Abstract
Purpose
Poor adherence to tyrosine kinase inhibitors (TKIs) could compromise the control of chronic myeloid leukemia (CML) and contributes to poorer survival. Little is known about how medication-related issues affect CML patients’ adherence to TKI therapy in Malaysia. This qualitative study aimed to explore these issues.
Patients and methods
Individual face-to-face, semistructured interviews were conducted at the hematology outpatient clinics of two medical centers in Malaysia from August 2015 to January 2016. CML patients aged ≥18 years who were prescribed a TKI were invited to participate in the study. Interviews were audio-recorded, transcribed verbatim, and thematically analyzed.
Results
Four themes were identified from 18 interviews: 1) concerns about adverse reactions to TKIs, 2) personal beliefs regarding the use of TKIs, 3) mismanagement of TKIs in daily lives, and 4) financial burden in accessing treatment. Participants skipped their TKIs due to ineffective emesis control measures and perceived wastage of medication from vomiting. Participants also modified their TKI therapy due to fear of potential harm from long-term use, and stopped taking their TKIs based on belief in curative claims of traditional medicines and misconception about therapeutic effects of TKIs. Difficulty in integrating the dosing requirements of TKIs into daily lives led to unintentional skipping of doses, as well as the risk of toxicities from inappropriate dosing intervals or food interactions. Furthermore, financial constraints also resulted in delayed initiation of TKIs, missed clinic appointments, and treatment interruptions.
Conclusion
Malaysian CML patients encountered a range of medication-related issues leading to a complex pattern of nonadherence to TKI therapy. Further studies should investigate whether regular contact with patients to improve understanding of treatment rationale, to elicit and address patients’ concerns about adverse reactions, and to empower patients with skills to self-manage their medications might promote better adherence to TKIs and improve CML patients’ outcome.
Introduction
Chronic myeloid leukemia (CML) is a rare type of cancer that occurs in 0.7–1.8 per 100,000 population annually.Citation1 Owing to effective treatment with tyrosine kinase inhibitors (TKIs), CML has become a chronic disease with a rising prevalence globally, expected to plateau at 35 times its annual incidence by 2050.Citation1 In Malaysia, ~740 new cases of leukemia are diagnosed annually,Citation2 of which CML accounts for 15%. Since the first TKI, imatinib, was launched in Malaysia in 2003, the overall survival rate of CML patients has reached 94.3% at 10 years.Citation3 It is estimated that about 1,500 CML patients are seeking treatment in Malaysia. Although the possibility of stopping TKI therapy in CML patients who have achieved deep molecular responses is a topic of active debate and investigation,Citation4,Citation5 lifelong treatment remains the current standard of care.Citation6
Although the survival of CML patients has increased markedly, some patients are not able to achieve a satisfactory response to a given TKI, while others respond to TKIs initially but the response is later lost.Citation6 These suboptimal treatment responses have been attributed to genetic variation in cellular drug uptake, development of genetic mutations, and poor adherence to TKIs.Citation6 It has been estimated that 3%–56% of CML patients are not adherent to their prescribed TKI therapy.Citation7 Several studies have indicated that poor adherence to TKIs compromises disease control,Citation8,Citation9 contributes to a higher mortality,Citation10 and also increases health care resource utilization and the economic burden.Citation11
A retrospective study in the United States of America found that CML patients on long-term TKI therapy are prone to developing certain medication-related issues such as side effects, drug interactions, and poor accessibility due to out-of-pocket payments.Citation12 Health care providers may not be fully aware of the challenges faced by CML patients in managing their TKI therapy. Discrepancies between the perspectives of health care providers and CML patients on adherence to TKIsCitation13,Citation14 and health-related quality of life have also been reported.Citation15 To understand the medicine-taking practices of CML patients, qualitative studies in the United Kingdom, Taiwan, and Australia have demonstrated some psychological factors that facilitate TKI adherence, including faith in clinicians,Citation16 belief in treatment efficacy,Citation17 and comparison to other patients in worse circumstances.Citation14 These studies also found that side effects, forgetfulness, complacency, lack of access to medical advice, and poor communication with health care providers are important barriers to TKIs adherence.Citation14,Citation16 However, given the role of sociocultural context in adherence to long-term medication,Citation18 patient’s perspective influencing adherence to TKIs reported in these developed countries may not be entirely applicable to other countries.
Malaysia is a developing country with a multiethnic population. The median age at diagnosis of CML (48 years)Citation19 is lower than in other countries (57–60 years).Citation1 It has previously been reported that approximately one third of CML patients in Malaysia have suboptimal adherence to imatinib.Citation20 This was significantly correlated with the duration of imatinib supply and also appeared to be linked to the number of concomitant medicines.Citation21 The current study seeks to understand the reasons for nonadherence to TKIs in Malaysian patients with CML by exploring medication-related issues using qualitative methodology.
Methods
Study design and settings
A qualitative approach was adopted to enable participants to express their thoughts freely, allowing the researcher a better opportunity to examine patients’ medication-taking experience in depth.Citation22 Individual face-to-face interviews were conducted at the hematology outpatient clinics of Ampang Hospital (AH) and University Malaya Medical Centre (UMMC) in Malaysia from August 2015 to January 2016.
UMMC is a tertiary teaching hospital partly financed by the Ministry of Education Malaysia. It is located in Kuala Lumpur, the main business and administrative region in Malaysia. AH is a public hospital located in a state adjacent to Kuala Lumpur; this national referral center for hematology is fully funded by the Ministry of Health Malaysia. At the time of the study, ~200 and 70 CML outpatients were obtaining TKIs via the Malaysia Patients Assistance Program at the two medical centers, respectively. Ethical approvals were granted by the Medical Research and Ethics Committee Ministry of Health (NMRR-14-1466-23315) and Medical Ethics Committee of University Malaya Medical Centre (MECID-201411-802) before commencement of the study.
Recruitment of participants
CML patients aged ≥18 years who were prescribed a TKI were invited to participate in the study when waiting at the clinic to see their doctors. Eligible patients were identified by screening the medical records. Patients who had cognitive or psychiatric disorders or who already had evidence of drug resistance were excluded. A purposive sampling strategy was usedCitation23 to include patients with a maximum variation of age groups, education levels, and treatment responses, and thus allowed a diverse range of medication-taking experiences.
Data collection
Semistructured interviews to explore participants’ experiences with TKI therapy were conducted in a room adjacent to the clinic before or after their appointments with the doctor. Before the interview, participants were provided with an information sheet about the study and informed of the study procedure, including the expected length of the interview (30–60 minutes). All participants provided written informed consent, including permission to audio-record the interview. Demographic information was collected from participants during the interview, and treatment-related information was obtained from their medical records.
All interviews were conducted by a researcher (BKT) as guided by an interview schedule that contained open-ended questions () aimed at exploring the views and preferences, practices, medication-taking issues, and concerns of patients in relation to their ability to use TKIs effectively and safely. This interview schedule was designed on the basis of the relevant literatureCitation24 and consultation with experts (SBT and PCB). It was piloted on three participants and then used in the main interviews with no amendments. Results from the pilot study were included in the final analysis.
Table 1 Interview schedule
Data analysis
A thematic analysis was performed based on the six phases of Braun and Clarke,Citation25 which included the following procedures: 1) all interviews were transcribed verbatim by a researcher who conducted the interviews (BKT) and repeatedly read to gain familiarity with the content. Interviews in Malay and Mandarin were translated into English by a professional translator and verified by BKT. All transcripts were imported into qualitative data analysis software – NVivo (version 10, QSR International Pty Ltd. Victoria, Australia). 2) Initial coding was performed by BKT and reviewed against the transcripts by PCB and SSC. 3) To ensure reliability of the analyses, two researchers (BKT and SB) independently searched for potential themes across the codes. Results of the two analysts were found to be in congruence. 4) Homogeneity of codes within each theme and heterogeneity of codes across the themes were reviewed by other members of the research team to ascertain absence of overlapping categories. Themes not supported by adequate data were collapsed into other relevant themes or discarded. 5) The themes were refined according to the features exhibited by the data. 6) The report was written, and selected quotes of participants were excerpted to support the themes.
Results
Data saturation, as determined by no appearance of new themes, occurred after 18 patients were interviewed. Ten of the participants were recruited out of 132 eligible patients from AH, and eight were recruited out of 59 eligible patients from UMMC. Of the 21 CML patients invited, three refused to participate due to a preference not to recall past treatment experiences (n=1), lack of time (n=1), or mild hearing impairment (n=1). Eight interviews were conducted in English, eight in Malay, and two in Mandarin. The median duration of the interviews was 41 minutes (range: 20–64 minutes).
The age of recruited participants was between 26 and 67 years, with nine aged <50 years, and nine aged <50 years. Participants mostly attained secondary education (n=9) or tertiary education (n=7), while two received primary education. The majority of CML patients were diagnosed in the chronic phase (n=15) with duration ranging from 3 months to 16 years. Most participants were on imatinib (n=13) as first-line therapy, while others had been started on imatinib but had switched over to nilotinib (n=5). Among the 15 participants who had been treated with TKIs for more than a year, disease status was as follows at the time of the interview: one patient had undetectable disease, seven had a major molecular response, six were not at the optimal benchmark of molecular disease control,Citation6 and one was in blast crisis ().
Table 2 Characteristics of participants
Four themes of medication-related issues affecting adherence to TKIs were generated from the perspectives and experiences of the participants as shown in .
Figure 1 Themes of medication-related issues affecting chronic myeloid leukemia patients’ adherence to tyrosine kinase inhibitors (TKIs).
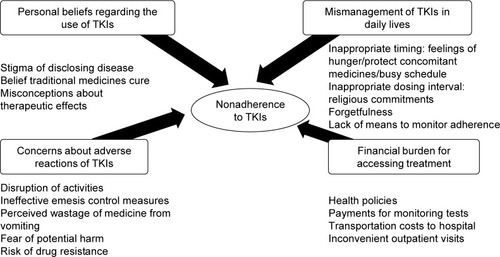
Concerns about adverse reactions to TKIs
Despite participants’ awareness that TKI can save their lives, many were psychologically reluctant to take the medicine because of negative emotions toward adverse effects they experienced or potential harm that they had read or heard about. Some participants delayed their TKI doses as they were worried that vomiting or discomfort may disrupt their daily activities. Participants also complained that supportive therapy such as antiemetic medications given by their treating physicians was not effective. Some even skipped their TKIs to prevent wastage of the medication from poorly controlled vomiting.
There were times that I was sick, even with the smell of medication, I felt nausea. I know the medication is expensive, (it would be) a waste if (I) were to take and vomit back out. I did not take. That’s the root cause that I progressed into stage 3. Unable to feed in the medication. [P16]
It will make you vomit, even if you take the medication that supposed to make you less nausea, but no [giggles] It doesn’t really help. I think the best option will be if they can reduce the side effect. Because that’s the reason why people stop taking medicine. If we take medicine every time then you go vomit, that’s a bit hard. [P17]
Participants who had experienced severe skin reactions expressed great concerns over their safety when rechallenged with the TKI after a gap. Furthermore, when adverse events such as renal failure, chronic back pain, or dysphagia from chronic esophagitis were reported, participants felt certain that the TKI was the cause and expressed ambivalence toward their ongoing therapy. Uncertainties related to the risk of drug resistance on continuous TKI therapy also worried the participants. Driven by the fear of potential harm from long-term TKIs, some participants modified their TKI regimens from routine to intermittent use without disclosing this to their health care providers.
The problem is we do not know when the condition will be stable because some of the drugs will cause resistance of your genes, so your genes at the end will eventually go mutated. And that’s very worrying for me, I don’t know when is the last day I need to take medications. [P12]
I was afraid that this medicine might cause side effects, so I dare not take it. In a week, I will take 5 days, skip 2 days. [P10]
Personal beliefs regarding the use of TKIs
Participants’ perceptions of the need to escalate their TKI dose when advised by their clinician, or to continue with therapy after their condition stabilized, varied and were influenced by their sociocultural background and disease knowledge. For example, to avoid the stigma of disclosing their disease through the visibility of taking TKIs in a workplace, some participants preferred less frequent dosing. Participants were also misled by family members and friends into believing the curative claims of traditional medicines, and some even stopped their CML treatment to try traditional medicines without consulting their health care providers.
The Malays they do believe that traditional medication could help, you know. After I was diagnosed for 2 months, a friend of mine told me that I could take lingzhi from China. Because from the readings that I made, it can cure even. Of course I would want to be cured right, so I told myself maybe this is the right thing. They said but I cannot take it together with the medication from the doctor, so I stopped the medication for a month. Then that’s it, my condition was worsening. [P15]
Many participants had misconceptions that the need to take their TKI could be judged according to their disease signs and symptoms. Consequently, participants experimented with stopping the TKI on their own after regaining their premorbid health status. In particular, one participant repeatedly discontinued his TKI even after relapse of his CML into blast crisis as he did not understand the need to continue therapy when test results were normal. Paradoxically, participants who were informed that they were not responding well to their TKI still claimed it was effective after they perceived some form of improvement in their symptoms. This phenomenon could be due to fear of further therapy, especially bone marrow transplantation.
They said your CML has really relapsed in third stage. I keep pleading, no, pleading, pleading to the doctor. Because I don’t know I’m really into a very serious stage already. Then I followed the advice from the doctors, then I took the medications again. Yes, now I stopped for 2 months already. But everything seems well. They still mention need to be stabilized, but they say so far your number (blood cell counts) seem alright, around that figure [snapped fingers]. [P12]
I’m not sure because by taking this medication, I feel well. Compared to the times I was sick, my weakness has reduced. Stomach has already healed, back to normal. I think this medication gives good effects, but doctor said it does not reflect well on my body. [P18]
Mismanagement of TKIs in daily lives
Participants felt that the dosing requirements of TKIs were too complicated to follow. Some participants who reported nausea on imatinib had not adhered to the requirement to take doses after meals. Instead, they delayed the medication timing on the basis of subjective feelings of hunger or to protect concomitant medications. Other participants revealed not taking their nilotinib on an empty stomach because the time needed for fasting interfered with their busy schedule, thus putting them at risk of adverse drug–food interactions. Some Muslim patients who adjusted their TKI schedules to fulfill religious commitments during the fasting month of Ramadan also reported toxicities due to inappropriate dosing intervals.
Actually nobody advise, doctor said it’s after food, but they didn’t specifically say directly after food. Initially I thought if I take everything together, then when I vomit, everything will come out, no point also I’m taking the medicine. So I just take my diabetes and blood pressure (pills) first, then after 1 hour only I take my Gleevac. So it [chuckles] it doesn’t work like that, I mean it’s getting worse. [P11]
During the fasting month, I took breakfast at 5 o’clock and [I] took [nilotinib]. On the night before, I took it at 11 o’clock, so 6 hours (dosing interval). Its overdose, I can feel my body aching, like what I have experienced initially. [P09]
Participants also expressed difficulties in managing their TKI regimens, leading to unintentional dose skipping. This was often attributed to human nature such as a sudden slip of the mind when distracted, or tiredness. When participants were unsure whether they had taken their TKI, most of them chose not to take it due to concern about overdose, although one participant mentioned the possibility of doubling the dose due to a lack of means to monitor adherence.
It’s just like a night routine for me. But you know sometimes even though it’s a routine, but we tend to forget. Sometimes, I didn’t take the medicine for you know, a few days straight. And when I came to the hospital, the blood test result was not good. [P15]
It’s the feeling that I seem to have taken, but it seems I have not, so at the end I took, maybe sometimes I have doubled (dose). I would just take whichever tablet that I have removed from the blister pack. Since many rows were opened, I have no clue whether I have taken or not. [P10]
Financial burden in accessing treatment
Obstacles in accessing CML treatment due to public health policies were other issues voiced by participants. Two participants, including a foreigner in Malaysia and a citizen who left the country to study abroad, could not be initiated on TKIs until 2 to 3 years into the diagnosis due to payment policy constraints in relation to the high price of TKIs. In addition, those who were not treated in a Ministry of Health facility struggled to cope with the out-of-pocket expenses for polymerase chain reaction tests for monitoring treatment responses to TKIs.
I was actually from Country X, before I got identification card, I was not entitled to Gleevac and Tasigna. So I received Gleevac and Tasigna a bit later. Hydroxyurea cannot change that marker, Gleevac can, but maybe (I was without Gleevac for) too long, so the drug was less [effective]. [P04]
Because the medicine I cannot stop, that’s my worries [chuckles] So how long … right? Now the cost of the hospital has increased. Before [this] my blood test BCR-ABL is free, now I need to pay hundred over. For private we struggle. [P07]
For participants from low-income families, transportation costs associated with travelling long distance to attend treatment follow-ups still posed a huge financial burden despite gaining access to TKIs at no cost. Work commitments also caused some participants to miss scheduled medical assessment, especially frequent monitoring for unstable responses, as well as medication refill visits, thereby resulting in interruptions of their TKI therapy.
Most of the people in village who always fall sick could not go to the hospital, this is the problem. Finance. Now, finance is really needed. We want to go to the hospital, there’s no vehicle, vehicle got to pay, that is difficult. Go once can, second time can, third time cannot go already because of insufficient finance. [P16]
Discussion
This study found that CML patients from two health care facilities in Malaysia encountered a range of medication- related issues. These led to a complex pattern of nonadherence behaviors to TKIs such as dose skipping, self-modification of dosing regimen and schedule, self-discontinuation of therapy, and even overadherence characterized by unintentional doubling of doses or refusal to be taken off ineffective TKIs. The four themes of medication-related issues that emerged from this study ranged from patients’ concerns and misconceptions regarding effects of TKIs through to practical obstacles in handling medication routines and treatment-related out-of-pocket expenses.
It has been reported that 23% of Malaysian CML patients taking imatinib experienced gastrointestinal side effects.Citation19 This accords with previous reports identifying nausea as a major cause of nonadherence to imatinib.Citation16,Citation26 Concern raised by patients over the long-term safety of TKIs is also in keeping with a German survey that found that the fear of harmful effects from TKIs ranked even higher than the fear of CML progression.Citation27 While Chen et alCitation17 reported that knowledge of potential for drug resistance served as a positive motivation for sustaining imatinib adherence among Taiwanese CML patients, the present study showed contradictory findings.
A previous survey found that 24% of patients with hematological cancers in Malaysia use complementary and alternative medicine to cure their cancers.Citation28 Generally, Asians believe that traditional medicines are from natural sources and are therefore safe. It is possible that our finding that some Malaysian CML patients prefer alternative medicine over TKIs could lead to poorer outcomes if they miss being treated in the chronic phase and instead take their TKI treatment only during the advanced phase, when disease burden is higher.Citation29
This study indicated that some Malaysian CML patients have a misconception that TKI therapy needs to be continued only while they are symptomatic. This suggests an incomplete understanding of the disease and also implies that patients tend to focus on immediate treatment experiences rather than potential repercussions since disease progression or treatment failure may not be immediately evident following nonadherence. Similar findings have been reported in other chronic disease states where patients appear less likely to adhere to their medication regimen in the absence of symptoms.Citation30 Scheduling regular patient contacts in between clinic visits with a member of the multidisciplinary health care team such as a pharmacist or a nurse so that potential adherence issues could be recognized, discussed, and addressed in a timely manner can be an effective method of improving adherence.Citation12,Citation31
The results of the present study echo those of a survey in Europe that found that 30% of CML patients reported difficulties in coping with the dietary restrictions and dosing schedule of their TKIs, which in turn correlated negatively with treatment adherence.Citation32 Strict adherence to the fasting requirement of nilotinib is necessary due to potential cardiotoxicity, including sudden cardiac death.Citation33 Therefore, it is essential that patients are fully guided on how to integrate TKIs into their personal schedules, taking into account any concomitant medications, and religious or other routines.
This study also highlighted a lack of means for patients to ascertain their own medication adherence. Developing appropriate patient reminders such as calendar charts or electronic remindersCitation34 may reduce forgetfulness and help patients stay organized with their prescribed TKI regimen, thereby avoiding both untoward consequences of treatment failure and accidental overdose.
In Malaysia, health care facilities that provide cancer services are located mostly in the major cities, and some are partially subsidized by the government. The current study demonstrates that this limits the accessibility and affordability of treatment, both of which are significant barriers to TKI adherence. This is consistent with a Japanese survey that found that CML patients with higher out-of-pocket medical expenses were more likely to discontinue or temporarily stop refilling their imatinib prescriptions.Citation35
This study is the first to focus on medication-related issues among CML patients treated on TKIs in Malaysia using qualitative methodology. The findings of this study offer insights into the issues encountered by CML patients in accessing treatment and in self-administration, self- management, and self-monitoring of TKIs that have important implications for the clinical effectiveness of this therapy. Consequently, measures addressing these issues need to be planned and implemented to support CML patients’ long-term adherence to TKIs.
One of the main limitations of this study lies with the translation of Malay and Mandarin transcripts, as the intended meaning conveyed by the patients may not be precisely relayed in English. In addition, we did not have the capacity to utilize Tamil, which is another major language in our region of Malaysia, so the views and experiences of primarily Tamil-speaking patients have not been studied. We also acknowledge the limited recruitment of patients to only two public hospitals in Malaysia. These limitations make it difficult to generalize the results to the experiences of CML patients receiving TKI therapy in private health care facilities, or from other regions in Malaysia, and constitute potential avenues for future research.
Conclusion
Malaysian CML patients encountered a range of medication-related issues leading to a complex pattern of nonadherence to TKIs. While drug resistance due to mutation is a non-modifiable factor limiting the optimal clinical benefits of TKIs, nonadherence is a major modifiable factor. Improving communication to ensure patients fully understand the significance of the treatment responses for their long-term outcomes, eliciting and addressing patients’ concerns about treatment tolerability and safety, and empowering patients with the skills to self-manage their medications might promote better adherence to TKIs and improve treatment outcome.
Author contributions
BKT, SBT, and LCC designed and BKT performed the study; BKT, SBT, PCB, SSC, SB, LCC, KMC, HNBKJ, and SCE analyzed the data; BKT drafted the manuscript; and LCC, SBT, PCB, KMC, SSC, SB, HNBKJ, and SCE revised the manuscript critically for important intellectual content and approved the final version to be published. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors express their deep gratitude to all patients who participated in the study. They also record their appreciation to Professor Nicholas Jackson from the Department of Pathology, Faculty of Medicine, University of Malaya, for his assistance in editing the manuscript, and to all the staff of the hematology clinics of UMMC and AH for their cooperation and assistance. In addition, they acknowledge the University of Malaya for funding this study under grant PG057-2015A. Lastly, they would like to thank the Director General of Health Malaysia for his permission to publish this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- HoglundMSandinFSimonssonBEpidemiology of chronic myeloid leukaemia: an updateAnn Hematol201594Suppl 2S241S24725814090
- OmarZAIbrahim TaminNSNational Cancer Registry Report 2007Kuala LumpurMinistry of Health Malaysia2011
- BeePCSekaranVNgRRKwehTYGanGGThe predictive value of early molecular response in chronic myeloid leukaemia patients treated with imatinib in a single real-world medical centre in a developing countrySingapore Med J201758311628111689
- MahonFXReaDGuilhotJDiscontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trialLancet Oncol201011111029103520965785
- RossDMBranfordSSeymourJFSafety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER studyBlood2013122451552223704092
- O’BrienSRadichJPAbboudCNChronic myelogenous leukemia, version 1.2014J Natl Compr Canc Netw201311111327134024225967
- NoensLHensenMKucmin-BemelmansIMeasurement of adherence to BCR-ABL inhibitor therapy in chronic myeloid leukemia: current situation and future challengesHaematologica201499343744724598855
- IbrahimAREliassonLApperleyJFPoor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapyBlood2011117143733373621346253
- MarinDBazeosAMahonFXAdherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinibJ Clin Oncol201028142381238820385986
- ChenTCChenLCHuangYBChangCSImatinib adherence associated clinical outcomes of chronic myeloid leukaemia treatment in TaiwanInt J Clin Pharm201436117218124242992
- DarkowTHenkHJThomasSKTreatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemiaPharmacoeconomics200725648149617523753
- LamMSCheungNImpact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemiaJ Oncol Pharm Pract201622674174826419691
- KekaleMTalvensaariKKoskenvesaPPorkkaKAiraksinenMChronic myeloid leukemia patients’ adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physiciansPatient Prefer Adherence201481619162725473270
- WuSCheeDUgaldeAButowPSeymourJSchofieldPLack of congruence between patients’ and health professionals’ perspectives of adherence to imatinib therapy in treatment of chronic myeloid leukemia: a qualitative studyPalliat Support Care201513225526324524212
- EfficaceFBrecciaMSausseleSWhich health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life GroupAnn Hematol20129191371138122543826
- EliassonLCliffordSBarberNExploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribedLeuk Res201135562663021095002
- ChenLCChenTCHuangYBChangCSDisease acceptance and adherence to imatinib in Taiwanese chronic myeloid leukaemia outpatientsInt J Clin Pharm201436112012724154825
- BurkhartPVSabatéEAdherence to long-term therapies: evidence for actionJ Nurs Scholarsh200335320714562485
- BeePCGanGGTaiYTHarisARChinEVeeraSNAn update on imatinib mesylate therapy in chronic myeloid leukaemia patients in a teaching hospital in MalaysiaSingapore Med J2012531576122252185
- BalashankerSChenLCChuaSSEvaluating imatinib adherence in a hospital setting in MalaysiaPoster presented at: EuroDURG ConferenceAugust 27–29, 2014Groningen, Netherlands
- BalashankerSChenLCTaylorCADeterminants of Patient Adherence to ImatinibPharmacoepidemiol Drug Saf201524S1493
- KuperAReevesSLevinsonWAn introduction to reading and appraising qualitative researchBMJ2008337a28818687727
- PalinkasLAHorwitzSMGreenCAWisdomJPDuanNHoagwoodKPurposeful sampling for qualitative data collection and analysis in mixed method implementation researchAdm Policy Ment Health201542553354424193818
- GaterAHeronLAbetz-WebbLAdherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemiaLeuk Res201236781782522364811
- BraunVClarkeVUsing thematic analysis in psychologyQual Res Psychol20063277101
- EfficaceFRostiGCottoneFProfiling chronic myeloid leukemia patients reporting intentional and unintentional non-adherence to lifelong therapy with tyrosine kinase inhibitorsLeuk Res201438329429823906625
- HefnerJCsefEJKunzmannVFear of progression in outpatients with chronic myeloid leukemia on oral tyrosine kinase inhibitorsOncol Nurs Forum201643219019726906130
- GanGGLeongYCBeePCComplementary and alternative medicine use in patients with hematological cancers in MalaysiaSupport Care Cancer20152382399240625876158
- PalandriFCastagnettiFTestoniNChronic myeloid leukemia in blast crisis treated with imatinib 600 mg: outcome of the patients alive after a 6-year follow-upHaematologica200893121792179618838477
- HalmEAMoraPLeventhalHNo symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthmaChest2006129357358016537854
- MoonJHSohnSKKimSNPatient counseling program to improve the compliance to imatinib in chronic myeloid leukemia patientsMed Oncol20122921179118521472487
- HirjiIGuptaSGorenAChronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspectiveHealth Qual Life Outcomes20131116724099272
- BaerMNilotinib risk evaluation and mitigation strategyClin Adv Hematol Oncol20119753954022402461
- VervloetMLinnAJvan WeertJCde BakkerDHBouvyMLvan DijkLThe effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literatureJ Am Med Inform Assoc201219569670422534082
- KodamaYMorozumiRMatsumuraTIncreased financial burden among patients with chronic myelogenous leukaemia receiving imatinib in Japan: a retrospective surveyBMC Cancer20121215222530992
