Abstract
Objective
As the multiple sclerosis (MS) disease-modifying drug (DMD) treatment options have expanded to include oral therapies, it is important to understand whether route of administration is associated with DMD adherence. The objective of this study was to compare adherence to DMDs in patients with MS newly initiating treatment with a self-injectable versus an oral DMD.
Methods
This retrospective database study used IMS Health Real World Data Adjudicated Claims – US data between July 1, 2010 and June 30, 2014. Adherence was measured by medication possession ratio (MPR), calculated as the total number of treated days divided by the total number of days from the first treated day until the end of 12-month follow-up. A binary measure representing adherence (MPR ≥0.8) versus nonadherence (MPR <0.8) to therapy was used. Logistic regression evaluated the likelihood of adherence to index DMD type (self-injectable vs oral). Covariates included patient baseline characteristics (ie, age, sex, comorbidities) and index DMD type.
Results
The analysis included 7,207 self-injectable and 1,175 oral DMD-treated patients with MS. In unadjusted analyses, the proportion of patients adherent to therapy (MPR ≥0.8) did not differ significantly between the self-injectable (54.1%) and the oral DMD cohorts (53.0%; P=0.5075). After controlling for covariates, index DMD type was not a significant predictor of adherence (odds ratio [OR] 1.062; 95% confidence interval [CI]: 0.937–1.202; P=0.3473). Higher likelihood of adherence was associated with male sex (OR 1.20; 95% CI: 1.085–1.335; P=0.0005) and age groups older than 18–34 years (ORs 1.220–1.331; P<0.01). Depression was associated with a lower likelihood of adherence (OR 0.618; 95% CI: 0.511–0.747; P<0.0001).
Conclusion
Male sex and age older than 18–34 years were significantly associated with a higher likelihood of adherence, while depression was associated with a lower likelihood of adherence. Index DMD type, stratified by the route of administration (self-injectable vs oral DMD), was not a significant predictor of DMD adherence.
Introduction
Multiple sclerosis (MS) is an inflammatory-mediated chronic neurodegenerative disease characterized by a range of symptoms including fatigue, impaired motor skills, blurred vision, bladder and bowel dysfunction, and cognitive impairment.Citation1 More than 2.3 million people are affected by MS worldwide.Citation1 The economic and humanistic burden of this chronic disease is substantial, particularly because patients with MS are affected at a young age, resulting in a greater loss of productivity and quality of life compared with other diseases.Citation2 Although there is no cure for MS, effective strategies are available to treat exacerbations (relapses), modify the disease course, manage symptoms, and improve function.Citation3 Along with the other essential components of comprehensive MS care, these treatments may help people manage their MS and enhance their comfort and quality of life.Citation4
Disease-modifying drugs (DMDs) have been shown to be efficacious in reducing relapse frequency in MS; however, many patients experience barriers to DMD treatment adherence. Published rates of adherence to DMDs range from 28% to 88%.Citation5–Citation8 Patients who are adherent to DMDs have been shown to have a decreased risk of relapse, fewer emergency room visits, fewer severe relapses, fewer hospitalizations, fewer neuropsychological issues, lower costs, and increased quality of life, compared with nonadherent patients.Citation9–Citation12
Adherence to therapy is rooted in a wealth of softer, often intangible, motivations that complicate its understanding and hinder its quantification.Citation13 MS-specific disease characteristics that may be related to adherence are believed to include long periods of disease remission, lack of disease predictability, inadequate knowledge of the disease or its treatments, fear of needles, side effects of therapies, cognitive impairment, and low self-efficacy.Citation14–Citation16
In order to effectively treat patients with MS, it is important to understand the various factors that are associated with adherence to DMDs. An understanding of the predictive value of the factors associated with adherence in MS could contribute positively to the overall planning of MS disease management programs and improved patient outcomes. Many studies of varying design and methodology have evaluated factors associated with DMD adherence in MS.Citation5,Citation9,Citation17–Citation47 Factors that have been shown to be associated with a statistically significant improvement in adherence to DMD treatment include older age, male sex, greater patient self-efficacy, reduced depression and anxiety, a positive relationship with the provider, less disability, and shorter duration of illness.
Self-injectable DMDs have historically been the most commonly used DMDs. In recent years, newer DMDs, including oral and infusion formulations, have been approved for the treatment of MS. To our knowledge, no existing studies in the published literature have examined the impact of route of administration on adherence to DMD therapy in MS. However, three studies comparing adherence of self-injectable versus oral DMDs have been presented at scientific conferences.Citation48–Citation50 The first study compared adherence and persistence across various dosing frequencies and routes of administration among patients with MS treated with DMDs.Citation50 The authors found that adherence to oral daily, oral twice daily, subcutaneous (SC) three times weekly, and intramuscular weekly regimens was significantly higher than adherence to SC every other day or SC daily regimen. The second study sought to determine if adherence and tolerability of oral DMDs was better than other DMD treatments in an MS center population.Citation48 A statistically significantly higher proportion of patients receiving self-injectable or intravenous DMDs reported not missing any doses compared with patients receiving oral DMDs. Fifty-five percent of patients receiving oral DMDs (n=89) reported no missed doses, 70.8% of patients taking SC or intramuscular DMDs (n=90) reported not missing any doses, and 93.3% of patients taking intravenous DMDs (n=30) reported no missed doses. The third study presented is the one described in this article.Citation49 The objective of this study was to compare adherence to DMDs between patients with MS newly initiating treatment with a self-injectable DMD versus an oral DMD using a large US administrative claims dataset.
Methods
Data source
This was a retrospective database study conducted using IMS Health Real World Data Adjudicated Claims – US data between July 1, 2010 and June 30, 2014. The IMS Real World Data Adjudicated Claims – US Database comprises commercial health plan information from managed care plans throughout the US, with adjudicated claims from more than 150 million unique enrollees since 2006. This anonymous, patient-centric, national managed care database includes all medical and pharmacy claims data for study patients, as well as demographic variables (age, sex, region of the US), eligibility by month, and the adjudicated payment for services. Ethics approval from an Institutional Review Board and informed consent were not required for this research database as per US Department of Health and Human Services Exemption 4 (E4). The research involved the study of existing data, and patients could not be identified directly or through identifiers linked to the subjects.
Patient population
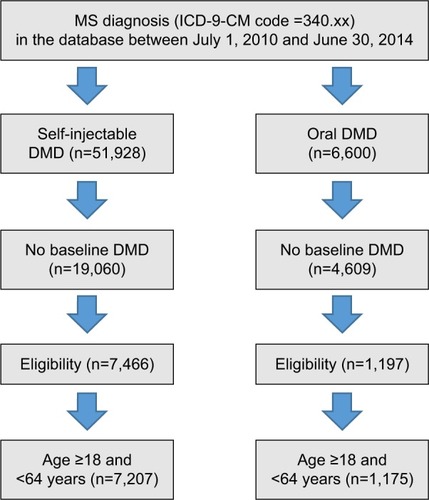
Eligibility criteria consisted of patients aged 18–64 years with at least one medical claim with a diagnosis for MS (International Classification of Diseases, Ninth Revision, Clinical Modification code: 340.xx) and at least one prescription for either a self-injectable or an oral DMD after MS diagnosis. The date of the first DMD prescription was defined as the index date. Patients were required to have continuous eligibility for at least 12 months before and 12 months after the index date (ie, eligible to receive health care benefits during the 24-month time period over which they were evaluated). As the goal was to examine patients new to therapy, any patient with a DMD 12 months prior to the index date was excluded.
Patients were divided into two treatment cohorts based on the index DMD route of administration: the self-injectable DMD cohort and the oral DMD cohort. Self-injectable and oral DMDs included in the study are listed in .
Table 1 Self-injectable and oral DMDs
DMD adherence, discontinuation, and switching
Primary measure: DMD adherence
“Adherence to medications” is the process by which patients take their medication as prescribed and is further divided into three quantifiable phases: “initiation”, “implementation”, and “discontinuation”.Citation51 Adherence was evaluated during the 12-month follow-up period using the medication possession ratio (ie, annual MPR), and results were stratified by the route of administration (ie, self-injectable or oral). The annual MPR was calculated by totaling the number of days supply between the first prescription claim and the last prescription claim issued during the 12-month follow-up period (ie, the numerator) and dividing by the total number of days in the 12-month follow-up period (ie, the denominator).
The calculation was restricted to ambulatory days (ie, days when the patient was not in the hospital). Adherence to the index DMD was defined as an MPR ≥0.8.Citation52
Secondary measures: DMD discontinuation and switching
DMD discontinuation and switching were also evaluated during the 12-month follow-up period as follows:
– Discontinuation was defined as the absence of the index DMD for a 90-day period during follow-up, without evidence of another DMD during that time.
– Switching was defined as the presence of any other (non-index) DMD during a 90-day period without the index DMD during follow-up.
Descriptive/univariate analyses
Baseline demographic and clinical characteristics were compared between index DMD cohorts, and included age, Charlson Comorbidity Index (CCI) score, and select common MS comorbidities based on a recent review of the published literature regarding comorbidities in patients with MSCitation53 (ie, anxiety, arthritis [osteoarthritis or rheumatoid arthritis], depression, diabetes [type I and type II], gastrointestinal disorders [constipation, diarrhea, dysphagia, gastroesophageal reflux disease, and irritable bowel syndrome], hypertension, and thyroid disease). All measures concerning DMD adherence, discontinuation, and switching were evaluated for patients in both cohorts. Patients without valid “days of supply” values (ie, missing or <0) on relevant prescriptions were excluded from analyses related to adherence, discontinuation, and switching. Statistical testing of differences between cohorts was evaluated with Fisher’s exact and Wilcoxon rank sum tests for binary/categorical and continuous measures, respectively. A P-value of <0.05 was used to determine statistical significance.
Multivariable analysis
Logistic regression was used to evaluate the likelihood of adherence (MPR ≥0.8) to the index DMD type (self-injectable or oral). Covariates included patient baseline characteristics (eg, age, sex, comorbidities) and index DMD type. Analyses were conducted using R version 3.2.1 software (The R Foundation, Vienna, Austria).Citation54
Results
Patient selection
A total of 7,207 patients with MS newly initiating a self-injectable DMD and 1,175 patients with MS newly initiating an oral DMD met the eligibility criteria ().
Baseline characteristics
Patient baseline characteristics for both index DMD cohorts, including age ranges, CCI score, and select common MS comorbidities, are shown in .
Table 2 Baseline demographic and clinical characteristics of patients with MS newly initiating a DMD
Patients in the self-injectable DMD cohort were younger compared with those in the oral DMD cohort (43.0 vs 44.9 years; P<0.0001). Mean CCI score was higher among patients on self-injectable DMDs compared with those on oral DMDs (0.55 vs 0.48; P=0.0242). The most common comorbidity at baseline in both cohorts was hypertension (21.7%–23.8% of patients), while the proportion with gastrointestinal disease was lower among patients on self-injectable DMDs compared with those on oral DMDs (17.0% vs 19.9%; P=0.0180).
DMD adherence, discontinuation, and switching
In unadjusted analyses, mean MPR was higher in the self-injectable DMD cohort versus the oral DMD cohort (0.69 vs 0.68, respectively; P=0.0002) (), while the proportion of patients adherent to therapy (MPR ≥0.8) did not differ significantly between the cohorts (54.1% vs 53.0%, respectively; P=0.5075) ().
Table 3 Adherence and discontinuation characteristics among patients with MS newly initiating a DMD: unadjusted bivariate analyses
No significant differences between the self-injectable and oral DMD cohorts were observed in the proportion of patients discontinuing (26.6% vs 28.2%, respectively; P=0.2710), the time to discontinuation (mean number of days: 118.0 vs 113.7, respectively; P=0.1341), or the time to switch (mean number of days: 163.1 vs 153.1, respectively; P=0.2519). A higher proportion of patients in the self-injectable DMD cohort switched to another DMD compared with the oral DMD cohort (9.9% vs 6.6%, respectively; P=0.0003). In both cohorts, the majority of patients who switched to another DMD switched to a self-injectable therapy ().
Multivariable analysis
Factors predictive of DMD adherence in the multivariable analyses are shown in . The logistic regression analysis shows that, after controlling for covariates, index DMD type (self-injectable DMD vs oral DMD) was not a significant predictor of adherence (odds ratio [OR] 1.062; 95% confidence interval [CI]: 0.937–1.202; P=0.3473). Male sex (OR 1.203; 95% CI: 1.085–1.335; P=0.0005) and age groups older than 18–34 years (ORs 1.220–1.331; P<0.01) were associated with a higher likelihood of adherence. The presence of depression at baseline was associated with a lower likelihood of adherence (OR 0.618; 95% CI: 0.511–0.747; P<0.0001).
Table 4 Multivariable analysis of potential predictors of adherence
Discussion
As the MS DMD treatment options have expanded to include oral therapies, it is important to understand whether the route of administration is associated with treatment adherence. In addition to the traditional factors of clinical efficacy, safety, and costs, differences in patient adherence to therapies in MS may be important in formulary decision making.Citation8 This study evaluated the impact of route of administration of DMD on patient adherence, and compared adherence between patients with MS newly initiating a self-injectable DMD versus an oral DMD.
The study findings showed that, in unadjusted analyses, the mean MPR was statistically significantly higher in the self-injectable DMD cohort compared with the oral DMD cohort; however, the proportion of patients adherent to therapy was similar between the two cohorts. A statistically significantly higher proportion of patients in the self-injectable DMD cohort switched therapies. There was no statistically significant difference in discontinuation rates between the self-injectable and the oral DMD cohorts.
In analyses adjusted for patient baseline characteristics (eg, age, sex, comorbidities) and index DMD type, the latter was not a significant predictor of DMD adherence, thus indicating that treatment adherence in patients with MS is complex and may not be exclusively influenced by the route of administration. Male sex and age older than 18–34 years were significantly associated with a higher likelihood of adherence, whereas depression at baseline was associated with a lower likelihood of adherence. These results are consistent with previously published studies which showed that male sex, older age, and less depression were associated with improved adherence.Citation5,Citation9,Citation17–Citation47 The adjusted analyses highlight the importance of consideration of covariates in nonrandomized analyses.
The finding that a statistically significantly higher proportion of patients in the self-injectable DMD cohort switched therapies could be due to the availability of oral DMDs. A recently published prospective, observational substudy of the MSBase registry by Warrender-Sparkes et al showed that, following the introduction of fingolimod, patients were more likely to discontinue treatments (hazard ratio 1.64, P<0.001) and switch to fingolimod (42.3% of switches).Citation47 It is important to note that the definition of discontinuation in the study by Warrender-Sparkes et al included patients who switched to another therapy, whereas the current study defined discontinuation as stopping therapy and not switching to another therapy.Citation47 There was no statistically significant difference in discontinuation rates between the self-injectable and the oral DMD cohorts in our study. Multivariable analyses would be helpful to explore these findings.
Lack of medication adherence remains a challenge among patients with MS. Maximizing adherence to MS therapies to improve a patient’s ability to gain the full benefit from their treatment is an important therapeutic goal. Improvements in adherence have the potential to reduce patient and payer burden in terms of improved clinical outcomes and lower nonpharmacy medical resource utilization.Citation8 It is important for the health care providers to identify and implement the strategies to overcome and monitor barriers to adherence in MS. These may include improved patient education and support programs, particularly those related to better informing patients about treatment benefits.Citation8
As the disease course of MS is unpredictable, and the patient’s attitudes and behavior patterns can change, encouraging adherence is a complex issue.Citation55 A patient’s support team needs to be aware of the potential factors affecting adherence and be sensitive to the patient’s needs in order to support the patient with the goal of establishing and maintaining adherence.Citation56 Interestingly, Lorefice et al found that caregivers believed that patients were more involved in choosing their therapy than the patients themselves did.Citation57 The importance of adherence with DMDs should be discussed as part of patient-centered medical care and shared decision making. Health information technology and comparative effectiveness research, two major components of health reform, have the potential to play important roles in promoting adherence to medications and expanding research on adherence interventions.Citation58
Limitations of this study should be considered when interpreting the study findings. The data were obtained for claims processing and payment, and not for research purposes.Citation59 Claims data are subject to possible coding errors and undercoding.Citation59 Services may not be captured in the claims database because the particular service is not covered by the plan sponsor or the service is “carved out” and not captured in the dataset (eg, mental health).Citation59 Data fields that are not required for reimbursement may be particularly unreliable.Citation59
When using prescription claims data, a number of different medication adherence measures can be examined, including length of therapy, persistence, days covered, gaps, and MPRs. The MPR is one of the most widely used measures of adherence using retrospective data.Citation52 The advantage of using the MPR as a measure of adherence is it provides a comprehensive estimate of the proportion of time that a patient had medication available during the observation period. However, the use of the MPR to evaluate adherence has been criticized by some researchers because it may overestimate the true rate of medication adherence.Citation52 The overestimation is most likely to occur when the patient receives early refills of the target medications, which may result in an “extra fill” during a defined measurement interval.Citation52 Also, since the MPR is being calculated for a class of medications (ie, DMDs), a switch between DMDs during the interval, with an overlap of the new drug with the prior drug, will inflate the MPR.Citation52
Annual MPR was utilized instead of a variable MPR (eg, denominator ending at the last treated day), which may impact results by underestimating adherence in either cohort. Although the analysis aimed to identify patients new to DMD therapy by requiring no DMD claim during the 12-month baseline period, it is possible that patients could have been treated with DMDs prior to baseline and discontinued for that period. Additionally, patients in the sample were not necessarily newly diagnosed patients with MS.
Adherence measures based on administrative prescription data can only capture medication availability. There are challenges associated with how to account for patients who change health plans or who are no longer in the database. Also, adjustment for baseline differences between treatment cohorts cannot ensure all confounders are accounted for. Our ability to control for differences in the treatment groups was limited to the variables available in the database. Finally, there is a lack of generalizability of data, given the inherent characteristics of claims databases and the use of an individual cohort. The patients included in the database received treatment in all regions of the US. However, care should be exercised when extrapolating these findings to other health care settings.
Conclusion
This real-world study of patients with MS demonstrated that after controlling for covariates, route of administration for DMD treatments (ie, self-injectable DMD or oral DMD) was not a significant predictor of adherence. Male sex and age older than 18–34 years were significantly associated with a higher likelihood of adherence, and depression at baseline was associated with a lower likelihood of adherence.
Acknowledgments
The authors thank Natalie Edwards of Health Services Consulting Corporation, Boxborough, MA, USA (supported by EMD Serono, Inc., Rockland, MA, USA [a business of Merck KGaA, Darmstadt, Germany]) for editorial assistance in drafting the manuscript, collating the comments of authors, and assembling tables and figures, and Caudex, New York, NY, USA (supported by EMD Serono, Inc., Rockland, MA, USA [a business of Merck KGaA, Darmstadt, Germany]) for editorial assistance with the manuscript.
Portions of the data in this manuscript were presented at:
The 2015 National Association of Specialty Pharmacy (NASP) Annual Meeting & Expo, September 29–October 1, 2015, National Harbor, MD, USA, as a poster with interim findings (the poster’s abstract was published on NASP’s website, abstract #4).
The 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), October 7–10, 2015, Barcelona, Spain, as a poster with interim findings (the poster’s abstract was published in Multiple Sclerosis Journal, abstract P1157).
Disclosure
MM and JM are employees of Boston Health Economics, Inc. MF is a former employee of Boston Health Economics, Inc. Boston Health Economics, Inc., received funding from EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany) to conduct the analyses. ALP is an employee of EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany). The authors received no funding for their participation in the writing of the manuscript. The authors report no other conflicts of interest in this work.
References
- National Multiple Sclerosis SocietyMultiple Sclerosis FAQs Available from: http://www.nationalmssociety.org/What-is-MS/MS-FAQ-sAccessed March 18, 2016
- SharacJMcCronePSabes-FigueraRPharmacoeconomic considerations in the treatment of multiple sclerosisDrugs201070131677169120731475
- National Multiple Sclerosis SocietyUnderstanding Multiple Sclerosis Available from: http://calmain.nationalmssociety.org/site/PageNavigator/CAL_Exercise_MS1_TreatedAccessed March 21, 2016
- National Multiple Sclerosis SocietyMedications Available from: http://www.nationalmssociety.org/Treating-MS/MedicationsAccessed March 21, 2016
- AgashivalaNWuNAbouzaidSCompliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort studyBMC Neurol20131313824093542
- BergvallNPetrillaAAKarkareSUPersistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysisJ Med Econ2014171069670725019581
- BrandesDWCallenderTLathiEO’LearySA review of disease-modifying therapies for MS: maximizing adherence and minimizing adverse eventsCurr Med Res Opin2009251779219210141
- MenzinJCaonCNicholsCWhiteLAFriedmanMPillMWNarrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosisJ Manag Care Pharm2013191 Suppl AS24S4023383731
- DevonshireVLapierreYMacdonellRThe Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosisEur J Neurol20101816977
- SteinbergSCFarisRJChangCFChanATankersleyMAImpact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort studyClin Drug Investig201030289100
- TanHYuJTabbyDDevriesASingerJClinical and economic impact of a specialty care management program among patients with multiple sclerosis: a cohort studyMult Scler201016895696320595246
- TanHCaiQAgarwalSStephensonJJKamatSImpact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosisAdv Ther2011281516121153000
- VarmingARHansenUMAndresdottirGHustedGRWillaingIEmpowerment, motivation, and medical adherence (EMMA): the feasibility of a program for patient-centered consultations to support medication adherence and blood glucose control in adults with type 2 diabetesPatient Prefer Adherence201591243125326366060
- CaonCSaundersCSmrtkaJBaxterNShoemakerJInjectable disease-modifying therapy for relapsing-remitting multiple sclerosis: a review of adherence dataJ Neurosci Nurs2010425 SupplS5S921049828
- HollandNWieselPCavalloPAdherence to disease-modifying therapy in multiple sclerosis: Part IRehabil Nurs200126517217612035685
- TreadawayKCutterGSalterAFactors that influence adherence with disease-modifying therapy in MSJ Neurol2009256456857619444532
- BergerBAHudmonKSLiangHPredicting treatment discontinuation among patients with multiple sclerosis: application of the transtheoretical model of changeJ Am Pharm Assoc (2003)200444444545415372865
- DorALageMJTarrantsMLCastelli-HaleyJCost sharing, benefit design, and adherence: the case of multiple sclerosisAdv Health Econ Health Serv Res20102217519320575233
- EvansCTamJKingwellEOgerJTremlettHLong-term persistence with the immunomodulatory drugs for multiple sclerosis: a retrospective database studyClin Ther201234234135022296946
- FraserCHadjimichaelOVollmerTPredictors of adherence to Copaxone therapy in individuals with relapsing-remitting multiple sclerosisJ Neurosci Nurs200133523123911668881
- FraserCPredictors of adherence to glatiramer acetate therapy in individuals with self-reported progressive forms of multiple sclerosisJ Neurosci Nurs200335316317012830664
- FraserCMorganteLHadjimichaelOVollmerTA prospective study of adherence to glatiramer acetate in individuals with multiple sclerosisJ Neurosci Nurs200436312012915233411
- GleasonPPStarnerCIGundersonBWSchaferJASarranHSAssociation of prescription abandonment with cost share for high-cost specialty pharmacy medicationsJ Manag Care Pharm200915864865819803554
- GryttenNAarsethJHEspesetKStoppers and non-starters of disease-modifying treatment in multiple sclerosisActa Neurol Scand2013127213314022924678
- HalpernRAgarwalSDembekCBortonLLopez-BresnahanMComparison of adherence and persistence among multiple sclerosis patients treated with disease-modifying therapies: a retrospective administrative claims analysisPatient Prefer Adherence20115738421423591
- JokubaitisVGSpelmanTLechner-ScottJThe Australian Multiple Sclerosis (MS) immunotherapy study: a prospective, multicentre study of drug utilisation using the MSBase platformPLoS One201383e5969423527252
- KozmaCMPhillipsALMeleticheDMUse of an early disease-modifying drug adherence measure to predict future adherence in patients with multiple sclerosisJ Manag Care Spec Pharm201420880080725062073
- LugaresiAFlorioCBrescia-MorraVPatient adherence to and tolerability of self-administered interferon beta-1a using an electronic autoinjection device: a multicentre, open-label, phase IV studyBMC Neurol201212722390218
- LuluSJulianLShapiroEHudsonKWaubantETreatment adherence and transitioning youth in pediatric multiple sclerosisMult Scler Relat Disord20143668969525798373
- MohrDCBoudewynACLikoskyWLevineEGoodkinDEInjectable medication for the treatment of multiple sclerosis: the influence of self-efficacy expectations and injection anxiety on adherence and ability to self-injectAnn Behav Med200123212513211394554
- PozzilliCSchweikertBEcariUOentrichWSupportive strategies to improve adherence to IFN beta-1b in Multiple Sclerosis – results of the BetaPlus observational cohort studyJ Neurol Sci20113071–212012621636099
- ReynoldsMWStephenRSeamanCRajagopalanKHealthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugsJ Med Econ2010131909820078189
- ReynoldsMWStephenRSeamanCRajagopalanKPersistence and adherence to disease modifying drugs among patients with multiple sclerosisCurr Med Res Opin201026366367420070144
- RioJPorcelJTellezNFactors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosisMult Scler200511330630915957512
- TarrantsMOleen-BurkeyMCastelli-HaleyJLageMJThe impact of comorbid depression on adherence to therapy for multiple sclerosisMult Scler Int2011201127132122096632
- TremlettHVander MeiIPittasFAdherence to the immunomodulatory drugs for multiple sclerosis: contrasting factors affect stopping drug and missing dosesPharmacoepidemiol Drug Saf200817656557618395883
- TurnerAPKivlahanDRSloanAPHaselkornJKPredicting ongoing adherence to disease modifying therapies in multiple sclerosis: utility of the health beliefs modelMult Scler20071391146115217967842
- TurnerAPWilliamsRMSloanAPHaselkornJKInjection anxiety remains a long-term barrier to medication adherence in multiple sclerosisRehabil Psychol200954111612119618711
- WicksPMassagliMKulkarniADastaniHUse of an online community to develop patient-reported outcome instruments: the Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ)J Med Internet Res2011131e1221266318
- ZeccaCRiccitelliGCCalabresePTreatment satisfaction, adherence and behavioral assessment in patients de-escalating from natalizumab to interferon betaBMC Neurol2014143824576156
- ZwibelHPardoGSmithSDenneyDOleen-BurkeyMA multicenter study of the predictors of adherence to self-injected glatiramer acetate for treatment of relapsing-remitting multiple sclerosisJ Neurol2011258340241120922405
- EvansCMarrieRAZhuFAdherence and persistence to drug therapies for multiple sclerosis: a population-based studyMult Scler Relat Disord20168788527456879
- JongenPJLemmensWAHuppertsRPersistence and adherence in multiple sclerosis patients starting glatiramer acetate treatment: assessment of relationship with care received from multiple disciplinesPatient Prefer Adherence20161090991727307711
- McKayKATremlettHPattenSBDeterminants of non-adherence to disease-modifying therapies in multiple sclerosis: a cross-Canada prospective studyMult Scler Epub2016629
- PaolicelliDCoccoEDi LecceVExploratory analysis of predictors of patient adherence to subcutaneous interferon beta-1a in multiple sclerosis: TRACER studyExpert Opin Drug Deliv201613679980526922837
- SaizAMoraSBlancoJTherapeutic compliance of first line disease-modifying therapies in patients with multiple sclerosis. COMPLIANCE StudyNeurologia201530421422224484756
- Warrender-SparkesMSpelmanTIzquierdoGThe effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosisMult Scler201622452053226199347
- DionneCGangulyRCamacAChavesCDo oral disease modifying agents (DMTs) improve adherence to MS treatment? A comparison of oral and injectable drugsPresented at: Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC)May 27–30; 2015Indianapolis, IN, USA
- MunsellMLocklearJCPhillipsALFreanMMenzinJAn assessment of adherence among multiple sclerosis patients newly initiating treatment with a self-injectable versus oral disease-modifying drugPresented at: Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC)May 27–30; 2015Indianapolis, IN, USA
- ShahSCoopermanTKachurSEvaluation of treatment adherence and persistence based on dosing frequency and route for self-administration of disease-modifying therapies in multiple sclerosisPresented at: Academy of Managed Care Pharmacy (AMCP) 27th Annual MeetingApril 7–10; 2015San Diego, CA, USA
- VrijensBDeGSHughesDAA new taxonomy for describing and defining adherence to medicationsBr J Clin Pharmacol201273569170522486599
- PetersonAMNauDPCramerJABennerJGwadry-SridharFNicholMA checklist for medication compliance and persistence studies using retrospective databasesValue Health200710131217261111
- MarrieRACohenJStuveOA systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overviewMult Scler201521326328125623244
- The R FoundationThe R Project for Statistical Computing Available from: https://www.r-project.org/Accessed March 22, 2016
- PattiFOptimizing the benefit of multiple sclerosis therapy: the importance of treatment adherencePatient Prefer Adherence201041920165593
- GlanzBHollandCGauthierSCognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosisMult Scler20071381004101017623735
- LoreficeLMuraGConiGWhat do multiple sclerosis patients and their caregivers perceive as unmet needs?BMC Neurol20131317724237586
- GelladWFGrenardJMcGlynnEAA review of barriers to medication adherence: a framework for driving policy options Available from: http://www.rand.org/content/dam/rand/pubs/technical_reports/2009/RAND_TR765.pdfAccessed March 16, 2016
- MotheralBBrooksJClarkMAA checklist for retrospective database studies – report of the ISPOR task force on retrospective databasesValue Health200362909712641858