Abstract
Background
Adherence to disease-modifying drugs (DMDs) is one of the key factors for achieving optimal clinical outcomes. Rebismart® is an injection device for subcutaneous administration of interferon beta-1a (INF β-1a) that is also able to monitor adherence objectively. The aim of this study was to describe adherence to INF β-1a using the said electronic autoinjection device and to explore the relationship between adherence and relapses in a Spanish cohort.
Methods
This is a retrospective observational study in which 110 Spanish patients self-administered INF β-1a subcutaneously using an electronic autoinjection device between June 2010 and June 2015. The primary end point was the percentage of adherence measured by Rebismart® to subcutaneous INF β-1a injections calculated as number of injections received in time period versus number of injections scheduled in time period. Other variables recorded were demographic and clinical data. Statistical analysis was performed using SPSS 19.0 software.
Results
Median adherence for the total study period was 96.5% (interquartile range [IQR]: 91.1–99.1). Similar values were observed during the first 6 months: 98.7% (IQR: 91.3–100), and the last 6 months: 97.6% (IQR: 91.1–99.8). Median duration of treatment was 979 days (IQR: 613.8–1,266.8). During the entire treatment period, 77.3% of patients were relapse free and mean annualized relapse rate was 0.14 (standard deviation: 0.33). Increased adherence was associated with better clinical outcomes, leading to lower relapse risk (odds ratio: 0.953; 95% confidence interval: 0.912–0.995). Specifically, every percentage unit increase in adherence resulted in a 4.7% decrease in relapse.
Conclusion
Patients with multiple sclerosis who self-injected INF β-1a with Rebismart® had excellent adherence, correlating with a high proportion of relapse-free patients and very low annualized relapse rate.
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, and degenerative disease of the central nervous system with a heterogeneous clinical course. MS usually begins with an acute inflammatory demyelinating episode turning to relapsing-remitting MS (RRMS) characterized by discrete acute attacks of worsening neurological function (relapses), which are followed by partial or complete recovery (remission). Residual disability may accumulate over time, and many patients with RRMS develop secondary progressive MS, which is characterized by progressive neurological decline.Citation1 Although MS remains incurable, treatment with disease-modifying drugs (DMDs) can reduce the frequency of disease exacerbations and may delay disability progression.Citation2,Citation3 Subcutaneous interferon β-1a (INF β-1a) has been shown to be effective in improving all three key measures of efficacy (relapse rate, disability progression, and magnetic resonance imaging measures of disease) when administered at a dosage of 22–44 µg three times weekly.Citation4,Citation5
Although treatment efficacy has been established, adherence to and persistence with pharmacotherapy in the long term remains one of the main challenges and is a key factor in predicting long-term outcomes. The World Health Organization defines adherence as the extent to which a person’s behaviour corresponds with agreed recommendations from a healthcare provider and stresses the importance of good adherence.Citation6 In MS, the need for long-term treatment and the frequently debilitating nature of the disease make treatment adherence particularly challenging. It has been reported that adherence to DMDs ranges from 61% to 97%,Citation7–Citation12 and poor adherence or treatment gaps are associated with a higher rate of relapse.Citation9,Citation13–Citation17
Adherence is difficult to assess, and methods for measuring it can be unreliable and highly subjective.Citation18 The ideal method for measuring adherence should be sensitive and specific, allowing for a quantitative, continuous, reliable, and reproducible measure in different situations as quickly and economically as possible. Autoinjection devices offer these features and can improve the injection experience and increase patient satisfaction.Citation19–Citation21
The aim of this study was to assess treatment adherence of Spanish patients with MS using Rebismart® for self-injection of subcutaneous INF β-1a and the association between adherence and the occurrence of MS relapses.
Materials and methods
Design and scope of the study
A retrospective observational study assessed all patients diagnosed with MS and treated with INF β-1a subcutaneously using the Rebismart® autoinjection device in Hospital Universitari i Politècnic La Fe since its marketing and approval in June 2010 until June 30, 2015. This device can electronically register administrations and can export and analyze records (date, time, fully, or partially administered dose) through the Mitra® (Merck Serono, Darmstadt, Germany) computer application.
In the Multiple Sclerosis Unit, Hospital Universitari i Politècnic La Fe, patients under interferon treatment are followed up by scheduled visit every 3 months, in all cases with a complete blood analysis including a complete leukocyte count, liver enzymes, and thyroid hormones. On every visit, a questionnaire with a list of adverse events is given to all patients to follow up any adverse symptom, with a special emphasis on flu-like and dermatologic events.
Outcome measures
The primary end point was the percentage of adherence obtained during the study period using the Mitra application and calculated as number of injections administered divided by number of injections that should have been administered during that period and multiplied by 100. Similarly, adherence was also calculated for the first 6 months of treatment and the last 6 months of treatment.
The other variables collected were 1) demographic variables associated with the patient: sex and age; 2) characteristics of patients at initiation of treatment with INF β-1a: evolutionary form of MS, score on the Expanded Disability Status Scale (EDSS), years of disease progression, and age at onset of treatment, and treatment at diagnosis; and 3) treatment-related variables: pattern, spacing doses, duration of treatment, relapses during treatment, adverse effects, discontinuation of treatment, causes of discontinuation, and subsequent treatment.
The data were obtained by reviewing patients’ electronic medical records (Orion Clinic®), the Outpatient Pharmaceutical Care Unit (UFPE in Spanish) computer application and the Mitra® program.
Statistical analysis
From the descriptive standpoint, quantitative variables were expressed as measures of central tendency and dispersion and qualitative variables as absolute and relative frequencies.
Possible associations were studied between adherence and sex, age, stage of disease, EDSS at baseline, time of disease progression, pattern, number of doses administered, days of exposure, and treatment discontinuation, using the Mann–Whitney U-test, Spearman’s rank correlation coefficient, and simple linear regression test. To analyze the association between occurrence of relapses (yes/no) and the rest of the compiled variables, we used the chi-square test, Fischer’s exact test, and multivariate analysis of binary logistic regression. The statistical program used was SPSS version 19.0.
Ethics
The local ethical review board of Hospital Universitari i Politècnic La Fe de Valencia approved the study protocol in accordance with the principles of the Declaration of Helsinki. Patient consent was not required to review their medical records.
Results
Demographic and clinical characteristics of patients
A total of 115 patients received INF β-1a with the Rebismart® electronic autoinjector device. Five patients were excluded from the study because of insufficient data. Of the 110 patients analyzed, 73 were women (66.4%) with a mean age at baseline of 38.8 years (standard deviation [SD]: 9.3). At the start of treatment with Rebismart®, most patients (93.6%) had RRMS and their clinical status based on EDSS was 1.5 (0–4.5). Median disease progression, understood as the time from diagnosis to inclusion in the study (June 6, 2015), was 10 years (interquartile range [IQR]: 7.0–14.3).
Most patients (80.9%) received DMDs for MS (INF β-1a subcutaneously without the Rebismart® electronic autoinjection device or other DMDs) before initiating treatment with Rebismart as detailed in .
Table 1 Demographic and clinical characteristics of patients (n=110)
Treatment characteristics and adherence
The most commonly prescribed pattern of INF β-1a at the time of starting the administrations with Rebismart was 44 µg/three times a week in 79.1% of patients. The median dose administered was 325.5 (IQR: 152.8–518.8), and the exposure time to Rebismart was 979 days (IQR: 613.8–1,266.8), as shown in . During the study period in 21.8% of patients, dose spacing was performed either due to adverse effects or due to clinical stability over a long period of time. Dosages resulting after the spacing were 44 µg/week (n=12 patients), 22 µg/three times a week (n=8), 22 µg/two times a week (n=3), and 44 µg/two times a week (n=1).
Table 2 Treatment characteristics and adherence (n=110)
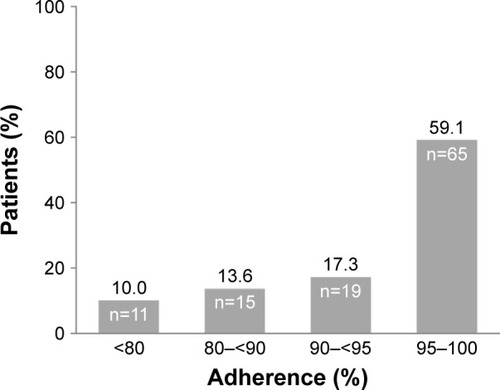
The median treatment adherence was 96.5% (IQR: 91.1–99.1), rising slightly to 98.7% (IQR: 91.3–100.0), taking into account only the first 6 months of treatment. Because of the importance of persistence in treatment outcomes, we also analyzed the adherence during the last 6 months, obtaining a median of 97.6% (IQR: 91.1–99.8). In summary, there were no statistically significant differences between adherence at the different times. A total of 59.1% of patients (n=65) had adherence >95%, 17.3% (n=19) were adherent in a range of 90%–95%, 13.6% (n=15) in a range of 80%–90%, and only 10% (n=11) showed adherence <80%, as shown in .
Figure 1 Distribution of patients according to level of adherence.
We also analyzed whether any of the demographic and clinical variables of patients was related to treatment adherence. No statistically significant differences in adherence were detected regarding sex (P=0.992), age (rs=0.183, P=0.055), the stage of disease (P=0.732), baseline EDSS score (rs=0.014, P=0.886), time of disease progression (rs=0.035, P=0.716), or previous treatment (P=0.496).
By contrast, other significant adherence differences were found among various prescription patterns (P=0.003) with a higher median adherence at 44 µg/week (100%, IQR: 94.7–100.0), followed by 44 µg/three times a week (96.5%, IQR: 91.2–98.6), 44 µg/two times a week (91.5%, IQR: cannot be calculated because there are only three cases in this category), and finally 22 µg/three times a week (91.1%, IQR: 74.4–96.6). We also observed statistically significant, weak, and directly proportional linear correlations between adherence and the number of doses administered (rs=0.274, P=0.004) and adherence and the number of days of exposure to Rebismart® (rs=0.287, P=0.002). Finally, discontinuation of treatment was another variable that showed a statistically significant association with adherence (P=0.002), with lower median adherence (94.3%, IQR: 88.2–97.2) in those patients who discontinued treatment versus those who continued it (97.7%, IQR: 92.3–99.5).
As most of the patients (79.1%) included in this study had a posology of 44 µg/three times a week, we also analyzed the same demographic and clinical variables to determine whether adherence was affected. Similar results were obtained when we compared either sex (P=0.854), age (rs=0.204, P=0.058), stage of disease (P=0.682), baseline EDSS score (rs=0.009, P=0.935), time of disease of progression (rs=0.034, P=0.757), and previous treatment (P=0.714). Moreover, the same significant adherence differences were obtained when we ana-lyzed the number of doses administered (rs=0.408, P=0.000), number of exposure days to Rebismart® (rs=0.291, P=0.006), and discontinuation of treatment (P=0.008).
Effectiveness of treatment
A total of 22.7% (n=25) of patients experienced a relapse during treatment with INF β-1a, with 2 being the maximum number of relapses in three patients. The average annual rate of relapses in our study cohort was 0.14 (SD: 0.33).
Binary logistic regression was used to analyze whether any of the variables collected was related to the occurrence of relapses. The only statistically significant relationships observed were with age at diagnosis (P=0.004), age at start of treatment (P=0.004), adherence in the first 6 months of treatment (P=0.010), and total adherence (P=0.027). In terms of age at diagnosis and early treatment, younger patients were those who were most likely to present a relapse during treatment. Regarding adherence, the data support the conclusion that adherent patients were less likely to develop a relapse (odds ratio [OR]: 0.953; 95% confidence interval [95% CI]: 0.912–0.995). Specifically, for each percentage unit increase in adherence, the likelihood of a relapse decreased by 4.7%. In addition, there was also a statistically significant weak and inversely proportional linear correlation between adherence and the annual relapse rate (rs=−0.244, P=0.018).
Safety profile
The most common side effects were flu-like syndrome (35.5%) and skin manifestations such as erythema (33.6%) and nodules (12.7%), as shown in . Most patients (60%) had adverse effects that were mild, with 18.2% (20 of 110) requiring a decrease in dose and 10.9% (12 of 110) discontinuation of treatment. Specifically, in these 12 patients, adverse effects responsible for the interruption of INF β-1a were nontolerance of flu-like syndrome (n=6), persistent hypertransaminasemia (n=4), leukopenia (n=1), and generalized urticarial lesions (n=1).
Table 3 Adverse reactions reported in patients
Suspensions of treatment
A total of 38.2% (n=42) of patients in treatment discontinued due to disease progression or relapse (11.8%), side effects (10.9%), pregnancy (8.2%), or change to oral treatments (7.3%). Of the 42 patients who discontinued treatment, 9 did so early (<6 months of treatment) due to adverse effects (n=4), relapses (n=3), and pregnancy (n=2).
A total of 78.6% (n=33) of patients who discontinued INF β-1a switched to other treatments: teriflunomide (n=10), glatiramer acetate (n=7), fingolimod (n=7), dimethyl fumarate in clinical trial (n=3), natalizumab (n=2), siponimod in clinical trial (n=2), Betaferon® (n=1), or cyclophosphamide (n=1).
Discussion
Treatment adherence is a key factor for ensuring the effectiveness and efficiency of DMDs in MS patients. The literature describes a wide range of adherence to DMDs that fundamentally depends on the measurement method used and the duration of the observation periods. The highest adherences described were obtained in patients who administered INF β-1a with the electronic Rebismart® autoinjector device, reaching 97% in the study by Bayas et al,Citation12 95% in Moccia et al,Citation22 or 92.6% in a very recently published article by Fernández et al.Citation23 These data are consistent with those obtained in this study, in which the median adherence to INF β-1a was 96.5%. The high percentage of compliance of the group of patients included in this study could be due to some particular Rebismart® characteristics. First, new electronic injection devices have been developed with the purpose to overcome specific problems associated with the injection (needle phobias, anxiety, pain at the injection site, reduced manual dexterity, and visual or cognitive impairment). When compared with manual injections, autoinjectors have been demonstrated to improve the injection tolerability and thus the satisfaction of the subject.Citation19 Second, Rebismart® incorporates a software that records injection details, providing the physician with objective data to monitor patient’s adherence and helping patients to avoid missed injections.Citation12 With this device, patients can experience what is known as the “Hawthorne effect”, in which patients change their behavior when they feel observed or monitored.Citation24 In this case, the patients knew that the Rebismart® device was recording all doses, and the data were reviewed by the Department of Neurology and Hospital Pharmacy. In addition, patients were informed of the instruction dated July 8, 2013, from the Department of Health of the Valencia Health Agency stating that it was mandatory that the patient bring the autoinjector device each time they come to the hospital pharmacy to pick up medication in order to facilitate monitoring of compliance in each dispensation.
Hence, the high adherence rates obtained with this device (provided free of charge by the pharmaceutical industry) help to make it an cost-effective device, since increased adherence was associated with a decrease in health care resources use, such as MS-related hospitalizations and emergency department visits. Lizán et alCitation25 estimated that this reduction in resource use led to a patient/year total cost reduction (excluding treatment cost) of up to 22%.
Among the most common reasons for failure in MS patients treated with interferon injections was forgetfulness.Citation26,Citation27 In this regard, the adherence of our patients was inversely correlated with the reduction in the frequency of injections. Patients with a prescribed weekly regimen were more adherent to treatment than those who had to inject several times a week, as described by Devonshire et al.Citation27 Therefore, it seems obvious that the availability of pegylated interferons of which the dosage is fortnightly could potentially improve adherence in our cohort of patients. Moreover, the presence of adverse effects is another common cause of lack of adherence. The analysis of our patients showed 82.73% of patients with adverse effects, the majority being mild, favoring no dose losses, and finding no statistically significant differences between adherence of patients with and without adverse reactions. This high number of side effects is consistent with the work by Fernández et al,Citation8 which reported that the majority of patients (77.5%) showed adverse effects, mainly cutaneous. However, some studies have found that events related to the injection site, anxiety about having to inject the drug or needle phobia, led patients to skip doses or even interrupt their treatment.Citation27,Citation28 For this reason, interventions aimed at facilitating self-injection and oral therapies may improve adherence in these patients.Citation27
Treatment with INF β-1a was effective, and 77.3% of the study population did not present any relapse during the course of using the drug, with median treatment duration of 979 days. These data are similar to those obtained by Bayas et al,Citation12 describing 79.5% of patients free of relapses for 12 months and much higher than the reported rates of 66.8% at 48 weeks and 53.3% at 96 weeks with the same formulation of IFN β-1a administered manually or using an autoinjector, respectively.Citation29,Citation30 Our annual relapse rate was half of that obtained by Bayas et alCitation12 after a follow-up of 12 months or even less if the patient stopped treatment with INF β-1a early.
In our study, the occurrence of relapses was correlated with adherence to INF-β 1a, with adherent patients less likely to develop a relapse. These findings are consistent with two previous retrospective studies. Oleen-Burkey et alCitation16 found that with increasing adherence (measured as medication possession ratio [MPR]) to glatiramer acetate decreased the odds of a relapse. Steinberg et alCitation13 compared the relapses of interferon-adherent patients (MPR >85%) with nonadherent patients and found an inversely proportional relationship between adherence and relapses. In the same vein but with a prospective study, Cohen et alCitation31 showed that patients treated with interferon or glatiramer acetate with adherence rates ≥0.9 were more likely to be free of relapses than patients with adherence rates ≤0.5.
In summary, adherence data captured objectively by the autoinjector, avoiding errors or recording oversights by the patient, allow us to have a high reliability of reported adherence and to characterize our population. We have managed to correlate high compliance with a high proportion of relapse-free patients and with very low relapse rates.
Limitations
Adherence to DMDs in MS is associated with better financial and health outcomes as a result of reduced risk of relapses, visits to emergency rooms, hospital admissions, and medical costs in general.Citation14,Citation15,Citation32 In our study, we have reviewed only the presence/absence of relapses as far as health outcomes are concerned, and most of these were resolved with megadoses of corticosteroids in a neurologist’s office or outpatient clinic without hospitalization. Quantification of the costs associated with medication to treat relapses and the use of other hospital resources have not been assessed.
Acknowledgments
The abstract of this paper was presented at the 19th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) as a poster presentation with interim findings. The poster’s abstract was published in Value in Health, vol. 19, issue 7, A435. http://dx.doi.org/10.1016/j.jval.2016.09.512.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeinshenkerBGBassBRiceGPThe natural history of multiple sclerosis: a geographically based study. I. Clinical course and disabilityBrain1989112pt 11331462917275
- JacobsLDCookfairDLRudickRAIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG)Ann Neurol19963932852948602746
- JohnsonKPBrooksBRCohenJACopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study GroupNeurology1995457126812767617181
- KapposLTraboulseeAConstantinescuCLong-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MSNeurology200667694495317000959
- PRISMS Study Group and the University of British Columbia MS/MRI Analysis GroupPRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MSNeurology200156121628163611425926
- De GeestSSabateEAdherence to long-term therapies: evidence for actionEur J Cardiovasc Nurs20032432314667488
- ReynoldsMWStephenRSeamanCRajagopalanKPersistence and adherence to disease modifying drugs among patients with multiple sclerosisCurr Med Res Opin201026366367420070144
- FernándezOAgueraEIzquierdoGAdherence to interferon beta-1b treatment in patients with multiple sclerosis in SpainPLoS One201275e3560022615737
- MenzinJCaonCNicholsCWhiteLAFriedmanMPillMWNarrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosisJ Manag Care Pharm2013191 suppl AS24S4023383731
- TremlettHLOgerJTen years of adverse drug reaction reports for the multiple sclerosis immunomodulatory therapies: a Canadian perspectiveMult Scler20081419410517881392
- WillisHWebsterJLarkinAMParkesLAn observational, retrospective, UK and Ireland audit of patient adherence to subcutaneous interferon beta-1a injections using the RebiSmart((R)) injection devicePatient Prefer Adherence2014884385124966669
- BayasAOualletJCKallmannBAdherence to, and effectiveness of, subcutaneous interferon beta-1a administered by RebiSmart® in patients with relapsing multiple sclerosis: results of the 1-year, observational SMART studyExpert Opin Drug Deliv20151281239125026098143
- SteinbergSCFarisRJChangCFChanATankersleyMAImpact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort studyClin Drug Investig201030289100
- TanHCaiQAgarwalSStephensonJJKamatSImpact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosisAdv Ther2011281516121153000
- IvanovaJIBergmanREBirnbaumHGPhillipsALStewartMMeleticheDMImpact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the USJ Med Econ201215360160922376190
- Oleen-BurkeyMADorACastelli-HaleyJLageMJThe relationship between alternative medication possession ratio thresholds and outcomes: evidence from the use of glatiramer acetateJ Med Econ201114673974721913796
- Santolaya PerrinRFernandez-Pacheco Garcia ValdecasasMArteche EguizabalLAdherence to treatment in multiple sclerosisFarm Hosp201236312412921798780
- LugaresiAAddressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation?Expert Opin Drug Deliv200969995100219637982
- CramerJACuffelBJDivanVAl-SabbaghAGlassmanMPatient satisfaction with an injection device for multiple sclerosis treatmentActa Neurol Scand2006113315616216441244
- DevonshireVArbizuTBorreBPatient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb studyBMC Neurol2010102820433746
- LugaresiADurastantiVGasperiniCCoSa Study GroupSafety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: the CoSa studyClin Neuropharmacol200831316717218520983
- MocciaMPalladinoRRussoCHow many injections did you miss last month? A simple question to predict interferon beta-1a adherence in multiple sclerosisExpert Opin Drug Deliv201512121829183526371561
- FernándezOArroyoRMartinez-YelamosSLong-term adherence to IFN Beta-1a treatment when using RebiSmart(R) device in patients with relapsing-remitting multiple sclerosisPLoS One2016118e016031327526201
- ZellerASchroederKPetersTJAn adherence self-report questionnaire facilitated the differentiation between nonadherence and nonre-sponse to antihypertensive treatmentJ Clin Epidemiol200861328228818226752
- LizánLComellasMPazSPovedaJLMeleticheDMPolancoCTreatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literaturePatient Prefer Adherence201481653166425525341
- TreadawayKCutterGSalterAFactors that influence adherence with disease-modifying therapy in MSJ Neurol2009256456857619444532
- DevonshireVLapierreYMacdonellRGAP Study GroupThe Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosisEur J Neurol2011181697720561039
- TremlettHVan der MeiIPittasFAdherence to the immu-nomodulatory drugs for multiple sclerosis: contrasting factors affect stopping drug and missing dosesPharmacoepidemiol Drug Saf200817656557618395883
- GiovannoniGBarbarashOCasset-SemanazFRNF Study GroupImmunogenicity and tolerability of an investigational formulation of interferon-beta1a: 24- and 48-week interim analyses of a 2-year, single-arm, historically controlled, phase IIIb study in adults with multiple sclerosisClin Ther20072961128114517692727
- GiovannoniGBarbarashOCasset-SemanazFRebif New Formulation Study GroupSafety and immunogenicity of a new formulation of interferon beta-1a (Rebif New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week resultsMult Scler200915221922818755819
- CohenBACoylePKLeistTOleen-BurkeyMASchwartzMZwibelHTherapy optimization in multiple sclerosis: a cohort study of therapy adherence and risk of relapseMult Scler Relat Disord201541758225787057
- ThomasNPCurkendallSFarrAMYuEHurleyDThe impact of persistence with therapy on inpatient admissions and emergency room visits in the US among patients with multiple sclerosisJ Med Econ201619549750526706292
