Abstract
Objective
Treatment options for psoriasis offer trade-offs in terms of efficacy, convenience, and risk of adverse events. We evaluated patients’ preferences with respect to benefit–risk in the treatment of psoriasis.
Methods
A discrete choice experiment was conducted in adults from the UK with moderate-to-severe psoriasis using an orthogonal design with 32 hypothetical choice sets. Participants were randomly assigned to one of two surveys with 16 choice sets. Patients’ preferences were investigated with respect to the following attributes: reduction in body surface area affected by psoriasis, treatment administration (frequency and mode of delivery), short-term diarrhea or nausea risk, and 10-year risk of developing melanoma or nonmelanoma skin cancer, tuberculosis, or serious infections. A mixed effects logistic regression model generated relative preferences between treatment profiles.
Results
Participants (N=292) had a strong preference to avoid increased risk of melanoma or nonmelanoma skin cancer (odds ratio [OR]: 0.44 per 5% increased 10-year risk) and increased risks of tuberculosis and serious infections (both ORs: 0.73 per 5% increased 10-year risk) and preferred once-weekly to twice-daily tablets (OR: 0.76) and weekly (OR: 0.56) or fortnightly (OR: 0.65) injections. Participants preferred avoiding treatments that may cause diarrhea or nausea in the first 2 weeks (OR: 0.87 per 5% increase) and preferred treatments that effectively resolved plaque lesions (OR: 0.93 for each palm area still affected).
Conclusion
All attributes were significant predictors of choice. Patients’ preference research complements clinical trial data by providing insight regarding the relative weight of efficacy, tolerability, and other factors for patients when making treatment choices.
Introduction
Psoriasis is a chronic, inflammatory disease that manifests as red, scaly plaques varying in size and symptom burden.Citation1,Citation2 Quality of life can be adversely affected, with both physical and psychological well-being severely affected.Citation1,Citation3,Citation4 Patient self-management can be challenging, with significant comorbidities such as arthritis, metabolic syndrome, atheromatous vascular disease, diabetes, obesity, and psychosocial impairment often complicating treatment outcomes.Citation5–Citation7 As no cure exists, treatment is aimed at working with the patient to maximize skin clearance while minimizing risk.Citation8–Citation10 A dialog is usually established between the clinician and the patient to explore the patient’s expectations and the range of treatment options available that suit the patient’s lifestyle and attitude compared to the therapeutic benefit–risk.Citation2,Citation11
Treatment options have expanded significantly in recent years, with conventional systemic oral therapies such as methotrexate and a range of effective injectable biologic options, including antitumor necrosis factor-α, anti-interleukin 12/23, and anti-interleukin 17 agents, now available to patients with moderate-to-severe psoriasis who are not benefiting from topical treatments or phototherapy.Citation2,Citation7,Citation9 However, biologic therapy may come with a higher risk of serious infection than other therapeutic options,Citation12 and one study found that biologic treatments are often initiated late in the treatment pathway, with a mean duration from psoriasis diagnosis to the first biologic of 22.1 years.Citation13
Both the National Institute for Health and Care Excellence (NICE)Citation2 and the American Academy of DermatologyCitation11 have placed increased emphasis on patient-centric care and patients’ preferences. Guidance from the latter, in particular, has moved away from systematic sequencing in favor of immediate treatment plans based on individual patient needs, placing a specific emphasis on patient education and input.Citation11 Using the various available patient-reported outcomes measurement tools for psoriasis,Citation14,Citation15 there has been a shift to improve the understanding of patients’ preferences and to incorporate these into the prescribing decision to improve overall therapeutic effectiveness in real-world clinical practice, especially as studies have shown considerable patient dissatisfaction with current treatment options, and a gulf has developed between patient expectations and the care they receive.Citation1,Citation16,Citation17
The aim of this discrete choice experiment (DCE) was to capture the preferences of patients with moderate-to-severe psoriasis regarding different aspects of their treatment, including mode and frequency of administration, adverse event risks, and therapeutic benefit. The impact of prior treatment history and health-related quality-of-life (HRQL) status on these preferences was also evaluated. This information can help different decision makers with health technology assessment, shared decision making, and benefit–risk assessment.
Methods
Study design and population
Participants aged ≥18 years with a formal psoriasis diagnosis and three or more palm areas or ≥3% of body surface area (BSA) affected were included. The BSA score was used because it is a relatively easy measure for patients to approximate and self-report and is recognized in clinical guidelines as a validated measure of psoriasis disease severity.Citation2,Citation18 All participants needed to be a resident of the UK, have adequate fluency in English, and have access to the Internet. The recruitment of current or past recipients of biologic therapy for psoriasis was capped at 30% of the total sample. Participants with self-reported acute illness or cognitive impairment were excluded. In addition, the survey included three mathematics-based screening questions to exclude people who were unable to understand the fractions and percentages used in the questionnaire by providing two or more incorrect answers. The study was conducted in accordance with the Declaration of Helsinki and, owing to the absence of an appropriate independent review board in the UK, the study protocol was referred to and approved by the Salus Institutional Review Board in Austin, TX, USA.
Survey development
The online DCE survey was developed in line with best practice guidance.Citation19,Citation20 To support the development of the survey, a brief literature review examined previous psoriasis research to evaluate the drivers of patients’ preferences in psoriasis and to identify the aspects of treatment that patients and physicians value. The review also informed the identification of products currently available and their characteristics.
Two rounds of face-to-face consultation were conducted with a leading consultant dermatologist who advised on the clinical appropriateness and patient relevance of each attribute selected, in addition to the levels of each attribute.
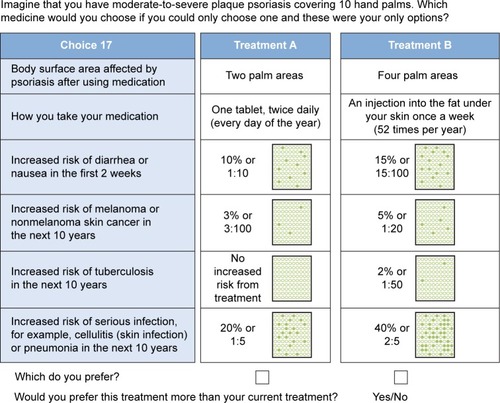
The final selection of attributes was based on characteristics (ie, treatment delivery methods) and clinically relevant properties (ie, safety and efficacy) of available treatments for patients with moderate-to-severe psoriasis who are not benefiting from topical treatments or phototherapy. A total of six attributes with four levels each were included in the final survey ().
Table 1 Treatment attributes and levels used in the discrete choice experiment
The attributes and levels were tested in a small pilot study (N=6) involving one-on-one cognitive debriefing telephone interviews. All participants provided their written informed consent for this study, and the interviews were audio recorded to aid analysis. This exercise was used to assess content validity of the DCE survey, including general comprehension, interpretation, and readability of the survey items and pictographs, and respondent burden (in terms of length and complexity of the items and the overall survey). During the interviews, participants were asked to discuss each attribute in detail to determine its relevance to treatment satisfaction. They were also asked to provide feedback on the language used in the attribute levels (ie, to interpret and explain the meaning of each attribute) and to describe the attributes in terms of importance. Content analysis was conducted on the interview data, and the survey was modified and finalized in line with the feedback obtained. The revisions were minor, and further rounds of cognitive debriefing were not deemed necessary.
Treatment profiles
Thirty-two hypothetical treatment profile pairs were created through combining the different attributes and levels. An orthogonal fractional factorial design was used to identify a minimum specification of combinations of attributes and levels that could define these hypothetical treatments. The combinations were then paired using the fold-over design outlined by Street et alCitation21 and estimated to be 99% efficient. These design elements were specifically included to support the analysis of the choice data and ensure more accurate data outcomes. The 32 treatment profile pairs were used to create two versions of the DCE survey with 16 sets each, Survey A and Survey B, to which participants were randomly allocated. In addition, Survey A and Survey B differed in the number of hand areas of their body that participants were asked to imagine were affected by plaque psoriasis when making their treatment choices; Survey A specified 15 palm areas and Survey B specified 10.
Participants in the UK were recruited by a specialist recruitment agency to complete the DCE survey online. Participants had to first meet the eligibility criteria via a self-report online screener that confirmed if they had been diagnosed with psoriasis (Yes = include), if they were currently prescribed treatment (Yes = include/No = exclude), and if they were residents of the UK (Yes = include/No = exclude). They were also asked to report whether they had ≥3% or <3% of BSA affected by psoriasis (<3% = exclude); this was later raised to ≥5% of BSA affected (<5% = exclude). The participants who were deemed eligible were directed to an online informed consent form and were asked to indicate that they had read and understood the document and agreed to participate in the online survey by ticking a box.
Participants were asked to choose between two treatment profiles consisting of combinations of attributes (). This was preceded by full instructions on the purpose of the DCE. Participants were also asked to complete a demographic questionnaire and the Dermatology Life Quality Index (DLQI)Citation14 to evaluate HRQL. The DLQI is scored from 0 to 30, with a score of 0–1 indicating that psoriasis has no effect at all on the quality of life, 2–5 indicating a small effect, 6–10 indicating a moderate effect, 11–20 indicating a very large effect, and finally 21–30 indicating an extremely large effect. Participants were also asked to rate the extent of their psoriasis lesions in terms of hand palm areas.
Statistical analysis
The sample size was not empirically determined; the aim was to include as many participants as possible within the limits of the criteria. Originally, 300 participants were recruited for the main DCE survey. However, during data cleaning, four duplicates were identified and removed from the data set. In addition, four participants were excluded for reporting an affected BSA <3% in the DCE, having previously passed the screener by reporting a BSA ≥3%. The survey results were therefore based on a final sample size of 292 participants.
Demographic and disease characteristics were collected and reported descriptively. Preference results from the DCE were analyzed for the overall population and stratified by DLQI score and previous biologic therapy exposure.
The DCE data from Survey A and Survey B were pooled and the efficacy attribute (BSA affected by psoriasis after using medication) was treated as a continuous variable. The DCE data were then analyzed using a mixed effects logistic regression model. Random effects were included for all attributes to account for participants’ relative preferences for specific attributes.Citation22 The mixed effects of logistic regression model was used to generate relative preferences between hypothetical treatment profiles using the odds ratio (OR) between profiles, calculated as the ratio of exponentiated coefficients.
The results of the regression analysis are presented as untransformed preference weights and exponentiated ORs with 95% confidence intervals (CIs), which describe the relative odds of selecting a treatment across attribute levels. Regression results for BSA are expressed per palm area difference, whereas results for adverse events are expressed as per 5% increase in risk.
The primary regression analysis utilized a standard dummy coding framework for categorical variables in which the coefficients for attribute levels were compared with a single reference category. In addition, an effects-coded model was fit, in which each attribute level is compared with a weighted average of all levels.Citation23 This analysis was conducted to allow for visual representation of preference weights and their variability across all attribute levels.
Results
Patient characteristics
In total, 292 participants with moderate-to-severe psoriasis completed the survey; key demographic and disease characteristics are reported in . The mean DLQI score was 10.5, corresponding to relatively large disease-related quality-of-life impairment; approximately one in five participants reported little impact (score ≤5), whereas 43% of participants reported a very large or extremely large impact (score ≥11).
Table 2 Participants’ demographics and treatment characteristics
Patients’ preferences
All attributes in the DCE analysis were significant (P<0.05) predictors of choice, indicating that participants considered all attributes when making their choices (). Once-weekly tablets were preferred over all other treatment scheduling options, including twice-daily tablets and weekly or biweekly injections. Participants also had a strong preference for avoiding an increased risk of melanoma or nonmelanoma skin cancer (NMSC) and were less likely to choose treatments with increased risks of tuberculosis and serious infections over the next 10 years than treatments that may cause diarrhea or nausea in the first 2 weeks after initiation. The analysis also explored measures of model performance and goodness of fit. Both the likelihood ratio test (Chi-square: 1,246.9, P<0.0001) and Wald test (Chi-square: 677.36, P<0.0001) were highly significant. Additionally, the Akaike information criterion score was also very high (5,029.29), indicating high goodness of fit of the model.
Table 3 Results of mixed effects logistic regression model for the main DCE in individuals with psoriasis reporting treatment preferences
When administration preferences were assessed using twice-daily tablets as the reference level in the main model, it was, as expected, preferred over weekly injections (OR: 0.74; 95% CI: 0.60–0.91) and less preferred than once-weekly tablets (OR: 1.32; 95% CI: 1.14–1.53), whereas fortnightly injections were not perceived as significantly different from having to take twice-daily tablets (OR: 0.84; 95% CI: 0.70–1.05). When stratifying the same model by previous exposure to biologic therapies as part of an exploratory sensitivity analysis, biologic-naïve patients showed a preference for avoiding weekly (OR: 0.61; 95% CI: 0.50–0.74) and fortnightly (OR: 0.80; 95% CI: 0.64–0.99) injections, whereas the route of administration did not influence treatment preferences in the biologic-experienced group.
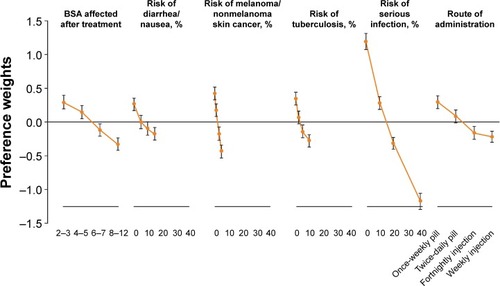
Mean preference weights and the corresponding 95% CIs are shown in , indicating the relative magnitude of preferences across attributes. Preferences for all attributes displayed consistent trends, with participants preferring treatments with increased efficacy and decreased risk of adverse events. The most notable preferences were observed for risk of serious infection, reflected by the magnitude of the range of preference weights across attribute levels. Note that this is due to the fact that attribute levels spanned a hypothetical range up to 40% risk of serious infection, compared with a maximum risk of 10%–15% for other comorbidities.
Figure 2 Preference weights (OR >1 vs <1) and 95% confidence intervals resulting from an effects-coded discrete choice experiment in individuals with psoriasis reporting treatment preferences.
Abbreviations: OR, odds ratio; BSA, body surface area.
Patients’ references: subgroup analysis
Preference results from the DCE were analyzed by the DLQI score and previous biologic therapy exposure. The key findings from the subgroup analyses of the DCE study are reported in . The influence of patients’ HRQL on their strength of preference for the attributes of psoriasis treatment was explored in patients with a DLQI score ≤10 (moderate to no effect on patients’ HRQL; n=166) and a DLQI score >10 (very large to extremely large effect on HRQL; n=126). Participants with a DLQI score >10 placed less value on treatment efficacy (OR: 0.97; 95% CI: 0.95–1.00), compared with those with a DLQI score ≤10 (OR: 0.90; 95% CI: 0.88–0.93). These findings were mirrored in the subgroup results comparing biologic-experienced participants (OR: 0.97; 95% CI: 0.94–1.00) with biologic-naïve participants (OR: 0.90; 95% CI: 0.88–0.92). Participants with a DLQI score >10 were also more tolerant of the risk of toxicities than were those with a DLQI score ≤10 (), an effect that was statistically significant (P<0.05) for the attribute describing infection risk (OR: 0.79; 95% CI: 0.76–0.81). Additionally, participants with a DLQI score ≤10 had a stronger preference for avoiding injectable treatments relative to once-weekly tablets (). Preference weights for the biologic-naïve participants (n=217) show marked differences from the participants with biologic experience (n=75). In participants with biologic experience, only the increased 10-year risk of serious toxicities (ie, risk of melanoma or NMSC), tuberculosis, and serious infections were significant predictors of treatment preferences (). Biologic-naïve participants considered all attributes and placed the greatest value on treatment efficacy (OR: 0.90; 95% CI: 0.88–0.93). They also preferred to avoid weekly and fortnightly injections compared with once-weekly tablets and to avoid the risk of all included toxicities in general (). There was some overlap between subgroups, defined by biologic therapy experience and DLQI score, with the majority of biologic-experienced patients also reporting a DLQI score >10 (60%; n=45/75).
Table 4 Results of mixed effects logistic regression model for DCE in individuals with psoriasis reporting treatment preferences, stratified by subgroup
Discussion
This prospective study was designed to determine the relative value individuals diagnosed with moderate-to-severe psoriasis place on attributes of treatment for the disease. The results revealed the importance of minimizing the extent of lesions, but also the views of participants regarding the long-term risks of side effects, such as melanoma or NMSC, tuberculosis, and serious infections. The survey also assessed the importance of issues related to the convenience of taking medication either orally or by injection. These attributes represent different issues that need to be considered when making treatment choices. The preference weights from the study show how important these issues are for a sample of individuals with psoriasis in the UK.
This research builds on the previous studies reporting patients’ preferences for the treatment of psoriasis.Citation24–Citation27 In a recent DCE conducted in German patients with moderate-to-severe psoriasis, Kromer et alCitation25 found that the safety of biologics was the most important attribute, followed by efficacy, but preferences varied with sociodemographic characteristics and working status. These results were consistent with an earlier report by Seston et alCitation27 who found that attributes such as adverse effects, time to improvement, and relapse time frames influence treatment choices of individuals with psoriasis. Although Seston et al found a preference for minimizing the risk of adverse events, Schaarschmidt et al, in a 2011 study,Citation26 reported that patients were willing to trade an increased risk of adverse events for better therapeutic outcomes. Unlike our study, neither study considered the previous treatment history of patients and how this might impact decision making. Schaarschmidt et alCitation26 concluded that incorporating patients’ preferences into the prescribing decision may facilitate increased adherence and lead to better treatment outcomes, a view shared by Umar et alCitation28 whose systemic review concluded that using patients’ preferences in decision making would likely improve both patient satisfaction and treatment outcomes.
In this study, participants showed a strong preference for avoiding treatments with serious long-term toxicities, such as melanoma or NMSC and serious infections, and for avoiding injectable therapies, especially if they had not previously used biologic therapies. These stated patient preferences may, in part, explain the protracted period of >22 years between diagnosis and initiation on biologic treatment reported by Fonia et alCitation13 in UK clinical practice. Other factors that may affect this delay include the current reimbursement criteria in terms of disease severity and the prerequisite of the number of treatments patients must have failed before being eligible for biologic therapies in the UK.Citation2,Citation29 A delay in referral from primary to secondary care, general challenges in access to specialist dermatology services throughout the UK, and the high cost of biologic treatment may also play a part.
Participants who reported that their psoriasis had a very large impact on their quality of life (DLQI score >10; n=126, 43.1%) had a higher likelihood of accepting treatment-related toxicities in relation to risk of serious infection and were less reluctant to receive injections. Overall, participants with no prior experience with biologic therapies were more averse to the risks of treatment toxicities compared with people with biologic experience. A trend was also observed for the biologic-experienced group to be more willing to accept injection treatments. This suggests that previous experience with biologic therapies or experiencing a very large effect on HRQL (DLQI score >10) may affect patients’ treatment preferences by reducing aversion to injections and increasing acceptance of adverse events and reduced efficacy. These two subgroups also stated preferences that appeared to place less differentiation between the hypothetical treatment options included in the DCE and less extreme preferences for all attributes considered. The similarities between the treatment preferences seen in participants with a DLQI score >10 and biologic-experienced participants can be expected because the majority of biologic-experienced patients (60%) also reported a DLQI score >10. Such an overlap is not surprising, given that the criteria for receiving biologic therapy in the UK include having a DLQI score >10. Moreover, the experience of failing on or switching between multiple therapies, including biologics, may affect patients’ expectations of future treatment, increasing their willingness to accept treatments with different modes of administration, such as injections, and altering their benefit–risk tolerances. This may apply particularly to participants in the current DCE who reported both biologic experience and a DLQI score >10. These factors in combination may be an indicator of being treatment refractory due to lack of efficacy or due to toxicities, thus leaving them few treatment options to consider in real life and therefore reducing their concerns about trying hypothetical treatments with less-favorable profiles.
In addition, the analyses indicated that people with better HRQL (DLQI score ≤10) placed more value on treatments that reduce the extent of BSA affected by psoriasis. Similar effects have been seen previously in other chronic diseases.Citation30–Citation32 It is possible that people who value treatments more highly are more likely to be adherent and therefore experience less disease burden.Citation30 Longitudinal research is needed to explore these effects in more detail. These methods may be able to provide fresh insights into what drives good outcomes in psoriasis.
The regulatory environment is also becoming increasingly accepting of evidence on patients’ treatment preferences during their decision-making processes. Indeed, the 2012 US Food and Drug Administration guidelines from the Center for Devices and Radiological Health on benefit–risk analysis clearly state the importance of considering patients’ preferred treatment when approving medical devices to market.Citation33 The Food and Drug Administration is also using patient feedback and preference DCE evidence to help to facilitate the development and use of patient-centric weight-loss devices for obese patients, a move away from its previous “one-size-fits-all” approach.Citation34,Citation35 In Belgium, DCE studies have been used to consider the importance of decision criteria among citizens with reference to reimbursement,Citation36 whereas the US Centers for Medicare and Medicaid Services uses patient experience surveys to link Medicare payments to health care quality via its Hospital Value-Based Purchasing program.Citation37 This has become an area of research for health technology assessment reimbursement agencies such as NICE in the UK, which has previously consulted on the use of multiple criterion decision analysis in their decision-making framework.Citation38 A recent collaboration between Myeloma UK and NICE to examine how patients’ treatment preferences can be quantitatively captured and incorporated alongside other data in decision modeling as part of the Health Technology Assessment program underscores the growing interest in understanding the impact and role of patients’ treatment preferences in the decision-making process in the UK.Citation39
Some limitations of this study should be considered. Patient self-reporting of psoriasis diagnosis and severity means there is potential room for error across patient comparisons. To best quantify the results, the number of attributes included in the survey needed to be finite. As a result, there may be other attributes that patients with moderate-to-severe psoriasis find important when choosing a treatment, but which we were not able to include. This is a constraint of the methodology. The pretest interviews supported the choice of attributes.
The choice survey is intended to simulate decisions in clinical practice, but of course it does not have the clinical or emotional consequences of actual decisions. Differences can arise between stated and actual choices. The results indicate the importance of different issues for patients, but this limitation should be considered when interpreting them. In addition, levels associated with each attribute may not reflect marketed therapies for psoriasis based on the available data. However, the dosing schedules for the injectable treatments reflected the most widely used biologic therapies for psoriasis in the UK at the time of this study.Citation40 The subgroup analyses were univariate and need to be interpreted cautiously. They were exploratory and descriptive in nature, which may have limitations. However, they can go some way to help us understand heterogeneity in the preference data.
To avoid well-known cognitive problems with evaluating small probabilities, we defined the risk exposure as the chance of each of three serious side effects over 10 years. The actual risk of serious infection may not be linear over time, but exploring preferences for potential nonlinearities in risk exposure was beyond the scope of this study.
The population recruited in this study appeared to be representative of the general psoriasis population in the UK in terms of age, sex, and disease severity.Citation41,Citation42 A comparison of participants’ demographics from this study shows some similarities with patients included in a prospective registry study in the UK and Ireland evaluating patients treated with biologic therapy.Citation40 However, some notable differences included a shorter mean disease duration (12.6 vs 19.0 years) and a lower mean DLQI score (10.5 vs 18.0) in this study. It is possible that the method for recruitment resulted in some selection bias, as most participants in the DCE had moderate disease. However, population-based studies of psoriasis using electronic medical records from the Health Improvement Network, which are representative of the general population in the UK, have reported that a higher proportion of psoriasis patients have moderate (~35%, BSA: 3%–10%) compared with severe (~13%, BSA >10%) disease,Citation41,Citation42 which is similar to the patient population recruited for our DCE. The study did not seek to correlate preferences for treatment attributes in patients with difficult-to-treat manifestations, such as nail, scalp, or palmoplantar psoriasis.
Conclusions
This study shows the importance of patients’ preferences toward the attributes of various potential treatment profiles for psoriasis and provides some insight into the extent to which people balance efficacy against risks of side effects and toxicities and even convenience issues. As regulators and reimbursement bodies begin to tangibly recognize the importance of patients’ preferences, it will be important for health care professionals to be aware and communicate benefit–risk information to patients. The data from this study could be used to help communicate how patients feel about the benefits and risks of treatments for psoriasis and promote shared care in treatment decision making. In addition, analyses were able to demonstrate how the preferences varied within the study sample. Preferences varied in terms of disease impact on HRQL and also in terms of whether people had received biologic therapy previously. The results show how experience with biologic therapy can reduce patients’ concerns about injection-based therapy. Many factors influence the quality of patient outcomes in chronic diseases. This type of research can complement clinical trial data by providing insight into the relative weight of efficacy, tolerability, and other factors for patients with psoriasis.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This research was funded by Celgene Ltd. The authors received editorial support in the preparation of the manuscript from Kathy Covino, PhD, of Peloton Advantage, LLC, and from Lynette Eyb, both funded by Celgene Corporation. The authors, however, directed and are fully responsible for all content of and editorial decisions for this paper.
Disclosure
FM is an employee of Celgene Ltd. AK was an employee of Celgene Corporation at the time of the study. LE, APB, KMJ, CP, and AJL report no conflicts of interest in this work.
References
- DubertretLMrowietzURankiAEuropean patient perspectives on the impact of psoriasis: the EUROPSO patient membership surveyBr J Dermatol2006155472973616965422
- National Institute for Health and Clinical ExcellencePsoriasis: Assessment and Management. Clinical Guideline No 153London, UKNational Institute for Health and Clinical Excellence2012
- ChoiJKooJYQuality of life issues in psoriasisJ Am Acad Dermatol200349Suppl 2S57S6112894127
- SampognaFTabolliSSoderfeldtBAxteliusBAparoUAbeniDMeasuring quality of life of patients with different clinical types of psoriasis using the SF-36Br J Dermatol2006154584484916634884
- AltobelliEPetrocelliRMaccaroneMRisk factors of hypertension, diabetes and obesity in Italian psoriasis patients: a survey on socio-demographic characteristics, smoking habits and alcohol consumptionEur J Dermatol200919325225619220983
- FarleyEMenterAPsoriasis: comorbidities and associationsG Ital Dermatol Venereol2011146191521317853
- MenterAGottliebAFeldmanSRGuidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologicsJ Am Acad Dermatol200858582685018423260
- GriffithsCEClarkCMChalmersRJLi Wan PoAWilliamsHCA systematic review of treatments for severe psoriasisHealth Technol Assess20004401125
- NickoloffBJNestleFORecent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunitiesJ Clin Invest2004113121664167515199399
- PathiranaDOrmerodADSaiagPEuropean S3-guidelines on the systemic treatment of psoriasis vulgarisJ Eur Acad Dermatol Venereol200923Suppl 2170
- MenterAKormanNJElmetsCAGuidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusionsJ Am Acad Dermatol201165113717421306785
- KalbREFiorentinoDFLebwohlMGRisk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR)JAMA Dermatol2015151996196925970800
- FoniaAJacksonKLereunCGrantDMBarkerJNSmithCHA retrospective cohort study of the impact of biologic therapy initiation on medical resource use and costs in patients with moderate to severe psoriasisBr J Dermatol2010163480781620662837
- FinlayAYKhanGKDermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical useClin Exp Dermatol19941932102168033378
- KitchenHCordingleyLYoungHGriffithsCEBundyCPatient-reported outcome measures in psoriasis: the good, the bad and the missing!Br J Dermatol201517251210122125677764
- LebwohlMGBachelezHBarkerJPatient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis surveyJ Am Acad Dermatol201470587188124576585
- SternRSNijstenTFeldmanSRMargolisDJRolstadTPsoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfactionJ Investig Dermatol Symp Proc200492136139
- SmithCHAnsteyAVBarkerJNBritish Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009Br J Dermatol20091615987101919857207
- BridgesJFHauberABMarshallDConjoint analysis applications in health – a checklist: a report of the ISPOR good research practices for conjoint analysis task forceValue Health201114440341321669364
- Reed JohnsonFLancsarEMarshallDConstructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task forceValue Health201316131323337210
- StreetDJBurgessLLouviereJJQuick and easy choice sets: constructing optimal and nearly optimal stated choice experimentsInt J Res Mark2005224459470
- LancsarELouviereJConducting discrete choice experiments to inform healthcare decision making: a user’s guidePharmacoeconomics200826866167718620460
- BechMGyrd-HansenDEffects coding in discrete choice experimentsHealth Econ200514101079108315852455
- KaufTLYangJCKimballABPsoriasis patients’ willingness to accept side-effect risks for improved treatment efficacyJ Dermatolog Treat201526650751325946139
- KromerCSchaarschmidtMLSchmiederAHerrRGoerdtSPeitschWKPatient preferences for treatment of psoriasis with biologicals: a discrete choice experimentPLoS One2015106e012912026058083
- SchaarschmidtMLSchmiederAUmarNPatient preferences for psoriasis treatments: process characteristics can outweigh outcome attributesArch Dermatol2011147111285129422106115
- SestonEMAshcroftDMGriffithsCEBalancing the benefits and risks of drug treatment: a stated-preference, discrete choice experiment with patients with psoriasisArch Dermatol200714391175117917875880
- UmarNYamamotoSLoerbroksATerrisDElicitation and use of patients’ preferences in the treatment of psoriasis: a systematic reviewActa Derm Venereol201292434134622278662
- National Institute for Health and Care ExcellenceNICE pathways: Psoriasis overview2016 Available from: https://pathways.nice.org.uk/pathways/psoriasisAccessed November 21, 2016
- HodgkinsPSwinburnPSolomonDYenLDewildeSLloydAPatient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experimentPatient201251334422077619
- LloydAMcIntoshEPriceMThe importance of drug adverse effects compared with seizure control for people with epilepsy: a discrete choice experimentPharmacoeconomics200523111167118116277551
- LloydAMcIntoshEWilliamsAEKapteinARabeKFHow does patients’ quality of life guide their preferences regarding aspects of asthma therapy? A patient-preference study using discrete-choice experiment methodologyPatient20081430931622272998
- Center for Devices and Radiological HealthFactors to consider when making benefit-risk determinations in medical device premarket approval and de novo classifications2012 Available from: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM296379.pdfAccessed June 8, 2016
- HoMPGonzalezJMLernerHPIncorporating patient-preference evidence into regulatory decision makingSurg Endosc201529102984299325552232
- LernerHWhangJNipperRBenefit-risk paradigm for clinical trial design of obesity devices: FDA proposalSurg Endosc201327370270723247746
- CleemputIDevrieseSKohnLIncorporating societal preferences in reimbursement decisions – relative importance of decision criteria according to Belgian citizens2014 Available from: https://kce.fgov.be/sites/default/files/page_documents/KCE_234_reimbursement_decisions_Report_0.pdfAccessed June 8, 2016
- Centers for Medicare and Medicaid ServicesMedicare program; hospital inpatient value-based purchasing program. Final ruleFed Regist20117688264902654721548401
- ThokalaPDuenasAMultiple criteria decision analysis for health technology assessmentValue Health20121581172118123244821
- UnderwoodGNICE to research patient preferences in HTA2016 Available from: http://www.pharmatimes.com/news/nice_to_research_patient_preferences_in_hta_1033029Accessed June 8, 2016
- IskandarIYAshcroftDMWarrenRBDemographics and disease characteristics of patients with psoriasis enrolled in the British association of dermatologists biologic interventions registerBr J Dermatol2015173251051825989336
- YeungHTakeshitaJMehtaNNPsoriasis severity and the prevalence of major medical comorbidity: a population-based studyJAMA Dermatol2013149101173117923925466
- TakeshitaJWangSShinDBEffect of psoriasis severity on hypertension control: a population-based study in the United KingdomJAMA Dermatol2015151216116925322196