Abstract
Introduction
Type 2 diabetes is a major burden for the payer, however, with proper medication adherence, diet and exercise regime, complication occurrence rates, and consequently costs can be altered.
Aims
The aim of this study was to conduct a cost-effectiveness analysis on real patient data and evaluate which medication adherence or lifestyle intervention is less cost demanding for the payer.
Methods
Medline was searched systematically for published type 2 diabetes interventions regarding medication adherence and lifestyle in order to determine their efficacies, that were then used in the cost-effectiveness analysis. For cost-effectiveness analysis-required disease progression simulation, United Kingdom Prospective Diabetes Study Outcomes model 2.0 and Slovenian type 2 diabetes patient cohort were used. The intervention duration was set to 1, 2, 5, and 10 years. Complications and drug costs in euro (EUR) were based on previously published type 2 diabetes costs from the Health Care payer perspective in Slovenia.
Results
Literature search proved the following interventions to be effective in type 2 diabetes patients: medication adherence, the Mediterranean diet, aerobic, resistance, and combined exercise. The long-term simulation resulted in no payer net savings. The model predicted following quality-adjusted life-years (QALY) gained and incremental costs for QALY gained (EUR/QALYg) after 10 years of intervention: high-efficacy medication adherence (0.245 QALY; 9,984 EUR/QALYg), combined exercise (0.119 QALY; 46,411 EUR/QALYg), low-efficacy medication adherence (0.075 QALY; 30,967 EUR/QALYg), aerobic exercise (0.069 QALY; 80,798 EUR/QALYg), the Mediterranean diet (0.057 QALY; 27,246 EUR/QALYg), and resistance exercise (0.050 QALY; 111,847 EUR/QALYg).
Conclusion
The results suggest that medication adherence intervention is, regarding cost-effectiveness, superior to diet and exercise interventions from the payer perspective. However, the latter could also be utilized by patients without additional costs, but medication adherence intervention requires trained personnel because of its complex structure. Interventions should be performed for >2 years to produce noticeable health/cost results.
Introduction
Type 2 diabetes is a major health care burden for the payer. In the scope of global estimates, the prevalence will rise and consequently the costs.Citation1,Citation2 The disease incidence, prevalence, progression, and complication occurrence are dependent on a number of factors: weight, fat distribution, fasting glucose, glycated hemoglobin (HbA1c), blood lipids, blood pressure, physical inactivity, family history, race, and age.Citation3 Therefore, accurate drug regime adherence and improved lifestyle regarding diet and exercise are the key to decelerate the disease progression and the incidence of complications. As a result, disease costs can be altered. Major trials were performed to assess the impact of lower blood glucose levels, lower blood pressure and lower lipid levels on disease progression and occurrence of microvascular and macrovascular complications.Citation4 The ACCORD, ADVANCE, and VADT studies focused on single risk factor enhancements (blood glucose and lipid lowering) and concentrated on achieving tight glucose control in a short time. However, primary results have not demonstrated any reduction of complications occurrence or mortality.Citation4–Citation9 In ACCORD’s follow-up study, it was revealed that lowering HbA1c to <7% actually reduced the mortality rate. Furthermore, the mortality rate increased linearly from 6% to 9% HbA1c.Citation10 The positive effect of a lower HbA1c on cardiovascular complications (the United Kingdom Prospective Diabetes Study [UKPDS], HOPE study) was also detected in long-term epidemiological studies.Citation11–Citation14 The intervention results in type 2 diabetes patients, however, strongly indicated that all three risk factors (glucose levels, lower blood pressure, and lower levels of cholesterol) should be targeted to achieve optimal diabetes progression reduction and complications occurrence reduction.Citation4 Consequently, the Steno study was conducted.Citation15,Citation16 In the study, HbA1c, total cholesterol, and serum triglycerides were used as primary outcome measures. The results confirmed that type 2 diabetes complication occurrence rate drops were significantly higher than those reported in studies employing single risk factor control interventions, suggesting that with proper multifactorial interventions, diabetes outcomes and payer burden can be altered.Citation4 As noted previously, interventions regarding medication adherence and lifestyle (diet and exercise) are suitable to alter multiple diabetes risk factors, especially the HbA1c, high-density lipoprotein (HDL), low-density lipoprotein (LDL), weight, and blood pressure values.Citation17–Citation20
The aim of the present study was therefore to conduct a cost-effectiveness analysis on real patient data and evaluate which medication adherence or lifestyle intervention to choose, when to apply it, and which one is less cost demanding for the payer with the help of quality-adjusted life-years (QALYs) gained and cost increment predictions.
Methods
To conduct a cost-effectiveness analysis of medication adherence and lifestyle interventions on real patient data, the literature was searched for relevant interventions and their efficacy, eligible simulation software, intervention costs, type 2 diabetes complication costs, and drug costs. The steps taken in the study are detailed as follows.
Search strategy and study selection for intervention assessment
Search key (type 2 diabetes) AND (exercise OR workout OR diet OR dietary OR diets OR adherence OR compliance) AND (intervention OR interventions) AND (meta OR review) were used on Medline database to search for possible interventions and their efficacies. Date of publication was not limited. Studies that clearly assessed medication adherence and lifestyle interventions and consequently allowed to quantitatively determine effectiveness of each intervention were included. Studies that encompassed interventions regarding behavior changes for intervention efficacy enhancing, interventions encompassing gestational diabetes, depression, diabetes prevention, risk of diabetes, schizophrenia, children, adolescents, type 1 diabetes, kidney diseases, dialysis, and transplantation were excluded. Based on the written protocol for review, one reviewer assessed titles and abstracts of all the retrieved articles. Abstracts that did not provide enough information regarding the eligibility criteria were retrieved for full-text evaluation. The full-texts of selected articles were also evaluated on the basis of the eligibility criteria. The final selection was checked and discussed with other authors.
Simulation model used
The UKPDS Outcomes model 2.0 was used for type 2 diabetes progression simulations.Citation21 The model predicts future events year by year, based on a series of risk equations, derived from the initial UKPDS cohort. The key aspect of the model is its ability to capture the clustering or interaction of different types of complications at an individual patient level. It also allows modification of various type 2 diabetes risk factor values for any given patient life year. Therefore, the model is suitable for the present cost-effectiveness assessment, as different durations of interventions could be simulated. Regarding the Fifth Mount Hood challenge, the UKPDS Outcomes model performs reasonably well when performing relative risk simulations. In addition, it also predicted higher cardiovascular mortality correctly with relation to aggressive HbA1c and blood pressure control, which the other models did not.Citation22
The simulation software allows the use of multiple computer processor cores. However, the results differ slightly regarding the number of cores used. Our calculations were conducted with Xeon 1231v3 processor with seven logical cores used, the number of loops value was set to 10,000 and the bootstraps value was set to 999. These settings produced stable results with a relative error of <5% (obtained from model results).
The study was performed from the Slovenian Health Care payer perspective. The latter consists of The Health Insurance Institute of Slovenia as compulsory health insurance and several complementary insurances. The patients were followed up for a lifetime. Outcomes were simulated in yearly cycles and included model-provided QALYs gained and costs in euros (EUR) as results, with a 5% annual discount rate for both. The outcomes were expressed as an incremental cost-effectiveness ratio (ICER) in EUR per QALY gained (EUR/QALYg) for each intervention type and their duration compared to no intervention.
Because of different intervention durations, we decided to simplify and assumed that each intervention lasted 1 year, although two diet and one exercise interventions were previously conducted for 2 years and 4 years, respectively.Citation23–Citation25 Therefore, to simulate an intervention effect for multiple years, the intervention had to be repeated annually. In our case, simulations were conducted for 1 and 2 years of intervention duration to capture the effects based on published studies and for 5 and 10 years to predict the possible effects with longer intervention duration. A longer intervention inclusion was possible because duration of intervention was not among predictors of dropout in long-term disease care program and short-term interventions according to published data.Citation26–Citation29
Cost-effectiveness of different interventions Patient data
Individual patient data were obtained in advance from the Slovenian pharmacist intervention study presented at the National Diabetes Conference in 2014 where 93 type 2 diabetes patients were recruited.Citation30 The mean (standard deviation) age of the patients was 65.9 (7.5) years which was therefore higher than that researched before in Slovenia [59 (10.1) and 62.2 years]; however, other diabetes risk factors were comparable.Citation31,Citation32 Average HbA1c values from our cohort were 0.1% higher; HDL 0.03 mmol/L, and LDL 0.61 mmol/L lower; and systolic blood pressure 2 mmHg higher, and therefore, despite different average age, the values were generally similar.Citation31,Citation32 Outcomes model 2.0 is based on new onset diabetes patients (mean age 53 years) who were followed-up for 25 years (intervention median of 16.8 and 17.7 years, post-trial 8.5 and 8.8 years, respectively). Therefore, a simulation with patients of higher age is possible.Citation33 The heart rate, WBC count, hemoglobin, and estimated eGFR were not obtained from the patients, because at the time of the Slovenian intervention study the required input values for UKPDS Outcomes model 2.0 were not published. Therefore, published mean values were used and were adjusted to individual patients for outcomes model internal equations.Citation34–Citation37 Patient demographic data are presented in the “Results” section.
Costs and utilities of diabetes complications and medications
Previously published type 2 diabetes costs from the Health Care payer perspective in Slovenia were used to assess the cost values of diabetes complications and medication.Citation38 The published costs were valid for the year 2011. Therefore, an update with recent Health Care payer data was conducted. All 2015 costs were lower than the 2011 costs, in particular the medication expenses. The assessed costs for the year 2015 are presented in . The prices of statins, ACE inhibitors, beta blocking agents, and acetylsalicylic acid fell by 60%, 40%, and 20%, respectively, from 2011 to 2014.Citation39 The cost of the antidiabetics and insulin, however, fell only by 6%.Citation39 The medication cost reduction calculation is provided in detail in the Supplementary materials.
For utility decrements, UKPDS Outcomes model 2.0-published values were used (presented in ).
Intervention costs
Previously published data were used to assess the intervention costs. For the medication adherence intervention, baseline costs of 329 EUR for 1 year of intervention duration (currency conversion on March 15, 2016: USD411.5) were used.Citation40 The costs were further inflated by higher drug consumption (52 EUR for high-efficacy adherence intervention and 13 EUR for low-efficacy adherence intervention). Higher adherence costs were derived from baseline drug costs (283.40 EUR) with the consideration of high and low adherence enhancement (20% vs 5%).Citation41 Cost of 232 EUR per patient was used for the diet intervention, whereas 800 EUR per patient was used for the exercise intervention.Citation42,Citation43
Sensitivity analysis
For the univariable sensitivity analysis, the impact of the variability of model parameters over their plausible ranges was tested. The one-way analysis for 2-year intervention duration was performed on discount rate (0% or 7%), intervention costs (−10%, +10%), and complication costs (−10%, +10%). The 2-year duration was chosen because it is supported by published data, whereas 5- and 10-year intervention durations are predictions.
Results
Description of studies selected for medication adherence and lifestyle intervention cost-effectiveness analysis
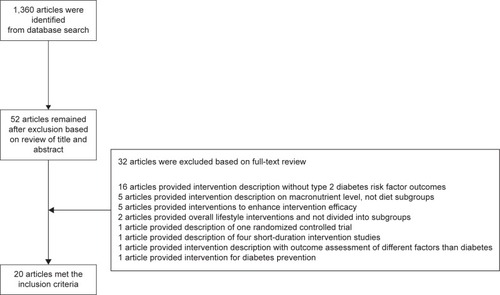
In the initial search on Medline (January 1, 2016), 1,360 potentially relevant citations were retrieved, from which 52 articles were subjected to full-text review after title and abstract screening. After the review, 20 met the inclusion criteria. One of them was attributed to medication adherence, ten to diet lifestyle interventions, and nine to exercise lifestyle interventions.Citation41,Citation44–Citation62 The interventions are presented in detail with a flow diagram in .
Medication adherence interventions
Retrieved studies encompassed the following intervention subtypes: simplification of drug regimen; patient education and information; intensified patient care, with reminders via mail, telephone, or handheld devices; Internet-based programs; home telehealth devices and also complex behavior approaches with or without group sessions.Citation41 The duration of the studies ranged from a few weeks to 2 years. Diverse intervention approaches prevented the authors from conducting a meta-analysis.Citation41 Therefore, highest and lowest ranges of HbA1c reduction (−1.5% to −0.5%) were utilized for the cost-effectiveness analysis.Citation41 Upper and lower HDL, LDL, blood pressure, and weight changes were derived from reviews that were cross-referenced with the selected articles, by us.Citation18–Citation20 In all the interventions, the common factor was that the intervention effects lasted for the duration of the intervention. After cessation, the effects disappeared.Citation41 It was assumed that the effect disappearance occurs shortly after conclusion of the intervention because after the UKPDS intensive therapy intervention had ended, the between-group difference in glycated hemoglobin was lost in the following year.Citation13 Different intervention efficacies for HbA1c, LDL, HDL, weight, and blood pressure are summarized in .
Table 1 Medication adherence and lifestyle intervention effects on HbA1c, HDL, LDL, weight, and blood pressure values
Diet lifestyle interventions
Diet interventions were divided into low carbohydrate, high protein, (very) low glycemic index, low-fat, ADA, conventional, the Mediterranean, and vegetarian diets.Citation44–Citation53 However, conventional, low-fat, ADA and low glycemic index diets were utilized as reference or control diets. Ajala et al conducted the first meta-analysis of different diets and reported their impact on glycemic control, weight, and blood lipids.Citation44 The meta-analysis was conducted with fixed effect model despite high heterogeneity among the included studies.Citation63 It also encompassed only studies longer than 6 months. The article comment further highlighted that very-low-carbohydrate and high-protein diets are not officially recommended because of their unconfirmed long-term effects.Citation63 Therefore, these diets were excluded from the cost-effectiveness analysis.Citation51,Citation53 Two review articles were published for the low-carbohydrate diet.Citation45,Citation52 The included low-carbohydrate studies utilized significantly different dietary interventions (carbohydrate intakes from >20 to 166 g/day) and control diets. No study was conducted with the usual diet control group. Consequently, no clear impact on diabetes risk factors could be established. Since the remaining studies suggested superior effect of the Mediterranean diet, the low-carbohydrate intervention was excluded from the cost-effectiveness analysis.Citation47 The vegetarian diets were effective in HbA1c reduction; however, the published meta-analysis didn’t present the impact on the remaining diabetes risk factors.Citation50 Vegetarian diet interventions were compared to meat diets, and none utilized the usual diet as comparison. Consequently, vegetarian diets were excluded from the cost-effectiveness analysis. The Mediterranean diet was the only intervention that was compared to the usual diet. In the Ajala et al study, the Mediterranean diet effect was assessed to lower HbA1c by −0.41%.Citation44 However, with random effects model utilization instead of the fixed effect, the HbA1c reduction amounted to −0.28% (using RevMan 5).Citation64 Similar effect was also reported in the Carter et al and the Huo et al meta-analyses (random effects model: −0.31%, −0.30%).Citation46,Citation48 All usual diet comparisons were based on the study of Toobert et al which stated a −0.40% HbA1c reduction, despite some publications reporting −0.34%.Citation47,Citation48,Citation65 Since the Huo et al study (nine studies, 1,178 patients) was the latest published, its reported efficacy was used in the cost-effectiveness analysis as the reported efficacy was similar to other studies and it reported the effects on other diabetes risk factors.Citation48 For the blood pressure diet impact, data from Nordmann et al (six trials, 2,650 patients) were used.Citation66
Interventions were applied on different age groups and body mass groups with different comorbidities. Intervention effects disappeared after the study conclusion. As noted previously, the obtained intervention efficacies (average with upper and lower confidence interval [CI] 95% values) on HbA1c, LDL, HDL, weight, and blood pressure are summarized in .
Exercise lifestyle interventions
Diabetes patients were generally subjected to aerobic, resistance, and combined exercise interventions.Citation54–Citation62 The duration of the intervention session was on average between 30 and 60 minutes, with 2–3 sessions per week and intervention duration of 12 weeks to 2 years. Similarly to diet interventions, the exercise studies were applied to different age groups and body mass groups with different comorbidities. Umpierre et al conducted a meta-analysis to assess the efficacy of aerobic (−0.73% HbA1c), resistance (−0.57%), and combined training (−0.51%).Citation54 However, the aerobic group included patients with higher baseline HbA1c and with three or more exercise sessions per week which suggested a higher effect.Citation67 The impact of baseline HbA1c and the number of sessions was afterwards confirmed by Umpierre et al, stating that each additional training day and baseline HbA1c unit resulted in 0.3% lower HbA1c values.Citation61 Armstrong et al furthermore specified that the combined exercise efficacy was assessed too low.Citation67 The latter was confirmed by Hayashino et al, Schwingshackl et al, and Chudyk et al where combined training resulted in highest HbA1c reduction efficacy in comparison with aerobic and resistance training.Citation55,Citation58,Citation62
Hayashino et al and Yang et al published comparable efficacy results for aerobic and resistance training on HbA1c reduction (−0.46% and −0.32%) and Chudyk et al published equivalent results of resistance training (−0.33%).Citation55,Citation60,Citation62 However, resistance training should not be conducted with resistance bands alone in order to be effective.Citation59 Hayashino et al, Yang et al, and Chudyk et al also published comparable results with exercise interventions on lipid profile.Citation55,Citation60,Citation62 The largest difference was observed among intervention effects on blood pressure. Hayashino et al published the lowest efficacy effect, which was followed by Figueira et al and Yang et al.Citation55,Citation57,Citation60 For the cost-effectiveness analysis, results from the Hayashino et al (41 included studies, 2,808 patients) meta-analysis were utilized because the study included results for all types of exercise interventions.Citation55 summarizes different intervention efficacies (average with upper and lower CI 95% values) on HbA1c, LDL, HDL, weight, and blood pressure.
Cost-effectiveness of different medication adherence and lifestyle interventions
As mentioned earlier, the analysis was conducted on real patient data. The patient demographic data are presented in . The quality-adjusted life expectancy for all diabetes patients without intervention was 7.769 QALYs. All interventions demonstrated higher quality-adjusted survival than the base case without intervention. The highest average survival was estimated with medication adherence intervention (high efficacy), followed by combined exercise, medication adherence (low efficacy), aerobic exercise, the Mediterranean diet, and resistance exercise. The first three interventions with 2-year intervention duration performed 0.061, 0.027, and 0.019 better than the base case without intervention and the ones with 10-year intervention duration performed 0.245, 0.119, and 0.075 better, respectfully. The total lifetime costs were also higher in all the cases, which resulted in an ICER between 9,984 EUR (medication adherence) and 148,424 EUR (resistance exercise). The simulated ICER values per QALY gained, QALY gained, and cost increments for all interventions and duration are presented in . In ICER values per QALY gained are presented.
Figure 2 Calculated incremental cost-effectiveness ratio ICER (EUR/QALYg) per each intervention compared to option without the intervention.
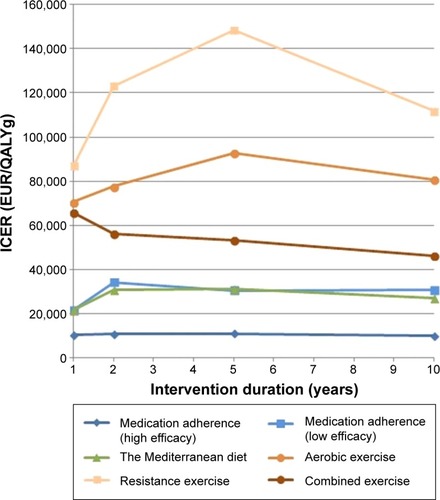
Table 2 Patients’ baseline characteristics used in the long-term simulation (n=93)
Table 3 Estimated QALYs gained (QALYg) and cost increments (EUR) per each intervention compared to option without the intervention and calculated ICER values
Aerobic and resistance intervention exercises present a concave, ICER per QALY gained, graph. Other interventions with the exception of combined exercise also result in a concave ICER chart which is considerably less curved. The chart shape is the result of higher QALY gained after 2 or 5 years of intervention which is more noticeable in the case of interventions with low performance. The ICER shape is also the outcome of natural deaths, which are correlated with intervention costs. With longer duration of type 2 diabetes, the intervention costs still rise, however, not linearly, and fall more with each year (occurrence of predicted natural deaths) of simulation and consequently the ICER value decreases.
Sensitivity analysis
One-way sensitivity analysis varying complication costs, intervention costs, and discount rates showed that the discount rate had the largest impact on the ICER. The impact of intervention costs was also significant, but the impact of complication costs was minimal.
Discussion
The results indicate that diabetes medication adherence and lifestyle interventions result in higher quality-adjusted life expectancy. Efficacy and intervention costs are an important factor for intervention cost-effectiveness. Regarding the cost savings aim of the present study, it has to be mentioned that no intervention produced net savings. Therefore, diabetes interventions are effective in the prevention of complications, but not in the reduction of payer burden.
Similar exercise intervention impact was detected previously as well, when the cost-effectiveness of community-based physical activity interventions was assessed.Citation68 The published QALY gain of different interventions (medication adherence and lifestyle) ranged from 0.014 to 0.14 QALYs, whereas our simulation results ranged from 0.009 to 0.245 QALY gained.Citation68–Citation70
While comparing medication adherence intervention cost-effectiveness with previously published data of 9,000 EUR/QALYg, it was found that our efficacy, cost, and patient data regarding this particular intervention were correct.Citation69 A similar intervention was also conducted on Dutch diabetes patients where the intervention cost-effectiveness resulted in ICER of 14,814 EUR/QALYg in patients with history of cardiovascular diseases and in ICER of 38,243 EUR/QALY in all patients, which is higher than our calculated values in the case of high-efficacy intervention but, however, similar to low efficacy medication adherence.Citation70
On the subject of exercise interventions, previously published studies reported cost per QALY outcomes of up to 68,557 USD/QALYg.Citation68 While considering higher intervention costs, the result is similar to our estimated values in exercise intervention cases (from 46,411 to 86,940 EUR/QALYg, excluding results over 100,000 EUR/QALYg).
While utilizing diabetes-specific nutritional meal replacements as part of the diabetes management program, the outcomes produced an incremental cost-effectiveness output of 47,917 to 50,414 USD/QALYg, whereas our estimated values for diet intervention ranged from 21,653 EUR/QALYg to 31,080 EUR/QALYg.Citation71
Limitations
The present cost-effectiveness diabetes intervention simulation was conducted on real patients. However, the raw data lack values for heart rate, WBC count, hemoglobin, and estimated glomerular filtration rate. Therefore, the simulation could estimate different absolute and possibly smaller relative differences in results. However, with a slight adjustment of average blood risk factor values in individual patients, the possibility of an error was lowered. Complex medication adherence studies also utilized lifestyle counseling among medication adherence education, however, without the usage of any diet and exercise interventions.Citation41
The meta-analyses of different interventions were conducted with different effects. The authors used fixed and random effects, meaning that the results are prone to errors. Furthermore, the authors generally did not explain the reasons why which a particular model was used. However, reviews and meta-analyses referenced in utilized random effects model for efficacy calculation which was suggested as preferable.Citation63
After 1 year of the UKPDS intervention discontinuation, the between-group differences in diabetes risk factor values were lost; however, the intervention effect persisted.Citation13 This effect was named “memory effect.”Citation13 In our simulation, after the conclusion of the intervention, we utilized diabetes risk factors predicted in the base case, meaning that the intervention trend was stopped and the future memory effect was not present because the UKPDS outcomes model does not utilize equations to compensate for the memory effect. It is therefore possible that longer interventions predicted higher complication outcomes.
Unaltered individual patient data from the pharmacist intervention study were used.Citation30 The results of this intervention study are presented elsewhere.Citation30
Which intervention to choose
While combining the results from high- and low-efficacy medication adherence intervention studies, the QALY and EUR/QALYg benefits exceed other interventions, indicating that medication adherence interventions are superior to diet and exercise interventions. However, when higher adherence results in significantly elevated drug costs or co-payments for the patient, then exercise and diet interventions are also important to consider since the literature suggests that with higher drug costs for the patient the adherence could fall.Citation72
The study was conducted with published intervention costs and an assumption that interventions need to be supervised. Nevertheless, diet and exercise interventions could also be conducted in patients with no supervision. Hypothetically, the patients could follow proper diet and exercise guidelines on the basis of common self-care which would result in no costs for the Heath Care payer. In the case of medication adherence, lack of supervision is not possible because the process consists of various steps (education sessions, drug reminders, blood tests, self-care reminders, and medication dispensing) where trained personnel are needed.
We should also consider that exercise is probably harder to implement in elderly patients. While examining average age in studies included in the Hayashino et al meta-analysis, most studies included patients <60 years with few carried out on patients >60 years.Citation55 Therefore, it is sensible to implement exercise interventions with younger patients (with low costs), and the diet intervention with the elderly, when medication adherence intervention is not preferred.
The results also indicate that intervention duration ≤2 years results in low ICER values; however, the QALY gained output is also low and similar between all interventions. Consequently, it is reasonable to conduct longer interventions particularly because QALY gains are more noticeable. It is insignificant which duration to choose for combined exercise, the Mediterranean diet, and medication adherence from the ICER standpoint because ICER decreases slightly, whereas for the aerobic and resistance exercise interventions, it is appropriate to choose interventions longer than 5 years because ICER decreases after 5 years of intervention.
Conclusion
The results suggest that from the payer perspective, medication adherence intervention is cost-effective and is superior to diet and exercise interventions. However, the latter could be utilized by patients without additional costs, whereas medication adherence intervention requires trained personnel because of its complex structure. Interventions should be performed for >2 years to produce noticeable health/cost results.
Supplementary materials
The following defined daily doses (DDD) and published Health Care payer expenses for different anatomic therapeutic groups (ATC) were utilized for the year 2011 and 2014.Citation1
ATC code C10AA, C10BA; 2011 901,671 DDD/2014 874,060 DDD; 2011 249,911 euro/2014 92,751 euro
ATC code C09A, C09B, C09C; 2011 1,216,343 DDD/2014 960,862 DDD; 2011 53,797 euro/2014 24,804 euro
ATC code C07; 2011 810,770 DDD/2014 772,186 DDD; 2011 91,755 euro/2014 70,754 euro
ATC code B01AC06; 2011 8,957,006 DDD/2014 12,843,360 DDD; 2011 529,577 euro/2014 559,528 euro
ATC code A10B; 2011 741,423 DDD/2014 662,937 DDD; 2011 194,237 euro/2014 163,045 euro
ATC code A10A; 2011 471,572 DDD/2014 575,191 DDD; 2011 502,277 euro/2014 577,481 euro.
Table S1 Costs used and utility values used in the cost-effectiveness analysis
Reference
- Slovenian National Institute of Public HealthDrug consume database from 2001 to 2014 Available from: https://partner.zzzs.si/wps/wcm/connect/94bb0e3f-3d23-4156-926f-47732415acd8/Zdravila+OZZ+2001_2014_i.xlsx?MOD=AJPERES&ContentCache=NONEAccessed December 1, 2015
Disclosure
The authors report no conflicts of interest in this work.
References
- ShawJESicreeRAZimmetPZGlobal estimates of the prevalence of diabetes for 2010 and 2030Diabetes Res Clin Pract20108741419896746
- ZhangPZhangXBrownJGlobal healthcare expenditure on diabetes for 2010 and 2030Diabetes Res Clin Pract20108729330120171754
- KoloverouEPanagiotakosDBPitsavosC10-Year incidence of diabetes and associated risk factors in Greece: the ATTICA study (2002–2012)Rev Diabet Stud20141118118925396406
- TandonNAliMKNarayanKMPharmacologic prevention of microvascular and macrovascular complications in diabetes mellitus: implications of the results of recent clinical trials in type 2 diabetesAm J Cardiovasc Drugs20121272222217193
- Action to Control Cardiovascular Risk in Diabetes Study GroupEffects of intensive glucose lowering in type 2 diabetesN Engl J Med20083582545255918539917
- Ismail-BeigiFCravenTBanerjiMAEffect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trialLancet201037641943020594588
- ADVANCE Collaborative GroupIntensive blood glucose control and vascular outcomes in patients with type 2 diabetesN Engl J Med20083582560257218539916
- BeulensJWPatelAVingerlingJREffects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trialDiabetologia2009522027203619633827
- DuckworthWAbrairaCMoritzTGlucose control and vascular complications in veterans with type 2 diabetesN Engl J Med200936012913919092145
- RiddleMCAmbrosiusWTBrillonDJEpidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trialDiabetes Care20103398399020427682
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet19983528378539742976
- UK Prospective Diabetes Study (UKPDS) GroupEffect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34)Lancet19983528548659742977
- HolmanRRPaulSKBethelMAMatthewsDRNeilHA10-Year follow-up of intensive glucose control in type 2 diabetesN Engl J Med20083591577158918784090
- GersteinHCPogueJMannJFThe relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysisDiabetologia2005481749175516059716
- GaedePVedelPLarsenNJensenGVParvingHHPedersenOMultifactorial intervention and cardiovascular disease in patients with type 2 diabetesN Engl J Med200334838339312556541
- GaedePLund-AndersenHParvingHHPedersenOEffect of a multi-factorial intervention on mortality in type 2 diabetesN Engl J Med200835858059118256393
- HuangXLPanJHChenDChenJChenFHuTTEfficacy of lifestyle interventions in patients with type 2 diabetes: a systematic review and meta-analysisEur J Intern Med201627374726655787
- SchedlbauerADaviesPFaheyTInterventions to improve adherence to lipid lowering medicationCochrane Database Syst Rev20103CD00437120238331
- MatthesJAlbusCImproving adherence with medication: a selective literature review based on the example of hypertension treatmentDtsch Arztebl Int2014111414724612495
- GrandySFoxKMHardyESHIELD Study GroupAssociation of weight loss and medication adherence among adults with type 2 diabetes mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes)Curr Ther Res Clin Exp201375778224465048
- HayesAJLealJGrayAMHolmanRRClarkePMUKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82Diabetologia2013561925193323793713
- PalmerAJClarkePGrayAMount Hood 5 Modeling GroupComputer modeling of diabetes and its complications: a report on the Fifth Mount Hood challenge meetingValue Health20131667068523796302
- ShaiISchwarzfuchsDHenkinYWeight loss with a low- carbohydrate, Mediterranean, or low-fat dietN Engl J Med2008359322924118635428
- EspositoKMaiorinoMICiotolaMEffects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trialAnn Intern Med2009151530631419721018
- LoimaalaAGroundstroemKRinneMEffect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitusAm J Cardiol2009103797297719327425
- FullertonBErlerAPöhlmannBGerlachFMPredictors of dropout in the German disease management program for type 2 diabetesBMC Health Serv Res201212822233930
- KirkALeeseGEncouraging physical activity interventions among people with type 2 diabetesJ Diabetes Nurs2009132631
- NamSDobrosielskiDAStewartKJPredictors of exercise intervention dropout in sedentary individuals with type 2 diabetesJ Cardiopulm Rehabil Prev20123237037823011489
- BenoitSRJiMFlemingRPhilis-TsimikasAPredictors of dropouts from a San Diego diabetes program: a case control studyPrev Chronis Dis20041A10 Epub2004915
- MartincBEffect of pharmacist’s intervention on glycemic control in type 2 diabetic patientsPresented at: “Preventive measures – the most effective means for controlling diabetes: National Conference on Diabetes Control”December 12, 2014Ljubljana, SloveniaRepublic of Slovenia Ministry of Health Available from: http://www.mz.gov.si/en/media_room/news/article/670/6912/f6d5fe577cd4459d97c19d207-b2a6b0d/Accessed June 1, 2015
- MrevljeFPiletičMSenčar BožičPInsulin detemir izboljša glikemično urejenost in ima nevtralen učinek na telesno maso v slovenski kohorti raziskave predictive – klinične izkušnje v Sloveniji [Insulin detemir improves glycemic organization and has a neutral effect on body weight in a cohort Slovenian predictive research – clinical experience in Slovenia]Zdrav Vestn200877699705 In Slovenian
- AndelMGrzeszczakWMichalekJDEPAC GroupA multinational, multi-centre, observational, cross-sectional survey assessing diabetes secondary care in Central and Eastern Europe (DEPAC Survey)Diabet Med200825101195120319046198
- HolmanRRPaulSKBethelMAMatthewsDRNeilHA10-Year follow-up of intensive glucose control in type 2 diabetesN Engl J Med20083591577158918784090
- CarnethonMRYanLGreenlandPResting heart rate in middle age and diabetes development in older ageDiabetes Care20083133533917959868
- TwigGAfekAShamissAWhite blood cells count and incidence of type 2 diabetes in young menDiabetes Care20133627628222961572
- ConwayBNMillerRGOrchardTJAre hemoglobin levels elevated in type 1 diabetes?Diabetes Care20103334134319918013
- RigalleauVLasseurCPerlemoineCEstimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of diet in renal disease study equation?Diabetes Care20052883884315793182
- NeratTKosMBurden of type 2 diabetes from the healthcare payer perspective in SloveniaSlovenian J Public Health201352162180
- Slovenian National Institute of Public HealthDrug consume database from 2001 to 2014 Available from: https://partner.zzzs.si/wps/wcm/connect/94bb0e3f-3d23-4156-926f-47732415acd8/Zdravila+OZZ+2001_2014_i.xlsx?MOD=AJPERES&ContentCache=NONEAccessed December 1, 2015
- OberjéEJde KinderenRJEversSMvan WoerkumCMde BruinMCost effectiveness of medication adherence-enhancing interventions: a systematic review of trial-based economic evaluationsPharmacoeconomics201331121155116824222477
- WilliamsJLWalkerRJSmallsBLCampbellJAEgedeLEEffective interventions to improve medication adherence in type 2 diabetes: a systematic reviewDiabetes Manag (Lond)20144294825214893
- DalzielKSegalLDe lorgerilMA Mediterranean diet is cost-effective in patients with previous myocardial infarctionJ Nutr200613671879188516772453
- Müller-RiemenschneiderFReinholdTWillichSNCost-effectiveness of interventions promoting physical activityBr J Sports Med200943707618971249
- AjalaOEnglishPPinkneyJSystematic review and meta-analysis of different dietary approaches to the management of type 2 diabetesAm J Clin Nutr20139750551623364002
- DysonPLow carbohydrate diets and type 2 diabetes: what is the latest evidence?Diabetes Ther2015641142426446553
- CarterPAchanaFTroughtonJGrayLJKhuntiKDaviesMJA Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: a network meta-analysisJ Hum Nutr Diet201427328029723790149
- EspositoKMaiorinoMIBellastellaGChiodiniPPanagiotakosDGiuglianoDA journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analysesBMJ Open201558e008222
- HuoRDuTXuYEffects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysisEur J Clin Nutr201569111200120825369829
- EmadianAAndrewsRCEnglandCYWallaceVThompsonJLThe effect of macronutrients on glycaemic control: a systematic review of dietary randomised controlled trials in overweight and obese adults with type 2 diabetes in which there was no difference in weight loss between treatment groupsBr J Nutr2015114101656166626411958
- YokoyamaYBarnardNDLevinSMWatanabeMVegetarian diets and glycemic control in diabetes: a systematic review and meta-analysisCardiovasc Diagn Ther20144537338225414824
- DongJYZhangZLWangPYQinLQEffects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: meta-analysis of randomised controlled trialsBr J Nutr2013110578178923829939
- Castañeda-GonzálezLMBacardíGascónMJiménez-CruzAEffects of low carbohydrate diets on weight and glycemic control among type 2 diabetes individuals: a systemic review of RCT greater than 12 weeksNutr Hosp20112661270127622411372
- ThomasDEElliottEJThe use of low-glycaemic index diets in diabetes controlBr J Nutr2010104679780220420752
- UmpierreDRibeiroPAKramerCKPhysical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysisJAMA20113051790179921540423
- HayashinoYJacksonJLFukumoriNNakamuraFFukuharaSEffects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trialsDiabetes Res Clin Pract20129834936023116535
- SanzCGautierJFHanaireHPhysical exercise for the prevention and treatment of type 2 diabetesDiabetes Metab201036534635120675173
- FigueiraFRUmpierreDCureauFVAssociation between physical activity advice only or structured exercise training with blood pressure levels in patients with type 2 diabetes: a systematic review and meta-analysisSports Med201444111557157225047852
- SchwingshacklLMissbachBDiasSKönigJHoffmannGImpact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysisDiabetologia20145791789179724996616
- McginleySKArmstrongMJBouléNGSigalRJEffects of exercise training using resistance bands on glycaemic control and strength in type 2 diabetes mellitus: a meta-analysis of randomised controlled trialsActa Diabetol201552222123024845604
- YangZScottCAMaoCTangJFarmerAJResistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysisSports Med201444448749924297743
- UmpierreDRibeiroPASchaanBDRibeiroJPVolume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysisDiabetologia201356224225123160642
- ChudykAPetrellaRJEffects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysisDiabetes Care20113451228123721525503
- MannJITe morengaLDiet and diabetes revisited, yet againAm J Clin Nutr201397345345423364020
- Review Manager (RevMan) [Computer program]Version 5.3CopenhagenThe Nordic Cochrane Centre, The Cochrane Collaboration2014
- ToobertDJGlasgowREStryckerLABiologic and quality-of-life outcomes from the Mediterranean Lifestyle Program: a randomized clinical trialDiabetes Care20032682288229312882850
- NordmannAJSuter-ZimmermannKBucherHCMeta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factorsAm J Med201112484185121854893
- ArmstrongMJBouléNGSigalRJExercise interventions and glycemic control in patients with diabetesJAMA20113066607
- RouxLPrattMTengsTOCost effectiveness of community-based physical activity interventionsAm J Prev Med20083557858819000846
- Jacobs-van der BruggenMAvan BaalPHHoogenveenRTCost-effectiveness of lifestyle modification in diabetic patientsDiabetes Care2009321453145819435958
- CleveringaFGWelsingPMvan den DonkMCost-effectiveness of the diabetes care protocol, a multifaceted computerized decision support diabetes management intervention that reduces cardiovascular riskDiabetes Care20103325826319933991
- RandolphSMustadVALeeJSunJEconomic analysis of a diabetes-specific nutritional meal replacement for patients with type 2 diabetesAsia Pac J Clin Nutr2010191720199981
- SimardPPresseNRoyLPersistence and adherence to oral antidiabetics: a population-based cohort studyActa Diabetol201552354755625524433