Abstract
The aim of the present study was to undertake a study on the prevalence of cytochrome P450 2D6 (CYP2D6) poor metabolizer alleles (*3, *4, *5, and *6) on a Montenegrin population and its impact on developing adverse drug reactions (ADRs) of β-blockers in a hospitalized cardiac patient population. A prospective study was conducted in the Cardiology Center of the Clinical Center of Montenegro and included 138 patients who had received any β-blocker in their therapy. ADRs were collected using a specially designed questionnaire, based on the symptom list and any signs that could point to eventual ADRs. Data from patients’ medical charts, laboratory tests, and other available parameters were observed and combined with the data from the questionnaire. ADRs to β-blockers were observed in 15 (10.9%) patients. There was a statistically significant difference in the frequency of ADRs in relation to genetically determined enzymatic activity (P<0.001), with ADRs’ occurrence significantly correlating with slower CYP2D6 metabolism. Our study showed that the adverse reactions to β-blockers could be predicted by the length of hospitalization, CYP2D6 poor metabolizer phenotype, and the concomitant use of other CYP2D6-metabolizing drugs. Therefore, in hospitalized patients with polypharmacy CYP2D6 genotyping might be useful in detecting those at risk of ADRs.
Introduction
According to the latest definition, formulated in 2010, adverse effect is a harmful and unintentionally caused reaction to a medicine.Citation1 Adverse drug reactions (ADRs) are a common cause of morbidity and mortality, with contribution of numerous risks factors. It is estimated that ADRs could be prevented iñ50% of cases.Citation2–Citation5
Of all cardiovascular drugs, most adverse effects are caused by β-blockers, angiotensin converting enzyme inhibitors, and calcium channel blockers.Citation6,Citation7
β-Blockers are recommended as a first-line agent for various diseases, including heart failure, hypertension, and angina, as well as after myocardial infarction.Citation8,Citation9 However, β-blocker therapy often produces variable responses among patients.Citation10,Citation11 Individual response to β-blockers in other clinical settings is substantially influenced by genetic variation in adrenergic signalingCitation12 and drug metabolism pathways, most notably in the cytochrome P450 2D6 (CYP2D6) enzyme.Citation13–Citation15
CYP2D6 is one of the most important metabolic enzymes, responsible for the biotransformation of 25% of all drugs, including most β-blockers.Citation16,Citation17 The observed interindividual variation in CYP2D6 enzyme activity is mainly due to its genetic polymorphism, which significantly affects the metabolism of ~50% of its substrate drugs.Citation18 More than 150 alleles lead to four distinct phenotypes of drug metabolism,Citation19 described as ultrarapid metabolizer, extensive metabolizer (EM), intermediate metabolizer, and poor metabolizer (PM). However, ~98% of the PM phenotype can be explained by three single-nucleotide polymorphisms (SNPs), ie, rs35742686 (2549delA), rs3892097 (1846G>A), and rs5030655 (1707delT) corresponding to alleles *3, *4, and *6, respectively, or a complete deletion of CYP2D6 (*5).Citation20–Citation22 In addition to the loss of function, CYP2D6 enzyme activity could be significantly decreased due to gene polymorphism, and the SNPs leading to this effect include rs1065852 (100C>T, CYP2D6*10)Citation23 present in 20% of the European population.Citation24
The aim of the present study was to examine the frequency of CYP2D6 PM alleles (*3, *4, *5, *6, and *10) in a Montenegrin population, and to determine its impact on developing ADRs of β-blockers in a hospitalized cardiac patient population.
Methods
A prospective study was conducted in the Cardiology Center of the Clinical Center of Montenegro and included 138 patients who had received any β-blocker in their therapy.
Before enlisting in the study, patients were given all necessary information about the study design, after which they signed an informed consent. The ethics committee of the Clinical Center of Montenegro approved the implementation of the research, which was conducted in accordance with the Declaration of Helsinki.
The exclusion criteria were as follows: age younger than 18 years and older than 80 years, dementia or other causes of disorientation, severe illness (eg, cardiogenic shock, pulmonary edema, etc), hematological disorders, significant renal and liver failure, short period of hospitalization (<3 days), and patient’s refusal to participate in the trial. In addition, coadministration with major CYP2D6 inhibitors was contraindicated (ie, fluoxetin, levomepromazine, lobelin, methadone, paroxetine, quinidine, and trifluperidol).Citation18
We defined ADRs according to the definition of the World Health Organization (WHO),Citation1 and for each adverse reaction determined the causal relationship with the drug according to the Naranjo algorithm.Citation25 Also, we determined the severity of the ADRs according to the criteria of the WHO, as well as the type (A: based on the drug’s pharmacologic properties; B: idiosyncratic, unexpected; C: chronic effects related to long-term drug use), according to the classification proposed by Edwards and Aronson.Citation26
For the study, a special questionnaire was designed (). It consisted of questions about the medication that the patient received during hospitalization in the Center of Cardiology.
The interview was conducted in three steps. First, the patients were asked the standard open question: “Have you noticed or felt any adverse reaction of the drug?” If the answer was yes, the ADRs were recorded. Then the patients were asked about complaints to the various organ systems: these questions helped the patients to “remember” the ADRs. Finally, the patients were asked for adverse reactions mentioned in the “Summary of Product Characteristics”, in relation to drugs administered during hospitalization. Data from the patients’ medical charts, laboratory tests, and other available parameters were observed and combined with the data from the questionnaire.
All patients who received β-blockers were CYP2D6 genotyped. On the basis of available literatureCitation27,Citation28 and CYP2D6 allele nomenclature,Citation29 for the purpose of the study, five variants were identified and included in the analysis. These variants are considered to be the most common genetic determinants of CYP3D6 PM phenotypes: CYP2D6*3 (2549delA, rs35742686), CYP2D6*4 (1846G>A, rs3892097), CYP2D6*5 (gene deletion), CYP2D6*6 (1707delT, rs5030655), and CYP2D6*10 (100C>T, rs1065852).
DNA was extracted from whole-blood samples using the PureLink® genomic DNA kit (Thermo Fisher Scientific, Waltham, MA, USA). Genotyping for CYP2D6*3, *4, and *6 was carried out using the real-time PCR method on a Mastercycler® ep realplexCitation2 (Eppendorf AG, Hamburg, Germany), with the use of TaqMan Genotyping Master Mix 2X (Thermo Fisher Scientific) and the corresponding TaqMan DME genotyping Assays (Thermo Fisher Scientific). Genotyping for CYP2D6*5 and *10 was conducted according to the previously described long-PCR tetra-primerCitation30,Citation31 and allele-specific PCRCitation32 methods, with minor modifications. Analyses were performed at the Faculty of Medical Sciences, University of Kragujevac.
Statistical data analysis was performed using IBM SPSS Statistics 22 (IBM Corporation, Armonk, NY, USA). Results are presented as frequency, percent, and mean ± standard deviation. For parametric data, independent sample t-test was used to test differences between groups. The Mann–Whitney U-test was used for obtaining the significance between ordinal data. The chi-squared test or Fisher’s exact test was used to test the differences between nominal data (frequencies), including Hardy–Weinberg equilibrium. The association between potential risk factors and ADRs was evaluated using binary logistic regression, expressing the strength of association by crude and adjusted odds ratio with 95% confidence intervals. A P-value of <0.05 was considered significant.
Results
One hundred and thirty-eight patients on β-blocker therapy were included in the study. Of those, 66 (47.8%) patients were treated with metoprolol (dose 25–100 mg), 36 (26.1%) with carvedilol (dose 6.25–50 mg), 33 (23.9%) with bisoprolol (dose 2.5–10 mg), and three (2.2%) with nebivolol (dose 5 mg). The basic characteristics of the patients and the frequencies of CYP2D6 variants, alleles, and genotypes are given in and , respectively.
Table 1 Baseline data of the patients
Table 2 Frequencies of CYP2D6 gene variations, alleles, and genotypes in Montenegrin patients on β-blocker therapy
ADRs to β-blockers were observed in 15 (10.9%) patients. Most ADRs were related to the use of carvedilol and metoprolol (six patients, ie, 8.0% each), followed by bisoprolol (three patients, ie, 4.0%). The most common ADRs were bradycardia (44.4%), dizziness (22.2%), headache (11.1%), hypotension (5.5%), cold lower extremities (5.5%), pain in the extremities (5.5%), and nightmares (5.5%). The characteristics of ADRs to β-blockers and the levels of intervention are given in .
Table 3 Characteristics of detected ADRs
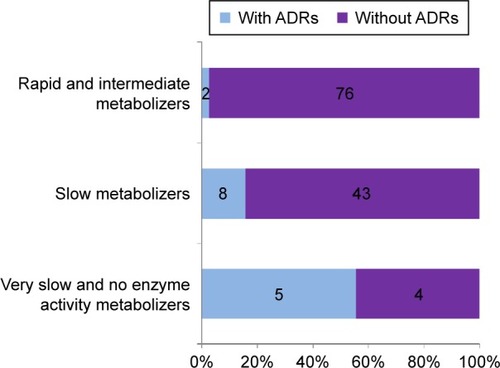
Genotyping of patients receiving β-blockers showed that more than half belonged to the group of EMs (54.3%). The frequency of PMs was 37.6%, 5.1% of respondents had no enzymatic activity, while 1.4% of patients had intermediate and very PM phenotypes. There was a statistically significant difference in the frequency of ADRs in relation to genetically determined enzymatic activity (P<0.001), with the occurrence of ADRs significantly correlating with slower CYP2D6 metabolism ().
Figure 1 Distribution of ADRs of β-blockers according to CYP2D6 enzyme activity.
The influence of different predictors on the outcome of interest (ie, ADRs to β-blockers) was analyzed in two steps. First, a univariate logistic regression model was used to assess the influence of single predictors, and a subsequent multivariate model analyzed the simultaneous influence of all predictors confirmed as significant in the single predictor analysis.
In the univariate logistic regression model, statistically significant predictors of ADRs to β-blockers were the number of days of hospitalization (P=0.019), enzyme activity (PMs [P=0.016], and very PM and metabolizer without enzyme activity [P<0.001] compared to EM and intermediate metabolizer as the reference category) and the concomitant use of other drugs that are metabolized by the same CYP enzyme system (P=0.001).
The multivariate logistic regression model included a statistically significant predictor from the univariate logistic regression model. As regards the CYP2D6 enzyme activity in the sample analyzed, it was necessary to compress a small number of variables into fewer categories: 1) EM and intermediate metabolizer and 2) PMs, very PMs, and metabolizers without enzyme activity. The multivariate logistic regression model was statistically significant (P<0.001), without significant multicollinearity between predictors. The interaction of predictors in the model was not statistically significant. In other words, the predictors were orthogonal (ie, they were not collinear), and the statistical interference on the model was reliable.
In the multivariate logistic regression model, statistically significant predictors of ADRs to β-blockers were longer hospitalization (P=0.008), slower enzymatic activity (P<0.001), and concomitant use of other drugs that are metabolized by CYP2D6 (P<0.001) ().
Table 4 Multivariate logistic regression with ADRs on β-blockers as the dependent variable
Discussion
β-Blockers are an important class of cardiovascular drugs used in hypertension, heart failure, and acute myocardial infarction, which represent one of four leading agents that reduce cardiovascular mortality and morbidity. Although >100 β-blockers have been developed, only ~30 are in clinical use.Citation33
Polypharmacy, a common practice in cardiovascular pharmacotherapy, may give rise to ADRs. The prevalence of cardiovascular drug-induced adverse reactions is 2.4 times higher compared to other drugs.Citation34 Numerous studies have shown that the administration of β-blockers is associated with higher incidences of ADRs compared to other cardiovascular drugs.Citation35,Citation36 The present study was conducted in order to analyze ADRs in patients with cardiovascular diseases treated with β-blockers, and to assess the modulating role of CYP2D6 SNPs.
The results of our study showed that ADRs to β-blockers appear in every ninth patient, which is twice compared to the data in the literature.Citation6 This may be partly due to the use of different drugs from this class studied or a different study design. The most common ADRs observed in our study population were bradycardia and dizziness, while other adverse effects, such as fatigue, cold hands, headache, stomach upset, constipation, and diarrhea, were rare. These results are in accordance with the data from the literature.Citation33–Citation35 As expected, the ADRs observed were mainly type A reactions (based on the drug’s pharmacologic properties), which could be explained by the study design, target population, and the well-known β-blocker safety pattern. In particular, the type A reactions are dose-related and more likely to occur in PMs.
It is well known that polymorphism of the CYP2D6 gene plays an important role in the biotransformation of β-blockers.Citation22,Citation37 Numerous studies have found the CYP2D6 genotype to be a major determinant of plasma concentration of metoprolol,Citation13,Citation38–Citation41 the most frequently prescribed β-blocker.Citation13 However, reports on the adverse effects of β-blockers in relation to CYP2D6 polymorphism are conflicting, as some authors observed that the PM genotype conveys significantly higher risk for the development of ADRsCitation12,Citation13,Citation42–Citation44 while others did not detect any significant difference in drug response among the CYP2D6 genotype groups.Citation39,Citation40,Citation45–Citation47 In a retrospective study, Wuttke et alCitation12 identified 24 patients treated with metoprolol who had experienced pronounced adverse effects. Genotyping revealed a fivefold higher frequency of PM in this group compared to the control population, with the CYP2D6 PM phenotype exhibiting up to tenfold higher plasma concentrations of metoprolol compared to EMs. Goryachkina et al,Citation13 investigating the effect of CYP2D6 polymorphism in 187 acute myocardial infarction patients on metoprolol treatment, observed the most pronounced bradycardia in genotypically PMs. A similar association was observed in several larger trials, including the MERIT-HFCitation44 and the Rotterdam Study,Citation42 reporting significantly lower heart rates and blood pressure in carriers of a CYP2D6*4 defective allele. On the other hand, there have been a number of reports showing no relationship between metoprolol response and the CYP2D6 genotype,Citation39,Citation40,Citation45,Citation47 but the lack of significance was usually due to the small sample size,Citation39,Citation40,Citation45,Citation47 lack of genotyping data on all important PM CYP2D6 SNPs,Citation45,Citation47 or the presence of confounding variables that are likely to obscure differences in clinical effects.Citation45
In the present study, we examined the frequency and effect of the most important CYP2D6 PM SNPs in patients treated with β-blockers. The results showed that most of our patients were EMs, while the least frequent were intermediate and very PMs. These data are consistent with those obtained in other white populations.Citation48–Citation52 As for the ADRs to β-blockers, they were significantly more common in carriers of the CYP2D6 genotype, which predicts poor metabolism. This corresponds well with the reports of higher incidence of ADRs in CYP2D6 PMs,Citation10,Citation18,Citation53–Citation55 indicating the importance of CYP2D6 genotyping in Montenegrin patients on β-blockers. Further studies, including genotyping for duplication of the functional CYP2D6 gene, might further reveal the possible effect of the ultrarapid metabolizer phenotype on the resistance to β-blockers therapy. In addition to the CYP2D6 activity, our study showed that ADRs to β-blockers are more likely to occur in patients with comedications and longer hospitalization, which fits into the well-known pattern. Significance in the multivariate regression model used without collinearity between predictors implies that the risk factors for the ADRs to β-blockers could be reliably identified.
As regards the possible limitations, β-blocker plasma monitoring could significantly improve the reliability of our study, although these drugs do not fulfill the criteria for routine therapeutic drug monitoring.Citation56
Conclusion
Our study showed that the adverse reactions to β-blockers could be predicted by the length of hospitalization, genotype-inferred CYP2D6 PM phenotype, and the concomitant use of other CYP2D6-metabolized drugs. Therefore, in hospitalized patients with polypharmacy that includes β-blockers, CYP2D6 genotyping might be useful in detecting those at risk of ADRs.
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (project no OI 175046) and a grant from the Ministry of Science, Montenegro (no 01-907).
Supplementary material
Disclosure
The authors report no conflicts of interest in this study.
References
- EUROPA [webpage on the Internet]Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 Amending, As Regards Pharmacovigilance, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use Text with EEA Relevance Available from: http://ec.europa.eu/health/files/eudralex/vol-1/dir_2010_84/dir_2010_84_en.pdfAccessed May 9, 2016
- ClassenDCPestotnikSLEvansRSBurkeJPComputerized surveillance of adverse drug events in hospital patientsJAMA199126620284728511942452
- PeyriereHCassanSFloutardEAdverse drug events associated with hospital admissionAnn Pharmacother200337151112503925
- HallasJHarvaldBGramLFDrug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of preventionJ Intern Med1990228283902394974
- PirmohamedMJamesSMeakinSAdverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patientsBMJ20043297456151915231615
- GholamiKZiaieSShalviriGAdverse drug reactions induced by cardiovascular drugs in outpatientsPharm Pract (Granada)200861515525170364
- MjörndalTBomanMDHäggSAdverse drug reactions as a cause for admissions to a department of internal medicinePharmacoepidemiol Drug Saf2002111657211998554
- ChobanianAVBakrisGLBlackHRThe seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 reportJAMA2003289192560257212748199
- PfefferMABraunwaldEMoyéLAEffect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE InvestigatorsN Engl J Med1992327106696771386652
- HummaLMTerraSGPharmacogenetics and cardiovascular disease: impact on drug response and applications to disease managementAm J Health Syst Pharm200259131241125212116890
- NagelePLiggettSBGenetic variation, β-blockers, and perioperative myocardial infarctionAnesthesiology201111561316132721918425
- WuttkeHRauTHeideRIncreased frequency of cytochrome P450 2D6 poor metabolizers among patients with metoprolol-associated adverse effectsClin Pharmacol Ther200272442943712386645
- GoryachkinaKBurbelloABolduevaSBabakSBergmanUBertilssonLCYP2D6 is a major determinant of metoprolol disposition and effects in hospitalized Russian patients treated for acute myocardial infarctionEur J Clin Pharmacol200864121163117318648788
- ShinJJohnsonJAPharmacogenetics of beta-blockersPharmacotherapy200727687488717542770
- BertilssonLDahlMLDalénPAl-ShurbajiAMolecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugsBr J Clin Pharmacol200253211112211851634
- DornGW2ndLiggettSBMechanisms of pharmacogenomic effects of genetic variation within the cardiac adrenergic network in heart failureMol Pharmacol200976346648019491328
- AklilluEPerssonIBertilssonLJohanssonIRodriguesFIngelman-SundbergMFrequent distribution of ultrarapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional CYP2D6 allelesJ Pharmacol Exp Ther199627814414468764380
- Ingelman-SundbergMGenetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversityPharmacogenomics J20055161315492763
- DaiZLChenHWuXYRelationship between cytochrome P450 2C19*17 genotype distribution, platelet aggregation and bleeding risk in patients with blood stasis syndrome of coronary artery disease treated with clopidogrelZhong Xi Yi Jie He Xue Bao201210664765422704413
- MarezDLegrandMSabbaghNPolymorphism of the cytochrome P450 CYP2D6 gene in a European population: characterization of 48 mutations and 53 alleles, their frequencies and evolutionPharmacogenetics1997731932029241659
- Ingelman-SundbergMSimSCGomezARodriguez-AntonaCInfluence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspectsPharmacol Ther2007116349652618001838
- ZhouSFPolymorphism of human cytochrome P450 2D6 and its clinical significance: part IIClin Pharmacokinet2009481276180419902987
- SakuyamaKSasakiTUjiieSFunctional characterization of 17 CYP2D6 allelic variants (CYP2D6.2, 10, 14A–B, 18, 27, 36, 39, 47–51, 53–55, and 57)Drug Metab Dispos200836122460246718784265
- CunninghamFAmodeMRBarrellDEnsembl 2015Nucleic Acids Res201543Database issueD662D66925352552
- NaranjoCABustoUSellersEMA method for estimating the probability of adverse drug reactionsClin Pharmacol Ther19813022392457249508
- EdwardsIRAronsonJKAdverse drug reactions: definitions, diagnosis, and managementLancet200035692371255125911072960
- BradfordLDCYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendantsPharmacogenomics20023222924311972444
- TehLKBertilssonLPharmacogenomics of CYP2D6: molecular genetics, interethnic differences and clinical importanceDrug Metab Pharmacokinet2012271556722185816
- CYPalleles [homepage on the Internet]CYP2D6 Allele Nomenclature Available from: http://www.cypalleles.ki.seAccessed May 9, 2016
- SteenVMAndreassenOADalyAKDetection of the poor metabolizer-associated CYP2D6(D) gene deletion allele by long-PCR technologyPharmacogenetics1995542152238528268
- NaveenATAdithanCSoyaSSGerardNKrishnamoorthyRCYP2D6 genetic polymorphism in South Indian populationsBiol Pharm Bull20062981655165816880622
- JohanssonIOscarsonMYueQYBertilssonLSjöqvistFIngelman-SundbergMGenetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylationMol Pharmacol19944634524597935325
- MansoorAHKaulUBeta-blockers in cardiovascular medicineJ Assoc Physicians India200957712
- LesarTSLomaestroBMPohlHMedication-prescribing errors in a teaching hospital: a nine year experienceArch Intern Med199715714156915769236558
- OlsenHKlemetsrudTStokkeHPTretlisSWestheimAAdverse drug reactions in current antihypertensive therapy: a general practice survey of 2586 patients in NorwayBlood Press1999829410110451036
- HussainAAqilMAlamMSKhanMRKapurPPillaiKKA pharmacovigilance study of antihypertensive medicines at a South Delhi hospitalIndian J Pharm Sci200971333834120490310
- ZhouSFPolymorphism of human cytochrome P450 2D6 and its clinical significance: part IClin Pharmacokinet2009481168972319817501
- RauTHeideRBergmannKEffect of the CYP2D6 genotype on metoprolol metabolism persists during long-term treatmentPharmacogenetics200212646547212172215
- ZinehIBeitelsheesALGaedigkAPharmacokinetics and CYP2D6 genotypes do not predict metoprolol adverse events or efficacy in hypertensionClin Pharmacol Ther200476653654415592325
- FuxRMörikeKPröhmerAMImpact of CYP2D6 genotype on adverse effects during treatment with metoprolol: a prospective clinical studyClin Pharmacol Ther200578437838716198657
- JinSKChungHJChungMWInfluence of CYP2D6*10 on the pharmacokinetics of metoprolol in healthy Korean volunteersJ Clin Pharm Ther200833556757318834373
- BijlMJVisserLEvan SchaikRHGenetic variation in the CYP2D6 gene is associated with a lower heart rate and blood pressure in beta-blocker usersClin Pharmacol Ther2009851455018784654
- RauTWuttkeHMichelsLMImpact of the CYP2D6 genotype on the clinical effects of metoprolol: a prospective longitudinal studyClin Pharmacol Ther200985326927219037197
- BattyJAHallASWhiteHLMERIT-HF Study GroupAn investigation of CYP2D6 genotype and response to metoprolol CR/XL during dose titration in patients with heart failure: a MERIT-HF substudyClin Pharmacol Ther201495332133024193112
- SharpCFGardinerSJJensenBPCYP2D6 genotype and its relationship with metoprolol dose, concentrations and effect in patients with systolic heart failurePharmacogenomics J20099317518419365402
- HamadehISLangaeeTYDwivediRImpact of CYP2D6 polymorphisms on clinical efficacy and tolerability of metoprolol tartrateClin Pharmacol Ther201496217518124637943
- AyyappadihasRDhanalekshmiUJestinHCYP 2D6*4 polymorphism and interindividual response variation to metoprolol in stage 1 hypertensive patients: no association in a rural Indian population?Turk J Med Sci201545235235726084127
- SachseCBrockmöllerJBauerSRootsICytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequencesAm J Hum Genet19976022842959012401
- FrommMKroemerHKEichelbaumMFImpact of P450 genetic polymorphism on the first-pass extraction of cardiovascular and neuroactive drugsAdv Drug Deliv Rev1997272–317119910837557
- DahlMLJohanssonIBertilssonLIngelman-SundbergMSjöqvistFUltrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basisJ Pharmacol Exp Ther199527415165207616439
- BertilssonLLouYQDuYLPronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquin and S-mephenytoinClin Pharmacol Ther19925143883971345344
- BozinaNJovanovićNLovrićMMedvedVClinical significance of a CYP2D6 poor metabolizer – a patient with schizophrenia on risperidone treatmentTher Drug Monit200830674875118806696
- WestonCFPharmacogenetics and cardiovascular disease managementHosp Med2004651059459815524338
- ManuntaPBianchiGPharmacogenomics and pharmacogenetics of hypertension: update and perspectives – the adducin paradigmJ Am Soc Nephrol2006174 suppl 2S30S3516565244
- PereiraNLWeinshilboumRMCardiovascular pharmacogenomics and individualized drug therapyNat Rev Cardiol200961063263819707183
- KangJSLeeMHOverview of therapeutic drug monitoringKorean J Intern Med200924111019270474