Abstract
Background
The effects of intra-aortic balloon pump (IABP) usage in patients with acute myocardial infarction remain controversial. This study sought to evaluate the outcomes of IABP usage in these patients.
Methods
Medline, EMBASE, and other internet sources were searched for relevant clinical trials. The primary efficacy endpoints (in-hospital, midterm, and long-term mortality) and secondary endpoints (reinfarction, recurrent ischemia, and new heart failure in the hospital) as well as safety endpoints (severe bleeding requiring blood transfusion and stroke in-hospital) were subsequently analyzed.
Results
Thirty-three clinical trials involving 18,889 patients were identified. The risk of long-term mortality in patients suffering from acute myocardial infarction was significantly decreased following IABP use (odds ratio [OR] 0.66, 95% confidence interval [CI]: 0.48–0.91, P=0.010). Both in-hospital and midterm mortality did not differ significantly between the IABP use group and no IABP use group (in-hospital: OR 0.87, 95% CI: 0.59–1.28, P=0.479; midterm: OR 1.12, 95% CI: 0.53–2.38, P=0.768). IABP insertion was not associated with the risk reduction of reinfarction, recurrent ischemia, or new heart failure. However, IABP use increased the risk of severe bleeding requiring blood transfusion (OR 2.05, 95% CI: 1.29–3.25, P=0.002) and stroke (OR 1.71, 95% CI: 1.04–2.82, P=0.035). In the thrombolytic therapy and cardiogenic shock subgroups, reduced mortality rates following IABP use were observed.
Conclusion
IABP insertion is associated with feasible benefits with respect to long-term survival rates in patients suffering from acute myocardial infarction, particularly those suffering from cardiogenic shock and receiving thrombolytic therapy, but at the cost of higher incidence of severe bleeding and stroke.
Introduction
Patients suffering from acute myocardial infarction (AMI) are at an increased risk for high mortality, particularly in the setting of AMI complicated by cardiogenic shock (CS), although both emergency revascularization (ERV) and thrombolysis have been widely used.Citation1 The intra-aortic balloon pump (IABP) has been widely used since it was first used clinically in 1968. It improves diastolic coronary blood flow and reduces both afterload and myocardial oxygen demand,Citation2 changes thought to have positive effects on myocardial recovery following AMI.Citation3,Citation4
According to the American College of Cardiology and American Heart Association (ACC/AHA 2012) guidelines, IABP was recommended for CS and its insertion was suggested at the completion of coronary angiography and revascularization (IIa).Citation5 The European Society of Cardiology guidelines also recommended IABP as a bridge to reperfusion for patients suffering from CS.Citation6 In recent years, several large randomized controlled trials (RCTs)Citation7–Citation10 and meta-analysesCitation11,Citation12 have demonstrated only limited or even no benefits with respect to midterm and long-term all-cause mortality in patients with AMI complicated by CS for whom early revascularization was planned. However, two other meta-analysesCitation13,Citation14 have demonstrated that IABP has a positive impact with respect to all-cause mortality in these patients for whom thrombolysis was used as a preferred reperfusion strategy. These conflicting data challenged the recommendations of the current guidelines and these aforementioned meta-analyses did not include all the available relevant clinical trials. Therefore, we sought to conduct an updated, comprehensive meta-analysis involving as many clinical trials as possible to evaluate the evidence pertaining to the performance of IABP performed as an adjunct therapy in patients suffering from an AMI complicated with CS or not.
Methods
Literature search
We searched Medline, EMBASE, and the Cochrane Controlled Trials Registry from their dates of inception until May 2015 for clinical trials comparing outcomes following IABP use with outcomes in the absence of IABP use (defined as the Control group) following AMI. To be certain all relevant studies were included, the electronic databases were searched using combinations of several relevant keywords, including intra-aortic balloon pump, counterpulsation, acute myocardial infarction, and clinical trials. All potentially relevant articles and references from published reviews and meta-analyses were subsequently screened for eligibility.
Inclusion and exclusion criteria
The articles were required to meet the following criteria: 1) adult patients suffering from AMI (age from 18 to 90 years), regardless of CS and 2) clinical trials comparing an IABP group and a control group. The exclusion criteria were as follows: 1) nonhuman or ongoing studies; 2) non-English language studies; 3) reviews or meta-analyses; 4) duplicated studies or different studies using the sample; and 5) patients supported by other cardiac assist devices.
Data extraction, synthesis, and quality assessment
Two investigators (FZG and GXF) independently reviewed all relevant articles to assess their eligibility, using standardized data-abstraction forms. Disagreements were resolved by a third investigator (CLW). We extracted the following data from each included study: the name of the trial or first author, publication year, inclusion and exclusion criteria, baseline demographics, and clinical outcomes at the hospital and/or during follow-up. All included studies were divided into two subgroups based on the design of each study, described as RCTs and observational clinical trials (Obs). To account for the effects of treatment, we also grouped the studies by type of reperfusion, as follows: no reperfusion, ERV alone, including either percutaneous transcoronary angioplasty or coronary artery bypass grafting, thrombolytic therapy alone (TT alone), and ERV plus TT. However, the patients with CS were selected as another subgroup to determine whether they responded to IABP insertion. Moreover, the patients with AMI were divided into two subgroups based on whether they underwent IABP insertion before or after reperfusion to determine the best opportunity for IABP insertion. The quality of all retrieved studies was assessed to ensure minimization of bias, but no formal scoring system was used.
Study endpoints
The primary efficacy endpoint was the rate of all-cause mortality, which was evaluated across three periods based on the follow-up durations of the included trials, including in-hospital mortality, midterm mortality (defined as mortality between 30 days and 2 months), and long-term mortality (defined as mortality after 6 months), and the secondary efficacy endpoints were new heart failure, reinfarction, and recurrent ischemia during hospitalization. The incidences of stroke and severe bleeding requiring blood transfusion in-hospital were evaluated as safety endpoints. The rates of all-cause mortality and new heart failure could be replaced by cardiac deathCitation15 and pulmonary edema,Citation16 respectively, if the included articles did not have the relevant data. The definitions of the clinical endpoints varied slightly among the included trials, and we evaluated the clinical endpoints using standardized definitions.
Statistical analysis
All endpoints were treated as dichotomous variables, which were analyzed using odds ratio (OR) with 95% confidence interval (CI). Statistical heterogeneity among the included studies was measured using the Cochrane’s Q test and the I2 statistic. When the P-value of Q test was <0.10 and/or the I2 was ≥50%, significant heterogeneity was considered and a random-effects model was selected. If not, the fixed-effects model and the Mantel–Haenszel method were used. Publication bias was examined via Egger’s test (P<0.1 for significant asymmetry).Citation17 To assess the stability of the overall treatment effects, sensitivity analyses (exclude one study at a time) were performed. All P-values were two-tailed, and a P-value <0.05 was considered statistically significant. This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statements,Citation18 and the data were analyzed using STATA 12.0 (StataCorp LP, College Station, TX, USA).
Results
Eligible studies and patient characteristics
After screening 1,841 initial articles using the electronic databases, 33 clinical trials were identified, including 15 RCTs (2,497 patients)Citation7–Citation10,Citation15,Citation19–Citation30 and 18 Obs (16,392 patients)Citation16,Citation31–Citation49 (). The baseline characteristics of the included RCTs and Obs are summarized in –.
Table 1 The characteristics of randomized controlled trials pertaining to the use of an intra-aortic balloon pump in the setting of AMI
Table 2 The characteristics of observational trials pertaining to the use of an intra-aortic balloon pump in the setting of AMI
Table 3 The baseline characteristics of randomized controlled trials pertaining to the use of an IABP in the setting of AMI
Table 4 The baseline characteristics of observational clinical trials pertaining to the use of an IABP in the setting of AMI
Primary efficacy endpoint
In-hospital mortality
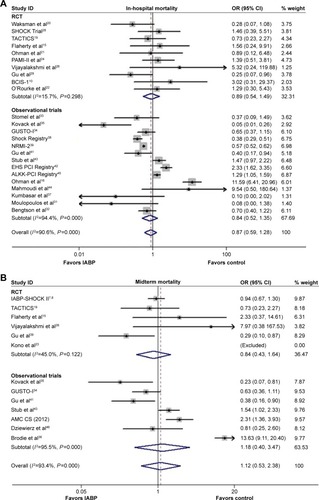
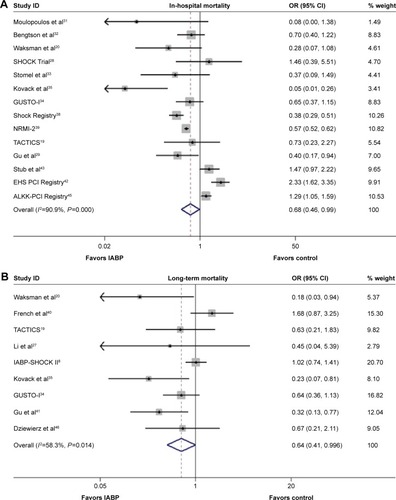
As shown in the , the overall risk of in-hospital mortality was not reduced significantly following IABP use (OR 0.87, 95% CI: 0.59–1.28, P=0.479; P<0.001, I2=90.6%); similar results were observed for both the RCTs and Obs (RCT: OR 0.89, 95% CI: 0.54–1.49, P=0.669; P=0.298, I2=15.7%; Obs: OR 0.84, 95% CI: 0.52–1.35, P=0.467; P<0.001, I2=94.4%). The results of the Egger’s test indicted that no publication bias was encountered (P=0.406, 0.325, 0.175 for Obs, RCT, and overall, respectively).
Figure 2 Forest plots of the primary efficacy endpoint of the included trials.
Abbreviations: CI, confidence interval; IABP, intra-aortic balloon pump; RCT, randomized controlled trial; ID, identification.
Midterm mortality
The comparison between IABP use and no IABP use demonstrated no significant differences with respect to the risk of midterm mortality (OR 1.12, 95% CI: 0.53–2.38, P=0.768; P<0.001, I2=93.4%; ), as well as no significant reductions in risk in the RCTs and Obs (RCT: OR 0.84, 95% CI: 0.43–1.64, P=0.609; P=0.122, I2=45.0%; Obs: OR 1.18, 95% CI: 0.40–3.47, P=0.760; P<0.001, I2=95.5%). No publication bias was observed based on the results of the Egger’s test (P=0.135, 0.843, 0.813 for Obs, RCT, and overall, respectively).
Long-term mortality
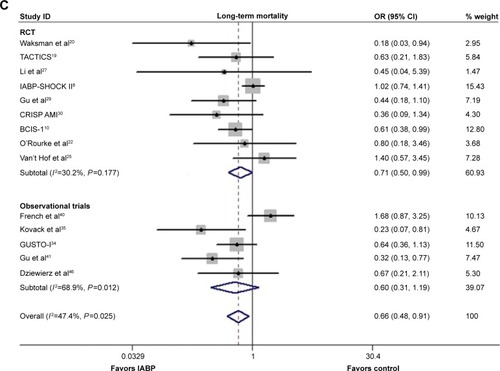
A significantly lower incidence of long-term mortality rate was observed in the IABP group compared with the control group (OR 0.66, 95% CI: 0.48–0.91, P=0.010; P=0.025, I2=47.4%; ), without publication bias (Egger’s test: P=0.132). A similar result was observed in the RCTs (OR 0.71, 95% CI: 0.50–0.99, P=0.046; P=0.177, I2=30.2%; Egger’s test: P=0.191), and no significant difference was observed in Obs (OR 0.60, 95% CI: 0.31–1.19, P=0.145; P=0.012, I2=68.9%; Egger’s test: P=0.359). A sensitivity analysis confirmed the beneficial effects of IABP with respect to the long-term mortality.
Secondary efficacy endpoints
Reinfarction
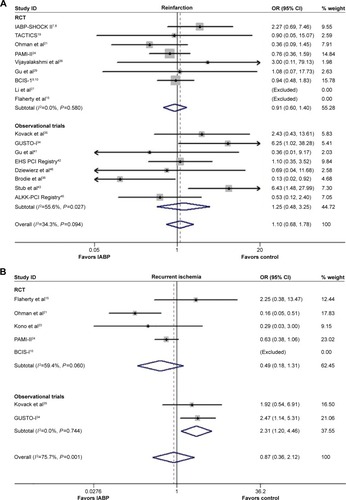
The impact of IABP insertion did not significantly differ from no IABP use with respect to the reduction of risk of reinfarction during hospitalization (OR 1.10, 95% CI: 0.68–1.78, P=0.706; P=0.094, I2=34.3%; ), and the results from the RCTs and Obs were similar (Obs: OR 1.25, 95% CI: 0.48–3.25, P=0.644; P=0.027, I2=55.6%; RCT: OR 0.91, 95% CI: 0.60–1.40, P=0.680; P=0.580, I2=0.0%). No publication bias was observed for reinfarction (Egger’s test: P=0.548, 0.667, 0.837 for Obs, RCT, and overall, respectively).
Figure 3 Forest plots of the secondary efficacy endpoint of the included trials.
Abbreviations: CI, confidence interval; IABP, intra-aortic balloon pump; RCT, randomized controlled trial; ID, identification.
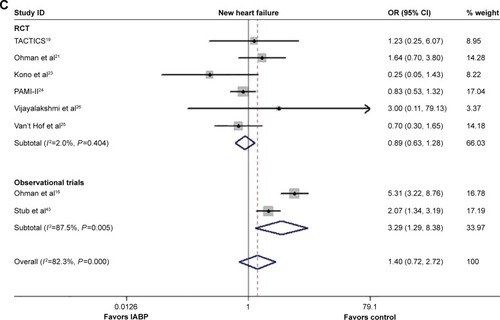
Recurrent ischemia
As depicted in , there was no significant difference between IABP use and no IABP use with respect to the risk of in-hospital ischemia recurrence (OR 0.87, 95% CI: 0.36–2.12, P=0.754; P=0.001, I2=75.7%), in either the RCTs or Obs (RCT: OR 0.49, 95% CI: 0.18–1.31, P=0.155; P=0.060, I2=59.4%; Obs: OR 2.31, 95% CI: 1.20–4.46, P=0.013; P=0.744, I2=0.0%). No publication bias was observed (P=0.855, 0.836 for RCTs and overall, respectively).
New heart failure
The overall incidence of new heart failure was similar between the two subgroups (OR 1.40, 95% CI: 0.72–2.72, P=0.320; P<0.001, I2=82.3%; ), as well as in the RCTs (OR 0.89, 95% CI: 0.63–1.28, P=0.541; P=0.404, I2=2.0%), whereas the risk of new heart failure increased significantly in the Obs (OR 3.29, 95% CI: 1.29–8.38, P=0.013; P=0.005, I2=87.5%). The results from the Egger’s test suggested no publication bias regarding the incidence of new heart failure (P=0.875, 0.866 for RCTs and overall, respectively).
Safety endpoints
Severe bleeding requiring blood transfusion
The incidence of severe bleeding requiring blood transfusion was higher in the IABP group than in control group (OR 2.05, 95% CI: 1.29–3.25, P=0.002; P=0.001, I2=78.5%; ); a similar result was observed in the Obs (OR 3.48, 95% CI: 2.09–5.79, P<0.001; P=0.003, I2=65.1%), whereas no significant difference was observed in RCTs (OR 1.14, 95% CI: 0.87–1.49, P=0.338; P=0.804, I2=0.0%). The Egger’s test was not suggestive of publication bias (P=0.582, 0.640, 0.880 for Obs, RCTs, and overall, respectively). The sensitivity analysis demonstrated that the inferior effects of IABP insertion in the setting of AMI on severe bleeding requiring blood transfusion were always observed by omitting a single study at a time.
Figure 4 Forest plots of the safety endpoints of the included trials.
Abbreviations: CI, confidence interval; IABP, intra-aortic balloon pump; RCT, randomized controlled trial; ID, identification.
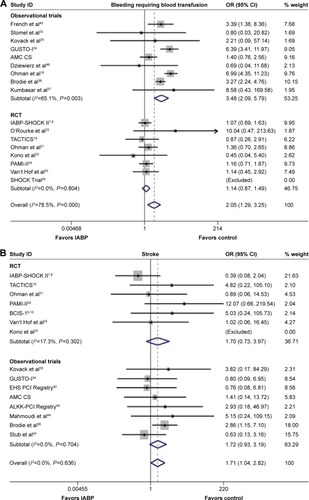
Stroke
IABP usage was associated with a higher in-hospital incidence of stroke (OR 1.71, 95% CI: 1.04–2.82, P=0.035, ) without any heterogeneity (P=0.636, I2=0.0%), which contrasted with the results of RCTs and Obs (OR 1.70, 95% CI: 0.73–3.97, P=0.220; P=0.302, I2=17.3% for RCTs; Obs: OR 1.72, 95% CI: 0.93–3.19, P=0.085; P=0.704, I2=0.0% for Obs). No publication bias was noted via Egger’s test (P=0.662, 0.142, 0.248 for Obs, RCTs, and overall, respectively).
Subgroup analysis
In the subgroup of patients suffering from AMI complicated by CS, significantly lower risks of in-hospital and long-term mortality associated with IABP use versus no IABP use were noted (OR 0.68, 95% CI: 0.46–0.99, P=0.045, ; OR 0.64, 95% CI: 0.41–0.996; P=0.048, ), but no significant difference compared with midterm mortality was observed (OR 0.86, 95% CI: 0.56–1.35, P=0.521).
Figure 5 Forest plots of the subgroup analysis of the included trials.
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; ERV, emergency revascularization; TT, thrombolytic therapy; CS, cardiogenic shock; IABP, intra-aortic balloon pump; ID, identification; NA, not available.
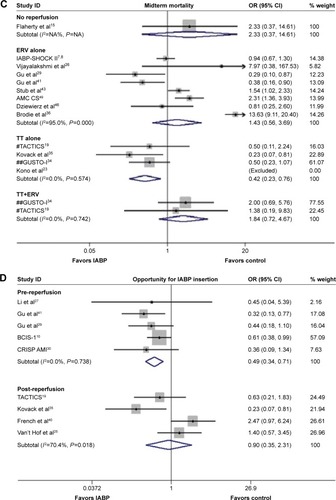
In the subgroup of reperfusion, the combination of IABP and TT alone resulted in a lower midterm mortality rate (OR 0.42, 95% CI: 0.23–0.76, P=0.004, ), although no significant differences were observed with respect to in-hospital and long-term mortality (in-hospital: OR 0.55, 95% CI: 0.21–1.39, P=0.206; long-term: OR 0.57, 95% CI: 0.28–1.17, P=0.128). Moreover, the combination of IABP and ERV alone was associated with a lower long-term mortality (OR 0.71, 95% CI: 0.49–1.03, P=0.072).
In the analysis pertaining to the opportunity for IABP insertion, a reduced risk of long-term mortality was observed among the patients suffering AMI for whom IABP was inserted before reperfusion compared with no IABP use (OR 0.49, 95% CI: 0.34–0.71, P<0.001, ), although no significant difference was observed between no IABP use and IABP insertion after reperfusion (OR 0.90, 95% CI: 0.35–2.31, P=0.825).
Discussion
The major finding of this comprehensive meta-analysis was that IABP insertion was associated with reduced long-term mortality in patients suffering from AMI compared with no IABP use at the cost of potential high risk of stroke and severe bleeding requiring blood transfusion. The subgroup analyses demonstrated that IABP insertion before reperfusion may be an optimal treatment for patients suffering from AMI complicated by CS or patients receiving TT.
Since IABP was first reported for clinical application in 1968,Citation4 it has been widely used in patients suffering from AMI, particularly patients following AMI complicated by CS.Citation2,Citation50 Both the ACC/AHA (2012)Citation5 and the European Society of Cardiology guidelinesCitation6 strongly recommended IABP as a bridge to reperfusion for patients suffering from AMI complicated by CS, recommendations derived from several observational clinical trials.Citation31,Citation34,Citation39 However, these recommendations have been challenged because of several recent meta-analysesCitation12 and RCTs,Citation7–Citation10,Citation19 which demonstrated that IABP for patients with AMI complicated by CS was not associated with reduced mortality, but with high potential risks of major bleeding and stroke. The IABP-SHOCK II trial, a randomized, open-label, multicenter trial involving 600 patients with CS following AMI who underwent early revascularization, demonstrated that IABP did not increase either 6- or 12-month survival rates compared with no IABP use.Citation7,Citation8 Moreover, the IABP SHOCK trialCitation28 demonstrated that IABP insertion exerted only modest or even no effects on the acute physiology and chronic health evaluation II score as a marker of disease severity, improvements in cardiac index, reduced inflammation, or reduced plasma brain natriuretic peptide levels compared with medical therapy alone. In summary, the current data did not support IABP as a routine treatment for patients with AMI regardless of whether the patients suffered from AMI complicated by CS, which called the above-mentioned guidelines into question. On the other hand, all these over-mentioned meta-analyses for IABP application did not include all available relevant citations and there was none of them grouped meticulously which might cover the beneficial efficacy of IABP on special patients. Therefore, we carried out this updated, comprehensive meta-analysis to explore which patients could benefit mostly from IABP insertion.
In spite of the advances in early revascularization,Citation28 the mortality rate of patients suffering from AMI complicated by CS remains high; IABP was empirically preferred with respect to the treatment of these patients. The thrombolysis and counterpulsation to improve survival in myocardial infarction (TACTICS) trialCitation19 demonstrated that IABP insertion for patients with AMI complicated by either hypotension or severe heart failure who were receiving thrombolysis was not associated with a significant risk reduction on 6-month mortality, but was associated with increased survival rates for patients in Killip classes III or IV (39% for combined therapy versus 80% for fibrinolysis alone). The data from the present study demonstrated the superior effects of IABP in patients suffering from AMI, particularly those complicated with CS, findings consistent with results of two previous meta-analyses.Citation13,Citation14 IABP may be the optimal choice for specific patients with AMI, but not for all patients with AMI.
In fact, the observed reductions in mortality secondary to IABP usage may be balanced by the increased rates of stroke and severe bleeding. More and more evidence, including the results from our study, has demonstrated that IABP insertion is associated with a higher incidence of severe bleeding and stroke. Four risk factors for complications following IABP insertion were identified from the Benchmark Registry:Citation51 age ≥75 years, female sex, peripheral arterial disease, and body surface area <1.65 m2. In the meta-analysis published by Sjauw et al,Citation13 a significantly increased rate of stroke secondary to IABP use was observed in patients suffering from AMI. Restricting activity due to IABP insertion for long periods may promote the development of venous thrombosis, resulting in an increased risk of all-cause death among affected patients. Moreover, several studiesCitation52,Citation53 have demonstrated that major bleeding was associated with an increased risk of death, indicating that bleeding was dangerous not only because of the hemorrhaging itself but also because it forced the discontinuation of the IABP and necessary antiplatelet therapy, resulting in higher rates of thrombotic events. Thiele et alCitation7,Citation8 pointed out that the higher in-hospital mortality in the AMI patients complicated with CS might depend on hemodynamic deterioration, multiorgan dysfunction, or systemic inflammatory response syndrome. One more possible related point was that some of the included patients were too serious to receive the implantation of IABP before their deaths or even had no access to implant this equipment in time if they were initially treated in a peripheral center. In contrast, the lower long-term mortality among these patients might be due to the successful insertion of IABP which positively affected myocardial recovery following AMI. Therefore, it may be more useful to determine which patients benefit most from IABP insertion, as opposed to studying the complications of IABP use.
In contrast to the recommendations of the ACC/AHA (2012) guidelines, the SHOCK trialCitation28 demonstrated that a higher rate of in-hospital mortality was observed among the patients suffering from AMI complicated by CS associated with IABP insertion following reperfusion compared with no IABP use (36.8% vs 28.6%). However, the balloon pump-assisted coronary intervention study (BCIS-1) trialCitation10 demonstrated that IABP insertion before reperfusion may result in a significantly reduced risk of long-term mortality among these patients. Therefore, a subgroup analysis was conducted in our study to address this controversy and determine the ideal opportunity for IABP insertion, as well as which patients may benefit most from IABP insertion. IABP usage was associated with a lower risk of mortality when inserted before reperfusion, a finding consistent with those of previous studies.Citation10,Citation27 Early IABP insertion promoted hemodynamic stability and reduced myocardial oxygen demand, which may be important in patients suffering from AMI complicated by CS.Citation2 Our results indicated that IABP insertion was associated with feasible benefits with respect to long-term survival in patients with AMI, particularly patients suffering from AMI complicated by CS and patients receiving thrombolytic therapy.
Several questions remained unanswered. First, there were not enough data to assess the optimal duration of IABP insertion for patients without severe complications, as well as for which patients the IABP may be removed at an earlier time. Most of current literature regarding the efficacy of IABP use consented to a duration of ~48 hours, although no absolute benefits regarding survival rates were demonstrated. A second dilemma involved the ideal opportunity for IABP insertion in patients with AMI complicated by hemodynamic compromise. The ACC/AHA (2012) guidelines recommend implantation at the completion of reperfusion.Citation5 Third, divergence regarding specifics about the reperfusion strategies was observed. Both ERV alone and TT alone demonstrated potential benefits in patients with AMI in this meta-analysis. Therefore, powerful randomized clinical trials comparing IABP use and no IABP use in patients suffering from AMI complicated by CS focusing on the optimal duration of IABP, most suitable time for IABP insertion, and more precise reperfusion strategies must be performed to guide clinical decision making.
Limitations
This study had several limitations. First, this meta-analysis was not based on individual patient data, and the small sample size of several included RCTs also made the evaluation of IABP’s efficacy easily influenced. Second, different studies used different endpoint definitions, which may have been the source of bias. Third, the lack of other potential confounding factors, such as the time of IABP insertion, duration of IABP placement, insertion details, including technology and choice of sheath with different sizes, as well as reperfusion strategies, did not allow us to explore the effects of IABP on patients with AMI or the underlying mechanisms of these effects. Additionally, there was no uniform or clear follow-up period.
Conclusion
IABP insertion is associated with feasible benefits with respect to long-term survival in patients with AMI, particularly those suffering from CS and receiving thrombolytic therapy, at the cost of higher risk of severe bleeding and stroke.
Author contributions
Z-GF and X-FG were involved in the design, literature searching, assessment of study quality, and drafted the manuscript. Disagreements were resolved by L-WC. X-BL and M-XS revised critically the manuscript. Z-GF and X-FG performed statistical analysis and critically revised the manuscript. QJ, HZ, and Y-ZR constructed the maps. S-LC and N-LT critically revised original study design and the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The study was supported by the Jiangsu Provincial Special Program of Medical Science (BL2013001).
Disclosure
The authors report no conflicts of interest in this work.
References
- GoldbergRJSpencerFAGoreJMLessardDYarzebskiJThirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspectiveCirculation200911991211121919237658
- ScheidtSWilnerGMuellerHIntra-aortic balloon counter-pulsation in cardiogenic shock. Report of a co-operative clinical trialN Engl J Med1973288199799844696253
- KernMJAguirreFBachRDonohueTSiegelRSegalJAugmentation of coronary blood flow by intra-aortic balloon pumping in patients after coronary angioplastyCirculation19938725005118425297
- KantrowitzATjonnelandSFreedPSPhillipsSJButnerANShermanJLJrInitial clinical experience with intraaortic balloon pumping in cardiogenic shockJAMA196820321131185694059
- AndersonJLAdamsCDAntmanEM2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarctionJ Am Coll Cardiol20136123e179e34723639841
- KolhPWijnsWDanchinNGuidelines on myocardial revascularizationEur J Cardiothoracic Surg201038S1S52
- ThieleHZeymerUNeumannFJIntraaortic balloon support for myocardial infarction with cardiogenic shockN Engl J Med201236714128722920912
- ThieleHZeymerUNeumannFJIntra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trialLancet201338299051638164524011548
- PereraDStablesRThomasMElective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trialJAMA2010304886787420736470
- PereraDStablesRClaytonTLong-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary interventionCirculation2013127220721223224207
- SuDYanBGuoLIntra-aortic balloon pump may grant no benefit to improve the mortality of patients with acute myocardial infarction in short and long termJ Pediatr Gastroenterol Nutr2015941917
- AhmadYSenSShun-ShinMJIntra-aortic balloon pump therapy for acute myocardial infarctionJAMA Intern Med2015175693125822657
- SjauwKDEngstromAEVisMMA systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines?Eur Heart J200830445946819168529
- RomeoFAcconciaMCSergiDThe outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: A comprehensive meta-analysisAm Heart J2013165567969223622904
- FlahertyJTBeckerLCWeissJLResults of a randomized prospective trial of intraaortic balloon counterpulsation and intravenous nitroglycerin in patients with acute myocardial infarctionJ Am Coll Cardiol1985624344463926848
- OhmanEMCaliffRMGeorgeBSThe use of intraaortic balloon pumping as an adjunct to reperfusion therapy in acute myocardial infarction. The Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study GroupAm Heart J19911213 Pt 18959011900381
- EggerMDaveySGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaborationBMJ2009339b270019622552
- OhmanEMNanasJStomelRJThrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS TrialJ Thromb Thrombolysis2005191333915976965
- WaksmanRWeissATGotsmanMSHasinYIntra-aortic balloon counterpulsation improves survival in cardiogenic shock complicating acute myocardial infarctionEur Heart J199314171748432295
- OhmanEMGeorgeBSWhiteCJUse of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction. Results of a randomized trial. The Randomized IABP Study GroupCirculation19949027927998044950
- O’RourkeMFNorrisRMCampbellTJChangVPSammelNLRandomized controlled trial of intraaortic balloon counterpulsation in early myocardial infarction with acute heart failureAm J Cardiol19814748158207010976
- KonoTMoritaHNishinaTAortic counterpulsation may improve late patency of the occluded coronary artery in patients with early failure of thrombolytic therapyJ Am Coll Cardiol19962848768818837563
- StoneGWMarsaleseDBrodieBRA prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty. Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial InvestigatorsJ Am Coll Cardiol1997297145914679180105
- Van’t HofAWLiemALde BoerMJHoorntjeJCSuryapranataHZijlstraFA randomized comparison of intra-aortic balloon pumping after primary coronary angioplasty in high risk patients with acute myocardial infarctionEur Heart J199920965966510208786
- VijayalakshmiKKunadianBWhittakerVJIntra-aortic counter-pulsation does not improve coronary flow early after PCI in a high-risk group of patients: observations from a randomized trial to explore its mode of actionJ Invasive Cardiol200719833934617712202
- LiJLXueHWangBSEffect of prolonged intra-aortic balloon pumping in patients with cardiogenic shock following acute myocardial infarctionMed Sci Monit2007136R270R274
- ProndzinskyRLemmHSwyterMIntra-aortic balloon counter-pulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndromeCritical Care Med201038115216019770739
- GuJHuWXiaoHProphylactic intra-aortic balloon pump reduces C-reactive protein levels and early mortality in high-risk patients undergoing percutaneous coronary interventionActa Cardiol201166449950421894807
- PatelMRSmallingRWThieleHIntra-aortic balloon counter-pulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trialJAMA2011306121329133721878431
- MoulopoulosSStamatelopoulosSPetrouPIntraaortic balloon assistance in intractable cardiogenic shockEur Heart J1986753964033732287
- BengtsonJRKaplanAJPieperKSPrognosis in cardiogenic shock after acute myocardial infarction in the interventional eraJ Am Coll Cardiol1992207148214891452920
- StomelRJRasakMBatesERTreatment strategies for acute myocardial infarction complicated by cardiogenic shock in a community hospitalChest1994105499710028162800
- AndersonRDOhmanEMHolmesDJUse of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: observations from the GUSTO-I Study. Global Utilization of Streptokinase and TPA for Occluded Coronary ArteriesJ Am Coll Cardiol19973037087159283530
- KovackPJRasakMABatesEROhmanEMStomelRJThrombolysis plus aortic counterpulsation: improved survival in patients who present to community hospitals with cardiogenic shockJ Am Coll Cardiol1997297145414589180104
- BrodieBRStuckeyTDHansenCMuncyDIntra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in high-risk patients with acute myocardial infarctionAm J Cardiol1999841182310404845
- KumbasarSDSemizESancaktarOYalçinkayaSErmişCDeğerNConcomitant use of intraaortic balloon counterpulsation and streptokinase in acute anterior myocardial infarctionAngiology199950646547110378822
- SanbornTASleeperLABatesERImpact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock?J Am Coll Cardiol2000363 Suppl A1123112910985715
- BarronHVEveryNRParsonsLSThe use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: Data from the National Registry of Myocardial Infarction 2Am Heart J2001141693393911376306
- FrenchJKFeldmanHAAssmannSFInfluence of thrombolytic therapy, with or without intra-aortic balloon counterpulsation, on 12-month survival in the SHOCK trialAm Heart J2003146580481014597928
- GuJHuWXiaoHFengXChenYZhangDIntra-aortic balloon pump improves clinical prognosis and attenuates C-reactive protein level in acute STEMI complicated by cardiogenic shockCardiology20101171758020924182
- ZeymerUBauerTHammCUse and impact of intra-aortic balloon pump on mortality in patients with acute myocardial infarction complicated by cardiogenic shock: results of the Euro Heart Survey on PCIEuroIntervention20117443744121764661
- StubDChanWClarkDAre intra-aortic balloon pumps harmful in cardiogenic shock?Heart Lung Circ201120S115
- MahmoudiMHauvilleCGagliaMAThe impact of intra-aortic balloon counter-pulsation on in-hospital mortality in patients presenting with anterior ST-elevation myocardial infarction without cardiogenic shockCardiovasc Revasc Med201213632833023062956
- ZeymerUHochadelMHauptmannKIntra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: results of the ALKK-PCI registryClin Res Cardiol2013102322322723179136
- DziewierzASiudakZRakowskiTKleczyńskiPZasadaWDudekDImpact of intra-aortic balloon pump on long-term mortality of unselected patients with ST-segment elevation myocardial infarction complicated by cardiogenic shockPostepy Kardiol Interwencyjnej201410317518025489303
- VisMMSjauwKDvan der SchaafRJPrognostic value of admission hemoglobin levels in ST-segment elevation myocardial infarction patients presenting with cardiogenic shockAm J Cardiol20079991201120217478141
- VisMMSjauwKDvan der SchaafRJIn patients with ST-segment elevation myocardial infarction with cardiogenic shock treated with percutaneous coronary intervention, admission glucose level is a strong independent predictor for 1-year mortality in patients without a prior diagnosis of diabetesAm Heart J200715461184119018035093
- SjauwKDEngströmAEVisMMEfficacy and timing of intra-aortic counterpulsation in patients with ST-elevation myocardial infarction complicated by cardiogenic shockNeth Heart J2012201040240922847042
- ThieleHAllamBChatellierGSchulerGLafontAShock in acute myocardial infarction: the Cape Horn for trials?Eur Heart J201031151828183520610640
- KimuraYOhbaKSumidaHA survival case of cardiogenic shock due to left main coronary artery myocardial infarction: successful cooperation with on-site percutaneous coronary intervention and helicopter emergency medical serviceIntern Med201251141845185022821098
- DoyleBJRihalCSGastineauDAHolmesDJBleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practiceJ Am Coll Cardiol200953222019202719477350
- ChhatriwallaAKAminAPKennedyKFAssociation between bleeding events and in-hospital mortality after percutaneous coronary interventionJAMA2013309101022102923483177