Abstract
Introduction
COPD is rapidly becoming one of the most challenging health problems worldwide, which is characterized by not fully reversible airflow limitation. Although a lot of treatment medications have been delivered, the treatment goals of COPD are often not achieved. Furthermore, few well-designed randomized controlled trials in the People’s Republic of China have been reported to evaluate the impact of pharmacist-managed clinic (PMC) on medication adherence and health-related quality of life in patients with COPD.
Methods
A prospective randomized controlled study (on a PMC group and a control group) was conducted between January 2015 and December 2015. A structured education about COPD was provided by a clinical pharmacist to the PMC group. Primary outcomes were medication adherence (assessed by medication refill adherence scores) and health-related quality of life (assessed by St George’s Respiratory Questionnaire). Secondary outcomes were exacerbation rate, hospitalization rate, and smoking behavior.
Results
A total of 244 patients were enrolled for our study. The PMC group showed a significantly greater improvement in medication adherence compared with the baseline (93.1±14.2 vs 78.8±12.3, P<0.01). When compared with the control group, there were more patients whose medication refill adherence score was ≥80 in the PMC group (83.3% vs 51.3%, P<0.01). The total St George’s Respiratory Questionnaire scores was found to be improved significantly in the PMC group (42.7±3.2 vs 52.4±5.2, P<0.05). There was a lower hospitalization rate in the PMC group, and more patients in the PMC group quit smoking (71.0% vs 52.2%, P<0.05).
Conclusion
The PMC may result in improvement of medication adherence and the health-related quality of life in patients with COPD. In the PMC group, a significant reduction in exacerbation rate, hospitalization rate, and smoking behavior was observed; therefore, our study provides support for a greater involvement of PMC in the care of patients with COPD.
Introduction
COPD is a common disease of respiratory system, which is characterized by non-fully reversible airflow obstruction. Acute exacerbations of COPD are important drivers of mortality and reduced quality of life in COPD patients and are the second most common reason for emergency hospital admission. According to a research published by the World Health Organization,Citation1 COPD has become the fourth leading cause of mortality in the US. It is estimated to become the fifth leading cause of disease burden in 2020. In the People’s Republic of China, the prevalence of COPD has been estimated at 8.2% among people aged 40 years.Citation2 COPD has thus become an important global health problem.
In order to administrate COPD effectively, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) updates its guidelines for diagnosis, management, and prevention every year.Citation3 The Chinese version of COPD guidelinesCitation4 is formulated based on the GOLD guidelines. As an important part of the management of stable COPD, the Chinese guidelines recommend the pharmacotherapy of patients be monitored closely, especially the medication adherence. Although the acute exacerbations of COPD are potentially preventable by suitable drug, it is very difficult for the patients to follow the prescribed regimens. There are many patients with COPD who cannot take drugs as prescribed or visit their physician.Citation5,Citation6
Some systematic reviews have demonstrated that the pharmaceutical care may improve the health outcomes of patients with cardiovascular diseasesCitation7 and diabetes mellitus.Citation8 However, in the People’s Republic of China, treatment for COPD is mainly provided by doctors or nurses, while clinical pharmacist involved occasionally. It was not until 2007 that the clinical pharmacy began to attain long-term development. In the pharmaceutical affairs management of medical institutions, five full-time clinical pharmacists are indispensable. Now, the roles of pharmacists have been expanded from dispensing prescriptions to providing pharmaceutical services to patients; these services include managing medication therapy, distributing educational materials, and monitoring the adverse drug reaction (ADR). COPD has been described as requiring 10% medication and 90% education. To the best of our knowledge, few well-designed clinical trials in the Zhejiang Province of the People’s Republic of China have been reported to evaluate the effectiveness of pharmacist-managed clinic (PMC) on medication adherence and health-related quality of life. The aim of this prospective randomized controlled study was to assess the impact of PMC on medication adherence and health-related quality of life in COPD patients.
Methods
Design and setting
We conducted a prospective randomized controlled study between January 2015 and December 2015. Patients were recruited from Tongde Hospital of Zhejiang Province, which is located in the Hangzhou City of the Zhejiang Province.
Inclusion criteria for patients included 1) age ≥35 years, 2) diagnosis of COPD, 3) regular visit to the pharmacist, 4) no previous diagnosis of uncontrolled psychiatric disease, and 5) no previous diagnosis of severe liver or kidney disease. Patients who were pregnant or analphabetic were excluded. Eligible patients were randomly assigned to either the PMC group or the control group. The randomization codes were generated by computer, and the clinical pharmacist was blinded to it.
In accordance with the GOLD guidelines, the patient’s adherence to maintenance therapy and the scores of St George’s Respiratory Questionnaire (SGRQ) were chosen as primary outcomes. Secondary outcomes were the acute exacerbation rate, hospitalization rate due to acute exacerbation, and smoking behavior. Adherence was assessed by medication refill adherence (MRA)Citation9,Citation10 score. The MRA score was calculated by the formula: the number of participation days/total number of days; the number of participation days was the period from the beginning date of the study to the date of second clinic visit. If the MRA score was ≥80, the patient was considered adherent.Citation11 The MRA was periodically assessed every 12 months. The SGRQ score is a supervised, self-managed measure, designed specifically for patients with chronic airways disease. The SGRQ has been validated to evaluate the health impairment and responsiveness to therapy in patients with chronic airflow limitation. It has 50 items, divided into three domains: impacts, activities, and symptoms.Citation12 For each domain, the score is from 0 to 100. The lowest score indicated that there was no effect on life completely, and a score of 100 indicated the poorest respiratory health of the patient. A change in the mean score of 4 units has been validated as a clinically significant threshold.Citation13,Citation14 The SGRQ score was periodically reassessed every 6 months.
The smoking status of patients was assessed through a questionnaire at the beginning and the end of this study, which included the following questions: Did you ever smoke?; How long did you smoke?; How many cigarettes do you smoke every day?; Do you still smoke now?; Are you trying to quit?; and Why did you cannot quit smoke?
Ethical approval
After approval by the research review committee from Tongde Hospital of Zhejiang Province, and written informed patient consent was obtained, we began to design the study.
Pharmacist-managed clinic
To maximize the efficacy and minimize the adverse drug events, the first PMC in the Zhejiang Province was established for respiratory therapy at Tongde Hospital of Zhejiang Province in January 2015. The PMC was opening on Wednesday every week. The pharmacist was mainly responsible for individualized education, and developing a comprehensive pharmaceutical care program. The patients in the control group received the usual care delivered by the doctor, but did not receive prescription services by the clinical pharmacist.
Patients in the PMC group were individually educated. At first, the pharmacist discussed with the patients about the definition of COPD, pathophysiology of the disease, the importance of medication adherence, and the importance of smoking cessation. Then, the pharmacist taught the patients on how to take the prescribed drugs and use the respiratory devices effectively, explained the possible ADR, the possible effect of drug combination, the importance of a well-balanced diet with sufficient intake of fresh fruits and vegetables, and the necessity of timely follow-up by physicians. At last, the pharmacist shared his phone number for the patients to consult conveniently. In order to help the patients understand easily the education plan, the pharmacist prepared many drug education materials,Citation14–Citation16 especially on how to use the inhaled device, and the patients were allowed to take it to their home. During telephone or network (eg, WeChat) counseling, the pharmacist asked the patients about the effect of medication, explained the examination results, the possible ADR, and reminded when the patients should visit their doctor.
All of the patient’s data including sex, age, diagnosis, and drugs used were entered into SPSS Version 19.0 (IBM Corporation, Armonk, NY, USA) for statistical analysis. The characteristics of patients in the PMC group and the control group were compared using chi-square tests. A P-value of <0.05 was considered statistically significant.
Results
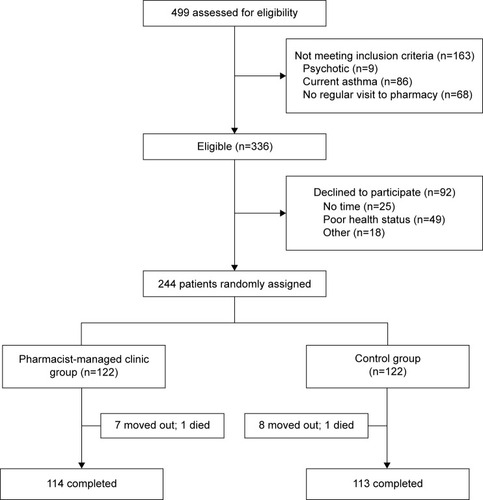
During the 12 months of the study, 499 patients were prescreened, of whom 336 were found eligible. Sixty-one percent (n=244) of patients agreed to participate, and were assigned randomly to the PMC group (n=122) or the control group (n=122). Almost 93% (n=227) of patients completed this study. The flow chart of inclusion and exclusion of patients is shown in .
There were similar clinical characteristics and socio-demographics between the study participants at baseline (). Most patients were elderly, females, married, and of low educational status. Mean age of the study population was 64.2±14.2 years in the PMC group and 64.6±14.5 years in the control group. More than half of the patients were found to have different comorbidities, such as diabetes and hypertension. The treatment medications were similar between two groups. Most of the patients were suffering from moderate or severe COPD, with a mean forced expiratory volume in 1 second/forced vital capacity of 54%. There was no significant difference in the number of hospital admissions at baseline between the two groups.
Table 1 Patient demographics and clinical characteristics at baseline
During the study, the patients in the PMC group and the control group were offered appropriate treatment medications (). At the baseline, mean MRA scores were 78.8±12.3 in the PMC group and 77.4±12.2 in the control group. After the trial, a significantly greater MRA scores in the PMC group were detected (93.1±14.2 vs 78.8±12.3, P<0.01), compared with the baseline. Additionally, when compared with the control group, there was a significant increase of patients whose MRA scores was ≥80 in the PMC group (83.3% vs 51.3%, P<0.01; ).
Table 2 Prescribed medications for COPD in the PMC and control group patients
Table 3 Medication adherence in the PMC and control group over the 12-month assessment period
At the baseline, the patients in both the PMC group and the control group were found to have approximately similar scores in SGRQ (50.9±4.7 vs 50.6±4.6, P=0.949). After 12 months, the total SGRQ scores improved significantly after the PMC intervention (42.7±3.2 vs 52.4±5.2, P<0.05), and the scores of the domains of symptoms, activities, and impacts in the PMC group were significantly improved, as shown in . The best improvement was noted in the impacts domain (30.8±2.4 vs 43.2±5.4, P<0.05).
Table 4 Changes in SGRQ scores at different time assessments
During the study, there were 51 independent acute exacerbations reported. Compared with 16 in the PMC group, 35 acute exacerbations were observed in the control group. Any exacerbation requiring an emergency department visit or hospitalization was regarded as severe, and there were significantly lower number of patients in the PMC group who had a severe exacerbation (14 vs 28, P=0.024). In addition, there were fewer hospitalizations in the PMC group (11 vs 35, P=0.015). At the beginning of this study, ~74% of patients self-reported to smoke, but after the trial, 83 (71%) patients in the PMC group and 59 (52.2%) patients in the control group quit smoking, as shown in .
Table 5 Severe exacerbations, hospitalizations, and smoking status over the 12 months
Discussion
In this study, we assessed the effectiveness of PMC in 244 patients with COPD. The main outcomes were selected based on GOLD guidelines and the disease management. The PMC significantly improved medication adherence, and scores of SGRQ and its subscales (symptoms, activities, and impacts), and decreased severe exacerbation and hos-pitalization rate.
Based on our study data, a significant difference between the two groups in medication adherence after 12 months was noted, which was most likely attributed to the intensive education received from a clinical pharmacist by the PMC group. This is consistent with a 12-month study of Khdour et alCitation10 in Northern Ireland, as the findings showed that clinical pharmacist-led education for patients with COPD led to a significant improvement in medication adherence. Therefore, positive health education can improve medication adherence of patients with COPD. A clinical pharmacist is well placed in the health care team to act as a link between the physicians and the patients.
With progression, COPD undoubtedly affects the ability of patients to perform normal daily activities, thereby affecting their quality of life.Citation17 As shown in , patients demonstrated a similar SGRQ scores at the baseline; however, SGRQ scores in the PMC group improved significantly after the trial. The findings of this present study on improved SGRQ scores in patients with COPD were inconsistent with those of Jordan et al;Citation18 for instance, the baseline mean scores on SGRQ for the patients in Jordan’s research were 36.8, which indicated that the patients may have had a good health status. In our study, the mean score on SGRQ was 50.9; therefore, the patients have had a poor quality of life, so there was a bigger margin for the patients to improve.
The reduction in severe exacerbations and hospitalization rate was another significant improvement in the present study. Since acute exacerbation of COPD can not only destroy health status, but also fasten the progression of disease,Citation19 it was an important treatment target for pharmacist intervention.Citation20 Some study demonstrated an education plan for reduction in hospitalization rate,Citation21 but the researchers emphasized usage of antibiotic and corticosteroid besides pharmacist interventions. In this present clinical trial, the reduction in hospital admissions may be attributable to the reduction in acute exacerbation.
The People’s Republic of China has been the world’s largest cigarette consumer and producer. It was directly beneficial for a patient to quit smoking, During this study, the motivation for smoking cessation for people with mild COPD was not strong, while the smoking cessation methods based on PMC intervention were effective for patients with moderate and severe COPD.
Limitations
While the present study has a number of methodological strengths, it has several limitations. This study utilized data from a single-center and was performed for a short term; therefore, our patients may not represent the general population of patients with COPD. Furthermore, there was not a gold standard to measure adherence; some methods are useful in daily practice (fast, easy, and cheap to apply), but may overestimate adherence.Citation22 And because some clinical trials are often accepted more frequently for motivating patients, the selection bias may not be fully excluded, which may affect the results.
Conclusion
This study was demonstrated to evaluate the impact of PMC on a wide range of clinical outcomes in patients with COPD. Both primary outcomes, that is, medication adherence and SGRQ scores on symptom and impact, were significantly improved in the PMC group. A trend toward reduction in severe exacerbations, hospitalization rate, and smoking status was also observed. To improve health-related quality of life in patients with COPD, our study clearly demonstrated the effectiveness of implementing a PMC in the People’s Republic of China. Clinical pharmacist can be a good support to the doctor in the management of patients with COPD.
Acknowledgments
The authors would like to acknowledge professor Xian-rong Xu, Dr Cheng Jiang, and Mrs Ting Xia for their involvement. This study was supported by Zhejiang Provincial Natural Science Foundation of China (LY14H280003) and Zhejiang Pharmaceutical Association (2012ZYY11).
Disclosure
The authors report no conflicts of interest in this work.
References
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- GOLDGlobal Initiative for Chronic Obstructive Lung Disease [updated January 18, 2015]. Available from: http://www.goldcopd.org/Accessed January 15, 2015
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in China: a large, population-based surveyAm J Respir Crit Care Med2007176875376017575095
- Chronic Obstructive Pulmonary Disease Study Group, Thoracic Society, Chinese Medical Association (CMACOPDS)Guideline for diagnosis and treatment of COPDZhonghua Jie He He Hu Xi Za Zhi2007301817 Chinese
- VestboJAndersonJACalverleyPMAAdherence to inhaled therapy, mortality and hospital admission in COPDThorax2009641193994319703830
- IngebrigtsenTSMarottJLNordestgaardBGLow use and adherence to maintenance medication in chronic obstructive pulmonary disease in the general populationJ Gen Intern Med2015301515925245885
- CarterBLRogersMDalyJZhengSJamesPAThe potency of team-based care interventions for hypertension: a meta-analysisArch Intern Med2009169191748175519858431
- CollinsCLimoneBLScholleJMColemanCIEffect of pharmacist intervention on glycemic control in diabetesDiabetes Res Clin Pract201192214515220961643
- HessLMRaebelMAConnerDAMaloneDCMeasurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measuresAnn Pharmacother2006407–81280128816868217
- KhdourMRKidneyJCSmythBMMcElnayJCClinical pharmacy-led disease and medicine management programme for patients with COPDBr J Clin Pharmacol200968458859819843062
- RodrigoGJPlazaVEfficacy and safety of a fixed-dose combination of indacaterol and glycopyrronium for the treatment of COPD: a systematic reviewChest2014146230931724556877
- NonatoNLDíazONascimentoOADreyseJJardimJRLisboaCBehavior of quality of life (SGRQ) in COPD patients according to BODE scoresArch Bronconeumol201551731532125622995
- WellingJBHartmanJETen HackenNHKloosterKSlebosDJThe minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPDEur Respir J20154661598160426493797
- BrownPBlumlBMKritzlerRWhite paper on expanding the role of pharmacists in chronic obstructive pulmonary diseaseJ Am Pharm Assoc2011512203211
- TommeleinETollenaereKMehuysEBousseryKPharmaceutical care for patients with COPD in Belgium and views on protocol implementationInt J Clin Pharm201436469770124858598
- SpruitMAAugustinIMVanfleterenLEDifferential response to pulmonary rehabilitation in COPD: multidimensional profilingEur Respir J20154661625163526453626
- BurtinCTer RietGPuhanMAHandgrip weakness and mortality risk in COPD: a multicentre analysisThorax2016711868726514408
- JordanREMajothiSHeneghanNRSupported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysisHealth Technol Assess201519361516
- McGeochGRWillsmanKJDowsonCASelf-management plans in the primary care of patients with chronic obstructive pulmonary diseaseRespirology200611561161816916335
- CrinerGJBourbeauJDiekemperRLPrevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society GuidelineChest2015147489494225321320
- Garcia-AymerichJHernandezCAlonsoAEffects of an integrated care intervention on risk factors of COPD readmissionRespir Med200710171462146917339106
- HansenRAKimMMSongLTuWWuJMurrayMDComparison of methods to assess medication adherence and classify nonadherenceAnn Pharmacother200943341342219261962