, and T groups were only found pre-interventionally. Patients in E group evaluated their QoL in emotional domain statistically significantly better than P group at 1st visit.
Score Differences Between Surgery Groups by Anatomic Units
T group in cheek unit, consisting only of 1 participant, was excluded from the analysis.
The findings suggest that the assessment of SCI by patients in different surgery groups varies significantly depending on tumor location. Particularly, the E group tends to evaluate the QoL in Social domain better compared to P group when the tumor is located on the cheek (1st visit) (p<0.05) and on the eyelid (3rd visit) (p<0.05).
SCI Score Differences Between Age Groups
Statistically significant differences between age groups were observed only in the SCI Appearance domain at the time of 2nd visit. Post hoc tests revealed that the 36–56 y group rated SCI Appearance subscale statistically significantly worse compared to 57–69 y group (p<0.05).
Score Differences Between Age Groups by Anatomic Units
The results revealed three sensitive areas: the eyelid during the initial visit, and the upper lip and nose during the 2nd visit. These differences are reflected in both total and subscale scores. Notably, following intervention, the areas of age-related concern shift, with prominent disparities observed in SCI Emotional scores for the upper lip region and SCI Social scores for the nose region at the 2nd visit. Interestingly, the SCI-II-Appearance and SCI-III did not reveal any significantly different anatomic areas of concern between age groups.
Post hoc tests to identify differing pairs were not feasible due to the 70–79 y group, comprising only 1 participant in the eyelid unit – when this group was removed, p-value according to ANOVA was no longer statistically significant.
SCI Score Differences Between Tumors Size Groups
The results indicate that significant differences between the SCI Total scores by tumor size groups were only found post-interventionally at the 2nd visit. Patients with tumors ranging from 6 to 10 mm exhibited generally higher SCI Total scores compared to those with tumors measuring 11–15 mm (p<0.05).
Score Differences Between Size Groups by Anatomic Units
Across the anatomic units, there were no statistically significant differences observed in SCI Total/Emotional/Social/Appearance scores at the 1st, 2nd, and 3rd visits between patients in different tumor size groups (p>0.05).
Discussion
In this study, the SCI was translated and culturally adapted to suit the Lithuanian patient population with facial NMSC. Following the rigorous methodology for PROM validation, an extensive investigation into various psychometric properties of the scale was conducted. The findings revealed that all assessed parameters, including internal consistency, structural validity, criterion validity, construct validity, discriminative convergent validity, sensitivity to change, responsiveness, measurement error, and reliability, surpassed acceptable thresholds. Our statistical analysis of the factorial structure corresponds to the model initially proposed by Rhee et al,Citation22 and subsequently confirmed by Samela et al,Citation25 validating the existence of three factors corresponding to Emotional, Social, and Appearance subscales. In contrast, the SpanishCitation23 and Melanoma-SCICitation34 versions demonstrated a two-factor structure. Two cross-loadings were included for items Q2 and Q3, revealing their interchangeability in both Emotional and Social subscales. Similar phenomenon regarding these two subscales was identified during the validation process in the Italian language. However, the Italian study found cross-loadings in the items Q5 and Q9.Citation25
Our study had a higher ratio of women to men compared to established literature, likely due to our methodology of including every consecutive patient meeting the inclusion criteria. The gender-specific behaviors and longer women's life expectancy could have led to an older average age among our participants. While this gender imbalance might have influenced the results, we believe our consecutive inclusion methodology minimizes potential selection bias. Therefore, the atypical gender distribution in our sample likely reflects the specific patient population at our center during the study period and the natural demographic variations in BCC incidence among older populations.
Primary differences of SCI scores were evaluated considering factors such as gender, age, tumor size, location, and surgery type. Sensitive groups throughout all 3 visits were identified.
Gender differences were evident, with men reporting higher overall SCI scores compared to women at all time points. This disparity was particularly notable in the emotional and appearance domains. These findings suggest that women may experience greater psychological and aesthetic distress related to facial BCC and its treatment. Clinicians should consider these gender differences when planning and providing post-operative care and support.
Age and tumor size were additional factors of QoL. Significant differences in the appearance domain were observed between age groups post-surgery, particularly between the 36 to 56 years group and the 57 to 69 years group. Patients with smaller tumors (6–10 mm) reported higher QoL than those with larger tumors (11–15 mm) at the second visit.
The segment analysis revealed notable variations in QoL of patients with tumors in various locations for different patient groups. Tumors in aesthetically sensitive areas such as the cheek, nose, and eyelid were associated with lower QoL scores both pre- and post-surgery. Although post hoc tests were mostly not feasible given the relatively small sample size, the identification of significant differences laid the groundwork for future studies. This highlights the importance of surgical precision and aesthetic considerations in these regions to minimize the impact on patients’ QoL.
The type of surgery performed also influenced QoL outcomes. Patients undergoing primary excision reported better emotional domain scores compared to those undergoing skin plasty pre-surgery. However, post-surgery, the differences between these groups were not statistically significant, suggesting that the initial psychological impact of more extensive surgeries may diminish over time. This finding indicates that while less invasive surgeries may offer immediate emotional benefits, all surgical treatments eventually contribute to improved QoL. This observation suggests that following the intervention, the appearance of the scar could be a more significant factor over the extent of the surgery performed. Interestingly, this hypothesis does not manifest in the scores of the Appearance domain, which exhibited no significant differences among patients with varying tumor sizes or locations.
Our findings highlight the great impact of surgery on the QoL of patients with facial NMSC. However, in contrast to previous findings,Citation12 we observed that the most significant improvement in QoL following the intervention is apparent during later follow-up visits rather than within the first month post-surgery. Specifically, one month after surgery, the improvements tend to be present only in the emotional aspect of patients’ lives.
Strengths
The notable strengths of this study lie within its prospective longitudinal design, focusing exclusively on patients with facial BCC. By including each participant consecutively, we avoided selection bias, which enhanced the credibility and relevance of our findings. The use of multiple validated instruments, including SCI, DLQI, and WHO-5, offers a comprehensive evaluation of both immediate and longer-term QoL from different perspectives enhancing the reliability and depth of the findings.
Furthermore, the study identified significant determinants of QoL, including gender, tumor location, and size. These findings are highly relevant for clinical practice worldwide, as they can guide the development of personalized treatment plans aimed at optimizing patient outcomes. The emphasis on tumors in aesthetically sensitive areas provides valuable insights into the psychological and emotional impacts of BCC, which are critical for improving patient care and support globally.
Additionally, the inclusion of a culturally adapted and validated Lithuanian version of the SCI ensures that the findings are grounded in the specific context of the patient population, which enhances the study’s relevance and applicability.
Limitations
While this study offers valuable insights, it has a few key limitations that should be noted. As a pilot study with a sample size of 100 patients, the findings may not be fully generalizable to all populations. Larger multi-center studies are needed to confirm these results and extend their applicability.
While we captured the QoL during the critical clinical period, a longer follow-up could offer further insights. Finally, the cultural adaptation of the SCI to Lithuanian patients, while essential, may limit the direct applicability of the findings to other cultural contexts.
These limitations highlight areas for further research to confirm and expand upon the study’s findings, ensuring their relevance and applicability in diverse clinical settings. Future research should balance validation efforts with a more extensive examination of clinical outcomes.
Conclusions
The Lithuanian version of the SCI can be confidently used in clinical practice and research settings to assess the impact of skin cancer on patients’ well-being, with three subscales offering detailed insights into emotional, social, and appearance-related distress.
Surgical NMSC treatment significantly improves QoL, with the most substantial impact being observed 6 months after surgery. Key determinants of QoL include gender, tumor location, and tumor size. Men, patients with smaller tumors, and those with tumors outside of aesthetically sensitive areas reported better QoL outcomes. Meanwhile, women, patients undergoing skin plasty and those with tumors located in aesthetically sensitive regions such as the cheek, nose, and eyelid presented with lower QoL.
These findings highlight the critical importance of early diagnosis, less invasive treatments, and tailored post-operative care in enhancing patient well-being. Further studies are needed to explore the multifactorial influences of sociodemographic, clinical, anthropometric, and scar-related variables on HRQoL in a bigger sample size.
Ethics Statement
The approval to conduct the study was issued by The Vilnius Regional Biomedical Research Ethics Committee, Approval No. 2022/11-1476-943. In alignment with the Declaration of Helsinki, all study participants provided written informed consent.
Disclosure
The authors declare no conflicts of interest in this work.
Data Sharing Statement
The data underlying this article will be shared by the corresponding author upon reasonable request.
Additional information
Funding
References
- Ciążyńska M, Kamińska-Winciorek G, Lange D, et al. Author Correction: The incidence and clinical analysis of non‑melanoma skin cancer. Sci Rep. 2021;11(1):15705. doi:10.1038/s41598-021-94435-7
- Leiter U, Keim U, Garbe C. Epidemiology of Skin Cancer: update 2019. Adv Exp Med Biol. 2020;1268:123–139. doi:10.1007/978-3-030-46227-7_6
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: A population-based study in Olmsted County. Mayo Clin Proc. 2017;92(6):890–898. doi:10.1016/j.mayocp.2017.02.015
- Rudolph C, Schnoor M, Eisemann N, Katalinic A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. J Dtsch Dermatol Ges J Ger Soc Dermatol JDDG. 2015;13(8):788–797. doi:10.1111/ddg.12690
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatology. 2015;151(10):1081–1086. doi:10.1001/jamadermatol.2015.1187
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303–317. doi:10.1016/j.jaad.2018.03.060
- Hu W, Fang L, Ni R, Zhang H, Pan G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer. 2022;22(1):836. doi:10.1186/s12885-022-09940-3
- Nguyen-Nielsen M, Wang L, Pedersen L, et al. The incidence of metastatic basal cell carcinoma (mBCC) in Denmark, 1997–2010. Eur J Dermatol EJD. 2015;25(5):463–468. doi:10.1684/ejd.2015.2546
- Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharm Eco. 2011;29(10):863–874. doi:10.2165/11589300-000000000-00000
- Liu Q, Sha M, Xue B, Shen L, Li G, Cheng X. Health-related quality of life and associated factors among non-melanoma skin cancer patients: a cross-sectional study. Ann Transl Med. 2023;11(3):150. doi:10.21037/atm-22-6654
- Gaulin C, Sebaratnam DF, Fernández-Peñas P. Quality of life in non-melanoma skin cancer. Australas J Dermatol. 2015;56(1):70–76. doi:10.1111/ajd.12205
- Stundys D, Ulianskaite G, Stundiene I, Grigaitiene J, Jancoriene L. The quality of life in surgically treated head and neck basal cell carcinoma patients: a comprehensive review. Cancers. 2023;15(3):801. doi:10.3390/cancers15030801
- Choi JH, Kim YJ, Kim H, Nam SH, Choi YW. Distribution of basal cell carcinoma and squamous cell carcinoma by facial esthetic unit. Arch Plast Surg. 2013;40(4):387–391. doi:10.5999/aps.2013.40.4.387
- Khalid A, van Essen P, Crittenden TA, Dean NR. The anatomical distribution of non-melanoma skin cancer: a retrospective cohort study of 22 303 Australian cases. ANZ J Surg. 2021;91(12):2750–2756. doi:10.1111/ans.17030
- Fahradyan A, Howell AC, Wolfswinkel EM, Tsuha M, Sheth P, Wong AK. Updates on the management of non-melanoma skin cancer (NMSC). Healthc Basel Switz. 2017;5(4):82. doi:10.3390/healthcare5040082
- Bondi T, Chaine A, Foy JP, Benassarou M, Bertolus C, Bouaoud J. Extensive head and neck skin cancers: carcinologic surgery as a cornerstone of treatment. J Stomatol Oral Maxillofac Surg. 2023;125(4):101737. doi:10.1016/j.jormas.2023.101737
- Thomson J, Hogan S, Leonardi-Bee J, Williams HC, Bath-Hextall FJ. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2020;11(11):CD003412. doi:10.1002/14651858.CD003412.pub3
- Rhee JS, Matthews BA, Neuburg M, Burzynski M, Nattinger AB. Creation of a quality of life instrument for nonmelanoma skin cancer patients. Laryngoscope. 2005;115(7):1178–1185. doi:10.1097/01.MLG.0000166177.98414.5E
- Lee EH, Klassen AF, Nehal KS, Cano SJ, Waters J, Pusic AL. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69(2):e59–67. doi:10.1002/14651858.CD003412.pub3
- Shao K, Zhang J, Pearl RL, Taylor L, Sobanko JF. Improving quality of life measurement in skin cancer patients: Clinical correlate for the skin cancer index. Dermatol Surg. 2018;44(8):1139–1140. doi:10.1097/DSS.0000000000001426
- Dobbs TD, Samarendra H, Hughes S, Hutchings HA, Whitaker I. Patient-reported outcome measures for facial skin cancer: a systematic review and evaluation of the quality of their measurement properties. Br J Dermatol. 2019;180(5):1018–1029. doi:10.1111/bjd.17342
- Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. Validation of a quality-of-life instrument for patients with nonmelanoma skin cancer. Arch Facial Plast Surg. 2006;8(5):314–318. doi:10.1001/archfaci.8.5.314
- de Troya-Martín M, Rivas-Ruiz F, Blázquez-Sánchez N, et al. A Spanish version of the skin cancer index: A questionnaire for measuring quality of life in patients with cervicofacial nonmelanoma skin cancer. Br J Dermatol. 2015;172(1):160–168. doi:10.1111/bjd.13173
- Hora EC, Lima MS, Siqueira HFF, et al. Cross-cultural adaptation of the skin cancer index into Brazilian Portuguese for patients with cervicofacial nonmelanoma skin cancer. off J Multinatl Assoc Support Care Cancer. 2023;31(10):590. doi:10.1007/s00520-023-08051-4
- Samela T, Raimondi G, Sampogna F, et al. Testing some psychometric properties of the Italian version of the Skin Cancer Index: A questionnaire for measuring quality of life in patients with non-melanoma skin cancer. Front Psychol. 2022;13:991080. doi:10.3389/fpsyg.2022.991080
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19(4):539–549. doi:10.1007/s11136-010-9606-8
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi:10.1016/j.jclinepi.2010.02.006
- Fattahi TT. An overview of facial aesthetic units. J Oral Maxillofac Surg. 2003;61(10):1207–1211. doi:10.1016/s0278-2391(03)00684-0
- Wild D, Grove A, Martin M, et al. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health J Int Soc PharmacoEcon Outcom Res. 2005;8(2):94–104. doi:10.1111/j.1524-4733.2005.04054.x
- Taber KS. The use of cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48(6):1273–1296. doi:10.1111/ajd.12205
- Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi:10.5116/ijme.4dfb.8dfd
- Jackson DL, Gillaspy JA, Purc-Stephenson R. Reporting practices in confirmatory factor analysis: an overview and some recommendations. Psychol Methods. 2009;14(1):6–23. doi:10.1037/a0014694
- Terwee CB, Bot SDM, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi:10.5116/ijme.4dfb.8dfd
- Moran C, Coroiu A, Körner A. Psychosocial distress in patients with cutaneous melanoma: Validation of the Skin Cancer Index (SCI). Supp Care Canc. 2021;29(2):1005–1014. doi:10.1007/s00520-020-05568-w