Abstract
Purpose
To define importance values assigned to attributes of biological agents (BAs) by Spanish patients with rheumatic diseases (rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis) and rheumatologists.
Patients and methods
This was an observational, cross-sectional design based upon a rank-based full-profile conjoint analysis. A literature review and four focus groups were undertaken to identify attributes and levels. An orthogonal matrix, combining the selected levels of attributes, was used to define scenarios. Participants ranked eight scenarios from 1 (most preferred) to 8 (least preferred). The relative importance (RI) of attributes was calculated. Multivariate regression analysis was performed to identify the characteristics that influenced the values of RI. A total of 488 patients (male 50.9%, mean age 50.6 [standard deviation {SD} 12.06] years, rheumatoid arthritis 33.8%, ankylosing spondylitis 32.4%, psoriatic arthritis 33.8%; mean time since diagnosis 12.6 [SD 8.2] years) and 136 rheumatologists (male 50.4%, mean age 46.4 [SD 9.1] years, mean time of practice 16.7 [SD 8.8] years) participated.
Results
The ideal BAs for patients and physicians, respectively, should allow pain relief and improvement of functional capacity (RI 39% and 44.7%), with low risk of adverse events (RI 24.9% and 30.5%), a long time prior to perceiving the need for a new dose (RI 16.4% and 12.4%), and self-administration at home (RI 19.7% and 12.5%), as identified through their preferences.
Conclusion
Although efficacy and safety are paramount for patients and rheumatologists to make a choice regarding BAs, the need for a low frequency of administration and the administration method also play a role as preference attributes for BAs.
Introduction
Rheumatic diseases (RDs) represent a multitude of chronic degenerative, inflammatory, and autoimmune conditions affecting millions of people worldwide.Citation1 In Spain, the prevalence of RD can reach 23%.Citation2 Three of the most prevalent RDs are rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA).Citation3–Citation6 All three pathologies are characterized by their potential to cause disability,Citation7 their negative influence on patients’ quality of life (QoL)Citation8 and functional capacity, and by the immense consumption of health care resources and loss of productivity they entail.Citation9
Traditional treatment of inflammatory RDs includes the use of symptom-modifying therapies (nonsteroidal anti-inflammatory drugs and corticosteroids), combined with nonbiologic disease-modifying antirheumatic drugs (DMARDs).Citation10,Citation11 The development of new biological therapies, particularly TNF inhibitors, has led to significant improvement in clinical outcomes, including symptoms, health-related QoL (HRQoL), and functioning.Citation12 However, this scenario is associated with a more complex decision-making process. These newer therapies present different routes of administration, increased or different toxicities, and higher financial costs, all of which may influence patient preferences and adherence to medications.Citation13
Assessing and including patient preferences within routine clinical practice has been related to an increment in medication adherence, improvements in treatment outcomes, and reduced health care costs.Citation13–Citation19 Since the first studies that examined patient preferences for biological agents (BAs) in RA were published,Citation20,Citation21 rheumatologists have started to use patient-focused outcomes to improve RA treatment.Citation22 Nevertheless, it is possible that RD outcomes could be improved further if rheumatologists were aware of how patients used and perceived newer medications,Citation23 thereby reinforcing the importance of understanding patients’ attitudes toward treatment and involving them in shared decision making.Citation13 The aim of this study was to define the importance values (preferences) assigned to the attributes of BAs by Spanish patients with the main RDs – RA, AS, and PsA – and by their rheumatologists.
Materials and methods
Design
An observational cross-sectional study was conducted in 41 Spanish hospitals, based on a conjoint analysis methodology. The conjoint analysis method is particularly useful for quantifying preferences for a diverse range of health applications. It consists of a composition method, in which the implicit values for an attribute of an intervention are derived from the overall score for a profile consisting conjointly of two or more attributes.Citation24 It is also used to understand patient preferences for health states, to value the various health states described by patient-reported outcomes and HRQoL scales,Citation25 and to assess patients’ willingness to accept the therapeutic risks associated with more effective treatments.Citation24 Conjoint analysis also offers a mechanism for patients to participate in the decision-making process.Citation26,Citation27
The conjoint analysis technique uses questionnaire data. In the present study, a rank-based full-profile conjoint was applied. In this method, individuals are first presented with, and then asked to give an ordinal ranking to the options of hypothetical scenarios involving different levels of characteristics, which have been identified as important to the question of interest.Citation28 Those options that achieve the highest ranking are viewed as the most important. Since it is considered easy to answer and to analyze, this method has become popular for eliciting preferences for health care interventions.Citation29
Following the International Society For Pharmacoeconomics and Outcomes Research good research practices for conjoint analysis,Citation24 a literature review was performed to identify the preliminary set of attributes and levels of BAs most frequently described in publications. Subsequently, a focus group with rheumatologists (n=5) and three focus groups with patients (n=5) – one for each pathology (RA, AS, and PsA) – were formed. Focus groups helped define, from the identified attributes, the final set of attributes and levels included in the study, reflecting both patients’ and professionals’ perspectives regarding BAs.
shows the four attributes of BAs included in the study, with their respective levels: administration method (subcutaneous self-administration at home, intravenous administration by a health care professional at hospital), risk of adverse events (AEs; high risk of AEs, low risk of AEs), pain relief (pain relief and improvement in functional capacity, no pain relief and no improvement in functional capacity), and duration of effect (1 week, 2 weeks, 4 weeks, 8 weeks).
Table 1 Attributes and levels included in the scenarios
For definition of the scenarios, an orthogonal design was used. A full-choice fractional factorial design was implemented using SPSS version 19.0. This orthogonal design, combining the levels of the attributes, resulted in eight scenarios, which described different alternatives of treatment with BAs for RA, AS, or PsA.Citation30
A self-completion hard-copy case-report form (CRF) was specifically designed to collect data from participants.Citation31 Patient CRFs included sociodemographic (age, sex, marital status, place of residence, level of studies, employment status, and other variables) and clinical variables (height, weight, date of onset of first symptoms, diagnosed rheumatic illness, date of diagnosis, disabling symptoms and complications associated with RD, comorbidities, current treatment, and previous treatment), as well as participants’ preferences. In patient CRFs, clinical and sociodemographic data were collected by rheumatologists taking part in the study during routine practice, while patients ranked the scenarios from 1 (most preferred) to 8 (least preferred). In addition, professionals self-completed another CRF based on their sociodemographic data (age, sex, work center, time of professional experience) and preferences. Professionals ranked the scenarios from 1 (most preferred to prescribe) to 8 (least preferred to prescribe). Missing data for variables are presented in .
Table 2 Patient sociodemographic and clinical variables
Participants
The study protocol was approved by the ethics Committee of the Bellvitge Hospital Universitari (Acta 01/13, reference EPA047/12). All participants in the study provided written consent. A total of 41 hospitals in the public health sector where BAs for RDs were prescribed were purposefully identified around the country, covering the whole Spanish territory. A rheumatologist at each selected hospital was required to recruit a minimum of four to five ambulatory patients for each condition under study (n=12–15), as well as a minimum number of rheumatologists (three to four) working in the same or a similar health care centre.
Patients’ inclusion criteria included having been diagnosed with RA, AS, or PsA at least 2 years prior to their inclusion and currently or previously (≤1 year ago) receiving BAs for a minimum of 1 year. Exclusion criteria included a need to translate questionnaire, coexistence of the studied RDs, incapacity to participate due to clinical, physical, or intellectual factors according to clinician judgment, and currently taking part in a clinical trial. Rheumatologists were required to have at least 3 years’ experience in the use of BAs, and were excluded if they practiced only in the private sector.
Statistical analysis
A descriptive statistical analysis was performed for sociodemographic, clinical, and treatment variables. A rank-ordered logit model was applied to estimate the preferences or partial utilities of each attribute, which indicated the perceived value of the feature. The relative importance (RI) of attributes was calculated from these partial utilities (the utility ranges of an attribute divided by the sum of the ranks of the four attributes of each individual), allowing the comparison of values between the two groups. To identify the clinical and sociodemographic characteristics that influenced the value of RI given to each attribute by both patients and rheumatologists, a multiple-regression analysis was performed where the values of importance were considered dependent and the clinical and sociodemographic variables independent variables. All variables were included in the analysis, but to reduce the number of independent variables and to avoid problems of collinearity, a stepwise algorithm was implemented. The software SPSS version 19.0 was used for all statistical tests, and a significance level of P<0.05 was assumed. To compare the values obtained in the different groups, the χ2 test was performed for qualitative variables. Parametric distributions were analyzed with analysis of variance, while nonparametric distributions were examined with the Kruskal–Wallis test.
Results
Descriptive analysis
A total of 488 patients were included, distributed equally among diseases (RA 33.8%, AS 32.4%, and PsA 33.8%). The sample mean age was 50.61 (standard deviation [SD] 12.06) years. In the RA sample, 73.8% were females, while in the AS and PsA groups, males accounted for 71.8% and 55.8%, respectively ().
Average sample weight and height were 75.8 kg and 166.5 cm, respectively, with significant differences between diseases. The mean Charlson index score was 0.4 (SD 0.7), with significant differences (P=0.001) among pathologies (RA 0.5 [SD 0.8], AS 0.2 [SD 0.6], and PsA 0.3 [SD 0.7]). At inclusion in the study, the mean time since the diagnosis of the RDs was 12.6 (SD 8.2) years, while the mean time since the onset of symptoms was 15 (SD 9.6) years. The most common disabling symptoms reported were joint pain (SD 51.8%) and limitation of functional capacity (34%) (). The sample mean body mass index was 27.3 kg/m2 (SD 4.8), and 43.9% of the population studied did not present comorbidities (secondary diagnoses). According to physician judgment, most of the patients did not have complications associated with their RD (68.9%), considered as an unfavorable evolution of the disease. The complications taken into account were amyloidosis, anemia, cardiac, intestinal, ocular, renal, lung, and neurological complications, and others, but only ocular (10%), intestinal (3.5%), and pulmonary (2.7%) complications showed significant differences between pathologies (). Of comorbidities reported, the most common were endocrine, nutritional, and metabolic conditions (22.7%), and these, together with neoplasia, were the comorbid conditions with marked differences between pathologies.
shows the BAs most frequently received by patients – mainly etanercept (27.3%), adalimumab (26.2%), and infliximab (23.2%) – with the mean duration of treatment being 49.7 (SD 36.7) months, as well as those BAs previously received – mainly adalimumab (10.7%), infliximab (9.4%), and etanercept (9%) – with an average duration of 43.8 (SD 37.5) months. With regard to rheumatologists, the sample consisted of 136 participants: 50.4% males with a mean age of 46.4 (SD 9.1) years. The mean time of professional experience was 16.7 (SD 8.8) years.
Table 3 Number and percentage of patients receiving treatment with BAs at the time of study inclusion, and previous treatment
Preferences for BA attributes
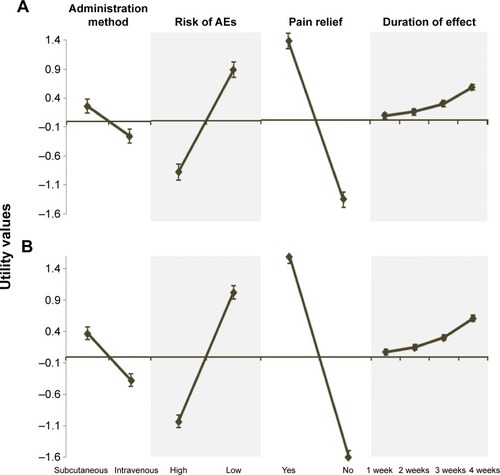
The conjoint analysis models proved to fit: Pearson’s R=0.991 (P<0.001) and Kendall’s τ=0.929 (P<0.001) for patients, and Pearson’s R=0.996 (P<0.001) and Kendall’s τ=1 (P<0.001) for rheumatologists. demonstrates that each attributes’ preferred levels for both patients and rheumatologists were similar: subcutaneous self-administration at home (utility values 0.26 and 0.37), low risk of AEs (utility values 0.81 and 1.03), pain relief and improvement of functional capacity (utility values 1.26 and 1.59), and duration of effect (time until perceiving the need for a new dose) of 8 weeks (utility values 0.53 and 0.61), respectively.
Figure 1 Patients’ (A) and rheumatologists’ (B) utility values.
Abbreviation: AEs, adverse events.
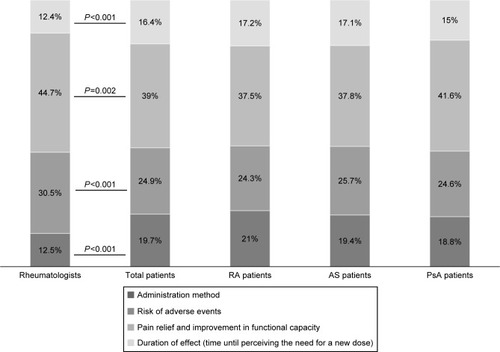
shows the RI given by both patients and rheumatologists to the attributes of BAs and for specific rheumatic conditions. Based on the utility values calculated by the model, both patients, with independence of the diagnosis, and physicians placed more importance on the pain relief and improvement in functional capacity attribute (RI 39% and 44.7%), followed by the risk of AEs (RI 24.9% and 30.5%), administration method (RI 19.7% and 12.5%), and duration of effect (time until perceiving the need for a new dose) (RI 16.4% and 12.4%), respectively. However, significant differences (P<0.002) were found on the RI given to the four attribute values by both groups of participants. Patients placed higher importance on the administration method and duration of effect (time until perceiving the need for a new dose) attributes, compared to rheumatologists, while the latter gave more importance to pain relief, improvement in functional capacity, and risk of AEs, than patients did.
Figure 2 Relative importance values given by both patients and rheumatologists to the attributes of biological agents and for specific rheumatic conditions.
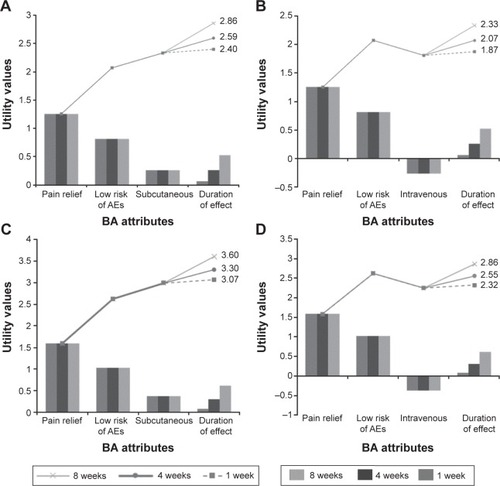
With regard to the time until perceiving the need for a new dose, both patients and professionals preferred 2–4 weeks over 1–2 weeks. Assuming that pain relief and risk of AEs were equal, patients’ and professionals’ utility values for BAs administered subcutaneously increased by 10% and 9% when the time until perceiving the need for a new dose was incremented from 4 to 8 weeks and by 20% and 17% when it was incremented from 1 to 8 weeks, respectively (). For BAs administered intravenously, the upturns for patients and professionals were 13% and 12%, and 25% and 23%, respectively (). These results demonstrate that both patients and rheumatologists gave higher importance to lower frequencies of administration or longer time until perceiving the need for a new dose.
Figure 3 Pareto diagrams representing patients’ and professionals’ utility values for subcutaneous and intravenous treatment alternatives.
Abbreviations: AEs, adverse events; BA, biological agent.
The ideal BA for both patients and professionals would be a drug that relieved pain and improved ability to perform daily activities, with a low risk of side effects, self-administered at home subcutaneously, and with longer time until perceiving the need for a new dose (8 weeks).
Multivariate-regression analysis
presents the results of the multivariate analysis, identifying the sociodemographic and clinical variables that affected the RI of each attribute. For patients, sex, pathology, symptoms, complications associated with the RD, and genitourinary comorbidities influenced the importance given to the risk of AEs. In general, females, patients diagnosed with AS, patients with articular rigidity, subjects without limitations to their functional capacity, and those with intestinal complications or genitourinary comorbidities gave greater importance to the risk of AEs. The variable that influenced the importance given to the relief of pain and improvement in functional capacity was disease symptoms. Patients presenting articular swelling and those without limitations in functional capacity gave less importance to the relief of pain. Mode of administration, age, sex, and the presence of intestinal complications influenced their preferences. Older patients, females, and those who presented an intestinal complication granted less importance to the mode of administration of BAs.
Table 4 Factors influencing patients’ and rheumatologists’ preferencesTable Footnotea
For rheumatologists, age and length of their professional experience influenced the importance given to the risk of AEs. Older professionals and rheumatologists with less time practicing the specialty granted less importance to the risk of AEs. Sex influenced the importance given to the relief of pain and improvement in functional capacity. Females gave less importance to the relief of pain and improvement in functional capacity.
Discussion
In recent years, BA options for patients with RDs have continued to expand, creating opportunities for improved outcomes, such as decreased pain, disability, and mortality. However, patients as well as physicians are faced with increasingly complex decisions about how and when a medication should be prescribed.Citation32 It has been reported that health care professionals more often rely on personal beliefs and experiences to make clinical decisions than on scientific evidence,Citation33–Citation35 and that those can differ from the views of their patients.Citation36 There is a need to provide patients with individualized treatment strategies and to enable their participation in medical decision making.Citation37 One essential factor that professionals must consider for reaching this goal is inquiring about patients’ preferences.Citation38
Results demonstrated that patients’ and professionals’ preferences were similar, the most to least preferred attributes being pain relief and improvement of functional capacity, risk of AEs, administration mode, and frequency of administration (time until perceiving the need for a new dose). The ideal treatment for both patients and professionals would be a BA that relieved pain and improved the ability to perform daily activities, with a low risk of AEs, self-administered at home subcutaneously, and with a greater time before perceiving the need for a new dose. Although efficacy and safety are key aspects for participants, both the frequency and method of administration play an important role as attributes of BAs in Spain. In this study, both patients and professionals preferred a low frequency of administration. Huynh et alCitation32 recently presented similar results. In an observational study performed in Denmark including RA patients naïve to and treated with BAs, as well as physicians and nurses, low treatment frequency was the preferred attribute for patients, followed by a more conservative route of administration. The authors concluded that the route and frequency of administration could influence patients’ adherence to and satisfaction with the treatment. Parallel outcomes were reported by Augustovski et al,Citation39 who performed a discrete-choice experiment on patients with RA naïve to BAs. These authors showed that avoidance of systemic AEs was the preferred attribute of BAs, followed by frequency of administration, efficacy, and route of administration.
In the present work, the RI given to the mode of administration and to the time until the perception of the need for a new dose was significantly greater in patients, while professionals placed more importance on the relief of pain, improvement in functional capacity, and risk of AEs. These differences could be due to patients granting more value to the characteristics regarding treatment comfort or convenience, while these aspects are not so valued by rheumatologists. These results are also in line with the previous findings of Huynh et al,Citation32 where RA patients and health professionals showed similar preferences regarding the route and frequency of administration of BAs, especially with those patients receiving subcutaneous treatment, but with differences in magnitude.
The importance of the administration route was also demonstrated in a study performed in Italy,Citation40 in which patients with RA addressed their perceptions of their current treatment and preferences for anti-TNF agents. The findings showed that 50.2% of patients preferred intravenous administration, mainly due to the reassuring effect of the presence of health care personnel, while 49.8% chose subcutaneous administration for its convenience. Moreover, in a study conducted in the UKCitation41 to assess the preferences of patients with RA receiving anti-TNF therapy or conventional DMARDs, the route of administration was the single feature of anti-TNF therapy that concerned patients the most. Subcutaneous injection was the first choice for those on anti-TNF therapy (41%) and those not yet receiving BAs (52.5%).
This analysis has some limitations, due to its design. The number and definition of attributes and factor levels is the critical step in any conjoint analysis, and although it followed the available guidelines, as it was based on a literature review and the opinion of patients and professionals, it may be subject to biases, due to participants’ cultural context or experience. Further research is needed to determine the relative effect of other potentially important attributes not included in this study, and to evaluate the stability of results across different populations. Moreover, patient preferences need to be explored in more depth in populations at a higher risk of poor communication with their physicians (those with low literacy, lower levels of education, and immigrants) and at risk of incomplete understanding or misunderstanding of the risks and benefits of these medications. Considering that this study was restricted to patients and rheumatologists from the Spanish public health system, its results must be extrapolated carefully to subjects from different backgrounds and cultures, patients with more severe diseases or institutionalized, or rheumatologists with less clinical experience than the included population, and must be interpreted within the context they were performed. Furthermore, a period of at least 1 year in biological DMARD (bDMARD) experience was chosen to select patients for this study, so it has to be considered that patients with early discontinuation of bDMARDs could have expressed different preferences.
For missing data, there are no objective criteria to clarify the maximum percentage of omissions that can be accepted, so each researcher must be responsible for their own decisions.Citation42 In the case of this study, given that all data were available for main variables (conjoint analysis), and that the maximum percentage of missing data for secondary variables was just 2.5%, missing values were not imputed, and results were calculated from available data. Nonetheless, this study provides very interesting results.
To the authors’ knowledge, it is the first time the preferences of treatment-experienced Spanish patients with different RDs, and rheumatologists, have been examined and their needs and perceptions regarding BAs identified and compared by means of a ranking-based conjoint analysis. Understanding patient needs provides the physician with the basis for the right therapeutic choice.Citation43,Citation44 At the same time, physician preference has been shown to be an important determinant of patient acceptance of biological therapy,Citation45 so the physician perspectives were also assessed in this study and compared to those of patients. This approach allowed us to detect differences between these two perspectives.
The knowledge produced in this analysis could contribute to a more informed decision-making process and a better choice of treatments.Citation46 Professionals should thus explore the preferences of patients by involving them in treatment decisions,Citation28 in order to achieve improvement in the quality of their care.Citation47
Conclusion
The assessment of patients’ and professionals’ preferences for the different attributes of BAs in the treatment of RDs is a necessary step toward improving results, by ensuring satisfaction and adherence findings that could contribute to better outcomes. Spanish patients with RDs’ and rheumatologists’ preferences for BAs were similar, preferring medication that relieves pain and improves ability to perform daily activities, with a low risk of AEs, self-administered at home subcutaneously, and with a greater time before perceiving the need for a new dose. Although efficacy and safety are key aspects for participants, both the frequency and method of administration play an important role as attributes of BAs.
Acknowledgments
This work was supported by Merck Sharp and Dohme. Two abstracts of this paper were presented at the ISPOR 17th Annual European Congress 2014 (PSY87 and PSY88) as poster presentations with interim findings. The poster abstracts were published in Value in Health, volume 17, issue 7 (http://www.valueinhealthjournal.com/article/S1098-3015(14)03648-1/pdf).
Participating investigators were: Alejandro Olivé Marqués (Hospital Universitari German Trias i Pujol, Barcelona, Spain), Alberto Alonso Ruiz (Hospital Universitario Cruces, Bilbao, Spain), Alberto Bermúdez Torrente (Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain), Alfredo Javier García González (Hospital Universitario 12 de Octubre, Madrid, Spain), Amalia Sánchez Rodríguez (Hospital Universitario Lucus Augusti, Lugo, Spain), Ana Sánchez Atrio (Hospital Universitario Príncipe de Asturias, Madrid, Spain), Antoni Rozadilla Sacanell (Hospital Universitari de Bellvitge, Barcelona, Spain), Antonia Ferreira Conejo (Hospital Don Benito Villanueva, Badajoz, Spain), Antonio Fernández Nebro (Hospital Regional Universitario de Málaga, Málaga, Spain), Antonio Juan Mas (Hospital Son Llàtzer, Majorca, Spain), Arturo Rodríguez de la Serna (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Asunción Acosta Pereira (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Berta Paula Magallares López (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Carlos Vázquez Galeano (Hospital Reina Sofía, Pamplona, Spain), Carmen Gómez Vaquero (Hospital Universitari de Bellvitge, Barcelona, Spain), Carmen Moragues Pastor (Hospital Plató, Barcelona, Spain), Eduardo Collantes Estévez (Hospital Universitario Reina Sofía, Córdoba, Spain), Elena Riera Alonso (Hospital Universitari Mútua de Terrasa, Barcelona, Spain), Enrique Júdez Navarro (Complejo Hospitalario Universitario de Albacete, Albacete, Spain), Erardo Meriño Ibarra (Hospital General San Jorge, Huesca, Spain), Eva Palero Díaz (Hospital General San Jorge, Huesca, Spain), Federico Navarro Sarabia (Hospital Universitario Virgen Macarena, Seville, Spain), Georgina Salvador Alarcón (Hospital Universitari Mútua de Terrasa, Barcelona, Spain), Indalecio Monteagudo Sáez (Hospital General Universitario Gregorio Marañón, Madrid, Spain), Jaime Calvo Alén (Hospital Sierrallana, Cantabria, Spain), Javier Calvo Catalá (Hospital General Universitario de Valencia, Valencia, Spain), Javier del Pino Montes (Hospital Universitario Salamanca, Salamanca, Spain), Joan Calvet Fontova (Hospital Universitari Parc Taulí, Barcelona, Spain), Joana Rovira Aguilar (Hospital Universitari Mútua de Terrasa, Barcelona, Spain), Joaquín Maria Belzunegui Otano (Hospital Universitario Donostia, San Sebastián, Spain), José Javier Pérez Venegas (Hospital del Servicio Andaluz de Salud de Jerez, Cádiz, Spain), José Rosas Gómez Salaz (Hospital de la Marina Baixa de Villajoyosa, Alicante, Spain), Juan José Alegre Sancho (Hospital Universitario Doctor Peset, Valencia, Spain), Juan Moreno Morales (Hospital General Universitario Santa Lucia, Murcia, Spain), Juan Víctor Tovar Beltrán (Hospital General Universitario de Elche, Alicante, Spain), Juana Sampedro Álvarez (Complejo Hospitalario de Toledo, Toledo, Spain), Leticia Lojo Oliveira (Hospital Universitario La Paz, Madrid, Spain), Lucía Pantoja Zarza (Hospital del Bierzo, León, Spain), Manel Pujol Busquets (Hospital Universitari Mútua de Terrasa, Barcelona, Spain), María García Manrique de Lara (Hospital Universitari Parc Taulí, Barcelona, Spain), Mariano Andrés Collado (Hospital General Universitario de Alicante, Alicante, Spain), Mario Agudo Bilbao (Hospital Universitario Marqués de Valdecilla, Santander, Spain), Miguel Ángel Abad Hernández (Hospital Virgen del Puerto, Cáceres, Spain), Montserrat Romera Baurés (Hospital Universitari de Bellvitge, Barcelona, Spain), Rafael Cáliz Cáliz (Hospital Universitario Virgen de las Nieves, Granada, Spain), Rosa Roselló Pardo (Hospital General San Jorge, Huesca, Spain), Rosario García de Vicuña Pinedo (Hospital Universitario de La Princesa, Madrid, Spain), Sagrario Bustabad Reyes (Hospital Universitario de Canarias, Canary Islands, Spain), Sara García Carazo (Hospital Universitario La Paz, Madrid, Spain), Silvia Martínez Pardo (Hospital Universitari Mútua de Terrasa, Barcelona, Spain), Trinidad Pérez Sandoval (Hospital de León, León, Spain), and Zulema Rosales Rosado (Hospital Clínico San Carlos, Madrid, Spain).
Disclosure
JM Nolla, M Rodríguez, E Martin-Mola, E Raya, and I Ibero report they have received remuneration for their contribution as experts regarding the subject of interest. L Lizán and M Prades work for an independent research entity, and have received remuneration for their contribution to the development and coordination of the original research project, as well as for writing this manuscript. G Nocea and B Aragon work at Merck Sharp and Dohme. The sponsor of the study, Merck Sharp and Dohme, has assumed all remuneration. However, the authors state that the research results described in this manuscript, as well as their analysis and interpretation, resulted from the free expression of opinion and from the agreement of the publication coauthors, and that no conflicts, either for obtaining or for disclosure of such results, existed. The authors report no other conflicts of interest in this work.
References
- ChoiHKNguyenUSNiuJDanaeiGZhangYSelection bias in rheumatic disease researchNat Rev Rheumatol201410740341224686510
- Ministerio de Sanidad Servicios Sociales e IgualdadEstrategia en Enfermedades Reumáticas y Musculoesqueléticas del Sistema Nacional de SaludMadridSanidad2013 Available from: http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/pdf/Estrategia_en_enfermedades_reumaticas_Accesible.pdfAccessed May 16, 2016
- MyasoedovaECrowsonCSKremersHMTherneauTMGabrielSEIs the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007Arthritis Rheum20106261576158220191579
- SieperJRudwaleitMKhanMABraunJConcepts and epidemiology of spondyloarthritisBest Pract Res Clin Rheumatol200620340141716777573
- WilsonFCIcenMCrowsonCSMcEvoyMTGabrielSEKremersHMTime trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based studyJ Rheumatol200936236136719208565
- GabrielSEMichaudKEpidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseasesArthritis Res Ther200911322919519924
- Al MainiMAdelowoFAl SalehJThe global challenges and opportunities in the practice of rheumatology: white paper by the World Forum on Rheumatic and Musculoskeletal DiseasesClin Rheumatol201434581982925501633
- LozaEJoverJARodriguezLCarmonaLMultimorbidity: prevalence, effect on quality of life and daily functioning, and variation of this effect when one condition is a rheumatic diseaseSemin Arthritis Rheum200938431231918336872
- SanghaOEpidemiology of rheumatic diseasesRheumatology (Oxford)200039Suppl 231211276800
- SmolenJSLandewéRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis201473349250924161836
- SinghJASaagKGBridgesSL2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritisArthritis Rheumatol201668112626545940
- KavanaughACohenSCushJJThe evolving use of tumor necrosis factor inhibitors in rheumatoid arthritis2004311018811884
- BartonJLPatient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapyPatient Prefer Adherence2009333534420016797
- LizánLComellasMPazSPovedaJLMeleticheDMPolancoCTreatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literaturePatient Prefer Adherence201481653166425525341
- Van den BemtBJvan LankveldWGHow can we improve adherence to therapy by patients with rheumatoid arthritis?Nat Clin Pract Rheumatol200731268118037928
- LopesPRozenbergSGraafJFernandez-VilloriaEMarianowskiLAerodiol versus the transdermal route: perspectives for patient preferenceMaturitas200138Suppl 1S31S3911390122
- JanzNKWrenPACopelandLALoweryJCGoldfarbSLWilkinsEGPatient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decisionJ Clin Oncol200422153091309815284259
- JahngKHMartinLRGolinCEDiMatteoMRPreferences for medical collaboration: patient-physician congruence and patient outcomesPatient Educ Couns200557330831415893213
- LinPCampbellDGChaneyEFThe influence of patient preference on depression treatment in primary careAnn Behav Med200530216417316173913
- HeigbergTKvienTPreferences for improved health examined in 1024 patients with rheumatoid arthritis: pain has highest priorityArthritis Rheum200247439139712209485
- FraenkelLBogardusSTConcatoJFelsonDTWittinkDRPatient preferences for treatment of rheumatoid arthritisAnn Rheum Dis200463111372137815020312
- StofferMASmolenJSWoolfADevelopment of patient-centered standards of care for rheumatoid arthritis in Europe: the eumusc.net projectAnn Rheum Dis201473590290523921994
- NashPNichollsDPerceptions of methotrexate use in rheumatoid arthritis by rheumatologists and their patients: an Australian survey studyInt J Rheum Dis201316665266124382276
- BridgesJFHauberABMarshallDConjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task ForceValue Health201114440341321669364
- MohamedAFHauberABJohnsonFRCoonCDPatient preferences and linear scoring rules for patient reported outcomesPatient20103421722722273431
- OpuniMBishaiDGrayGEMcIntyreJAMartinsonNAPreferences for characteristics of antiretroviral therapy provision in Johannesburg, South Africa: results of a conjoint analysisAIDS Behav201014480781519533322
- FraenkelLConjoint analysis at the individual patient level: issues to consider as we move from a research to a clinical toolPatient20081425125320401337
- FarrarSRyanMRossDLudbrookAUsing discrete choice modelling in priority setting: an application to clinical service developmentsSoc Sci Med2000501637510622695
- RyanMScoottDAReevesCEliciting public preferences for healthcare: a systematic review of techniquesHealth Technol Assess200155118611262422
- JohnsonFRLancsarEMarshallDConstructing experimental designs for discrete choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task ForceValue Health201316131323337210
- BellarySKrishnankuttyBLathaMSBasics of case report form designing in clinical researchPerspect Clin Res20145415916625276625
- HuynhTKOstergaardAEgsmoseCMadsenORPreferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritisPatient Prefer Adherence2014208939924470758
- MayerJPitermanLThe attitudes of Australian GPs to evidence-based medicine: a focus group studyFam Pract199916662763210625142
- HadleyJAWallDKhanKSLearning needs analysis to guide teaching evidence-based medicine: knowledge and beliefs amongst trainees from various specialitiesBMC Med Educ200771117493274
- HayMCWeisnerTSSubramanianSDuanNNiedzinskiEJKravitzRLHarnessing experience: exploring the gap between evidence-based medicine and clinical practiceJ Eval Clin Pract201414570771319018899
- van TuylLHPlassAMLemsWFDiscordant perspectives of rheumatologists and patients on COBRA combination therapy in rheumatoid arthritisRheumatology (Oxford)200847101571157618710900
- FraenkelLFriedTRIndividualized medical decision making: necessary, achievable, but not yet attainableArch Intern Med2010170656656920308644
- FraenkelLMcGrawSWhat are the essential elements to enable patient participation in medical decision making?J Gen Intern Med200722561461917443368
- AugustovskiFBeratarrecheaAIrazolaVPatient preferences for biologic agents in rheumatoid arthritis: a discrete choice experimentValue Health201316238539323538191
- ScarpatoSAntivalleMFavalliEGPatient preferences in the choice of anti-TNF therapies in rheumatoid arthritis: results from a questionnaire survey (RIVIERA study)Rheumatology (Oxford)201049228929419920093
- WilliamsELEdwardsCJPatient preferences in choosing anti-TNF therapiesRheumatology (Oxford)200645121575157617085468
- MedinaFGalvánMImputación de datos: teoría y práctica [Data imputation: theory and practice]2007 Available from: http://www.eclac.org/publicaciones/xml/9/29949/LCL2772e.pdfAccessed May 16, 2016 Spanish
- Cunha-MirandaLCostaLRibeiroJSNEAR study: Needs and Expectations in Rheumatoid ARthritis – do we know our patients [sic] needs?Acta Reumatol Port201035331432320975634
- MarshallNJWilsonGLapworthKKayLJPatients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: a qualitative studyRheumatology (Oxford)20044381034103815150436
- CurtisJRChenLHarroldLRNarongroeknawinPReedGSolomonDHPhysician preference motivates the use of anti-tumor necrosis factor therapy independent of clinical disease activityArthritis Care Res (Hoboken)201062110110720191497
- HewlettSSmithAPKirwanJRValues for function in rheumatoid arthritis: patients, professionals, and publicAnn Rheum Dis2001601092893311557648
- SayREThomsonRThe importance of patient preferences in treatment decisions – challenges for doctorsBMJ2003327741454254512958116
