Abstract
Background
Pulmonary arterial hypertension (PAH) is a devastating disease and ultimately leads to right heart failure and premature death. A total of four classical targeted drugs, prostanoids, endothelin receptor antagonists (ERAs), phosphodiesterase 5 inhibitors (PDE-5Is), and soluble guanylate cyclase stimulator (sGCS), have been proved to improve exercise capacity and hemodynamics compared to placebo; however, direct head-to-head comparisons of these drugs are lacking. This network meta-analysis was conducted to comprehensively compare the efficacy of these targeted drugs for PAH.
Methods
Medline, the Cochrane Library, and other Internet sources were searched for randomized clinical trials exploring the efficacy of targeted drugs for patients with PAH. The primary effective end point of this network meta-analysis was a 6-minute walk distance (6MWD).
Results
Thirty-two eligible trials including 6,758 patients were identified. There was a statistically significant improvement in 6MWD, mean pulmonary arterial pressure, pulmonary vascular resistance, and clinical worsening events associated with each of the four targeted drugs compared with placebo. Combination therapy improved 6MWD by 20.94 m (95% confidence interval [CI]: 6.94, 34.94; P=0.003) vs prostanoids, and 16.94 m (95% CI: 4.41, 29.47; P=0.008) vs ERAs. PDE-5Is improved 6MWD by 17.28 m (95% CI: 1.91, 32.65; P=0.028) vs prostanoids, with a similar result with combination therapy. In addition, combination therapy reduced mean pulmonary artery pressure by 3.97 mmHg (95% CI: −6.06, −1.88; P<0.001) vs prostanoids, 8.24 mmHg (95% CI: −10.71, −5.76; P<0.001) vs ERAs, 3.38 mmHg (95% CI: −6.30, −0.47; P=0.023) vs PDE-5Is, and 3.94 mmHg (95% CI: −6.99, −0.88; P=0.012) vs sGCS. There were no significant differences in all-cause mortality and severe adverse events between prostanoids, ERAs, PDE-5Is, sGCS, combination therapy, and placebo.
Conclusion
All targeted drugs for PAH are associated with improved clinical outcomes, especially combination therapy. However, all these drugs seem to show less favorable effects on survival in the short-term follow-up, suggesting further clinical trials are required.
Introduction
Pulmonary arterial hypertension (PAH) is a life-threatening disease associated with elevated pulmonary vascular resistance (PVR), ultimately leading to right heart failure and premature death.Citation1 Recent data from the National Institutes of Health in the United StatesCitation2 showed that the five-year survival rate from the time of a diagnostic right-sided heart catheterization was only 57%,Citation3 and the treatments for PAH were very limited and expensive. Apart from the use of support measures (such as long-term oxygen therapy, diuretics, oral anticoagulants, and digoxin), targeted therapies including prostanoids, endothelin receptor antagonists (ERAs), phosphodiesterase 5 inhibitors (PDE-5Is), and soluble guanylate cyclase stimulator (sGCS) are also recommended by current guidelines and expert consensus.Citation1,Citation4,Citation5 These targeted drugs have been proven to alleviate symptoms and to improve exercise capacity and hemodynamics compared to placebo by several randomized controlled clinical trials (RCTs)Citation6–Citation11 and meta-analyses.Citation12–Citation14 However, direct head-to-head comparisons are lacking, and traditional meta-analysis methods do not allow adequate assessment of the comparative effectiveness of all therapies. Therefore, we performed this network meta-analysis of all relevant randomized clinical trials to comprehensively compare the efficacy of targeted drugs for PAH treatment.
Methods
Literature search
Literature comparing targeted drugs for patients with PAH was acquired through searching Medline, EMBASE, and Cochrane Controlled Trials Registry data from January 1990 to December 2015. In order to search and include all relevant studies, we used combinations of various key words, including: pulmonary arterial hypertension, pulmonary hypertension, prostanoids, prostacyclin analogues, ERAs, PDE-5Is, sGCS, epoprostenol, iloprost, beroprost, treprostinil, bosentan, ambrisentan, macitentan, sildenafil, tadalafil, vardenafil, riociguat, and clinical trial. References from reviews and selected articles were further screened.
Inclusion and exclusion criteria
The inclusion criteria were: 1) the study was a RCT; 2) the sample population was definitely diagnosed as PAH according to current guidelines; 3) at least one of the prostanoids (epoprostenol, iloprost, beroprost, and treprostinil), ERAs (bosentan, ambrisentan, and macitentan), PDE-5Is (sildenafil, tadalafil, and vardenafil), sGCS (riociguat), and combination therapy were used, regardless of drug dosage forms; and 4) there was at least 8 weeks clinical follow-up. The following were excluded: 1) non-English language studies; 2) studies with duplicate publication, or different studies from the same sample origin; 3) crossover, 2×2 factorial, and pediatric studies; and 4) studies of sitaxsentan as it has been withdrawn from the market due to severe liver toxicity.
Data extraction and quality assessment
All relevant articles were independently reviewed by two investigators (GXF and ZJJ) to assess the eligibility of the article and abstract with standardized data abstraction forms, and disagreement was resolved by a third investigator (JXM). The following data were extracted from each included study: study’s name, first author, publication date, baseline demographics, and clinical outcomes at follow-up. The quality of the retrieved studies was assessed by Jadad score.Citation15
Study end points
The primary effective end point of this network meta-analysis was a 6-minute walk distance (6MWD). The secondary end points included mean pulmonary arterial pressure (mPAP), PVR, all-cause mortality, and clinical worsening events. The definitions of clinical worsening events were slightly different across studies, and we used the trial-specific definitions for each study. The safety end point was severe adverse events (SAEs), mainly including PAH-related adverse events and treatment-related adverse events.
Statistical analysis
This network meta-analysis was conducted with Stata software 14.0 (StataCorp, College Station, TX, USA) using the network family of commands.Citation16–Citation19 The network meta-analysis was performed to obtain estimates for outcomes of primary and secondary end points, presented as odds ratios (OR) for dichotomous variables or weighted mean differences (WMD) for continuous variables with 95% confidence intervals (CIs). The plot of a network of drugs was a visual representation of the evidence base and offered a concise description of its characteristics. It consisted of nodes representing the drugs being compared and edges representing the available direct comparisons (comparisons evaluated in at least one study) between pairs of drugs.Citation16 The common heterogeneity was explored in every network by comparing the magnitude of the τ value for the network with an empirical distribution of heterogeneity variances specific to the types of outcome and treatments. An inconsistency model was used only when the P-value of χ2 test was more than 0.05. Otherwise, a consistency model was performed.Citation17,Citation19 A node-splitting method was also used, separating evidence for a particular comparison into direct and indirect evidence, excluding one direct comparison at a time, and estimating the indirect treatment effect for the excluded comparison.Citation18 All P-values were two-tailed with the statistical significance set at <0.05.
Results
Eligible studies and patient characteristics
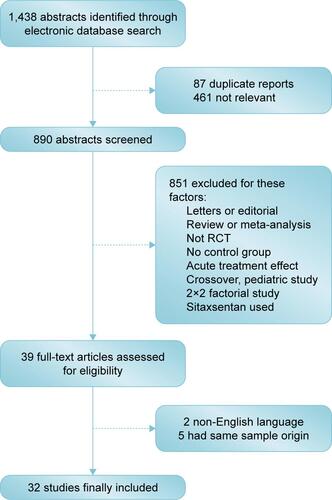
Thirty-two eligible studiesCitation2,Citation6–Citation11,Citation20–Citation44 including a total of 6,758 patients, were identified in the present network meta-analysis (). The quality of each study was good according to the Jadad score.
The baseline characteristics are summarized in . Four important classes of drugs for PAH (prostanoids, ERAs, PDE-5Is, and sGCS) and combination therapy were compared with placebo and with each other. Networks of eligible comparisons for the primary outcomes (6MWD) are presented in . Most enrolled patients were female and in the New York Heart Association/World Health Organization (NYHA/WHO) Functional Class II/III. The predominant etiology was idiopathic and/or familial PAH, and the median period of study follow-up was 16 weeks (range from 8 to 115 weeks).
Figure 1 Network of available drugs for PAH.
Abbreviations: ERAs, endothelin receptor antagonists; PAH, pulmonary arterial hypertension; PDE-5Is, phosphodiesterase 5 inhibitors; sGCS, soluble guanylate cyclase stimulator.
6MWD
A 6MWD was reported in 32 RCTs. As shown in , there was a statistically significant improvement in 6MWD associated with each of the four drug classes compared with placebo, with a consistency model used (τ=8.72, χ2=7.79, P=0.455). Prostanoids, ERAs, and sGCS showed a comparable 6MWD for PAH patients. Compared with prostanoids, PDE-5Is improved 6MWD by 17.28 m (95% CI: 1.91, 32.65; P=0.028), meanwhile combination therapy improved it by 20.94 m (95% CI: 6.94, 34.94; P=0.003). Furthermore, combination therapy was superior to ERAs for improving 6MWD by 16.94 m (95% CI: 4.41, 29.47; P=0.008).
Figure 2 Pooled WMD and 95% CIs determined by network meta-analysis for 6MWD of targeted drugs (A) or oral targeted drugs (B) for PAH.
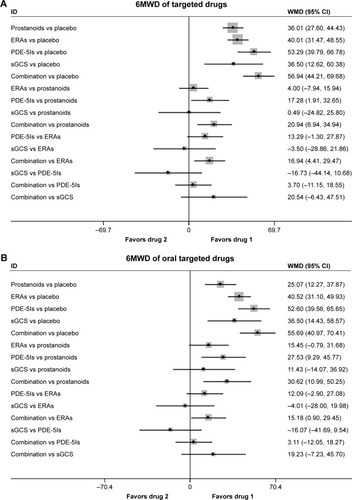
In 22 studies, oral targeted drugs including oral prostanoids, oral ERAs, oral PDE-5Is, sGCS, and combination therapy were also associated with a more improved 6MWD than placebo, with a consistency model used (τ=7.37, χ2=11.2, P=0.130; ). Compared with oral prostanoids, oral PDE-5Is and combination therapy improved 6MWD by 27.53 m (95% CI: 9.29, 45.77; P=0.003) and 30.62 (95% CI: 10.99, 50.25; P=0.002), respectively.
Mean pulmonary artery pressure and pulmonary vascular resistance
There were significant reduced mPAP found in prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy (17 studies, ), with an inconsistency model used (τ=0.27, χ2=8.65, P=0.003). ERAs increased mPAP by 4.27 mmHg (95% CI: 1.03, 7.51; P=0.010) than prostanoids, while PDE-5Is had a similar mPAP change compared with prostanoids, ERAs, and sGCS. In addition, combination therapy reduced mPAP by 3.97 mmHg (95% CI: −6.06, −1.88; P<0.001) vs prostanoids, 8.24 mmHg (95% CI: −10.71, −5.76; P<0.001) vs ERAs, 3.38 mmHg (95% CI: −6.30, −0.47; P=0.023) vs PDE-5Is, and 3.94 mmHg (95% CI: −6.99, −0.88; P=0.012) vs sGCS.
Figure 3 Pooled WMD and 95% CIs determined by network meta-analysis for mean pulmonary artery pressure of targeted drugs (A) or oral targeted drugs (B) for PAH. Pooled WMD and 95% CIs determined by network meta-analysis for pulmonary vascular resistance of targeted drugs (C) or oral targeted drugs (D) for PAH.
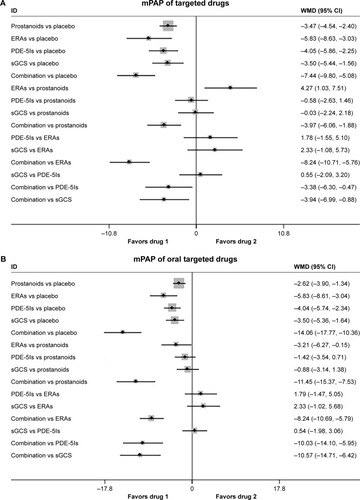
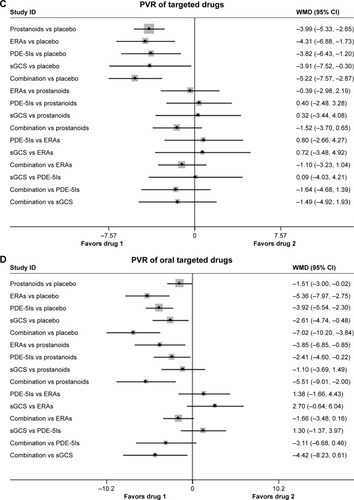
As shown in (n=11 studies), oral targeted drugs could decrease mPAP significantly more than placebo, with no significant heterogeneity found (τ≈0). Of note, oral prostanoids, oral ERAs, oral PDE-5Is, and sGCS had approximately comparable mPAP changes with each other. Combination therapy was associated with reduced mPAP by 11.45 mmHg (95% CI: −15.37, −7.53; P<0.001) vs prostanoids, 8.24 mmHg (95% CI: −10.69, −5.79; P<0.001) vs ERAs, 10.03 mmHg (95% CI: −14.10, −5.95; P<0.001) vs PDE-5Is, and 10.57 mmHg (95% CI: −14.71, −6.42; P<0.001) vs sGCS.
In 16 studies, targeted drugs including prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy were associated with more reduced PVR than placebo, with a consistency model used, regardless of the forms of drug administration (τ=1.71, χ2=0.36, P=0.550; ; τ=1.01, χ2=3.17, P=0.075; ). Moreover, the mean values of the reduction in PVR were ~14.7%, 9.8%, 19.2%, 28.2%, and 22.4% for prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy respectively (~ mean 17.5% for single targeted drug), while the values of PVR were increased by 3.4% in terms of placebo. However, prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy were comparable in reduced PVR with each other.
All-cause mortality
All-cause mortality was reported in 24 studies. There were no significant differences in all-cause mortality between prostanoids, ERAs, PDE-5Is, sGCS, combination therapy, and placebo (odds ratio [OR]: 0.59, 95% CI: 0.34, 1.03; P=0.063 for prostanoids; OR: 0.65, 95% CI: 0.29, 1.46; P=0.295 for ERAs; OR: 0.60, 95% CI: 0.18, 1.97; P=0.397 for PDE-5Is; OR: 0.39, 95% CI: 0.07, 2.08; P=0.271 for sGCS; OR: 0.54, 95% CI: 0.17, 1.74; P=0.301 for combination therapy; respectively; ), with a consistency model used (τ=0.21, χ2=4.69, P=0.320).
Figure 4 Pooled OR and 95% CIs determined by network meta-analysis for all-cause mortality of targeted drugs (A) or oral targeted drugs (B) for PAH.
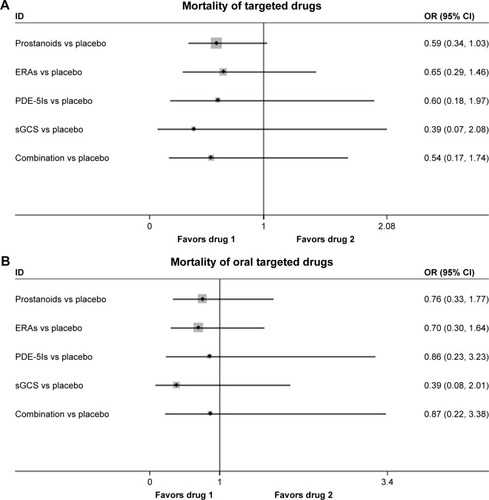
Among the 15 studies, oral prostanoids, oral ERAs, oral PDE-5Is, sGCS, and combination therapy could not decrease all-cause mortality more than placebo (OR: 0.76, 95% CI: 0.33, 1.77; P=0.528 for oral prostanoids; OR: 0.70, 95% CI: 0.30, 1.64; P=0.415 for oral ERAs; OR: 0.86, 95% CI: 0.23, 3.23; P=0.824 for oral PDE-5Is; OR: 0.39, 95% CI: 0.08, 2.01; P=0.262 for sGCS; OR: 0.87, 95% CI: 0.22, 3.38; P=0.840 for combination therapy, respectively; ), with a consistency model used (τ=0.14, χ2=3.06, P=0.383).
Clinical worsening events
In 22 studies, there were significantly lower rates of clinical worsening events in prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy than in placebo (), with a consistency model used (τ=0.30, χ2=8.09, P=0.325). However, prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy were comparable in clinical worsening events with each other.
Figure 5 Pooled OR and 95% CIs determined by network meta-analysis for clinical worsening events of targeted drugs (A) or oral targeted drugs (B) for PAH.
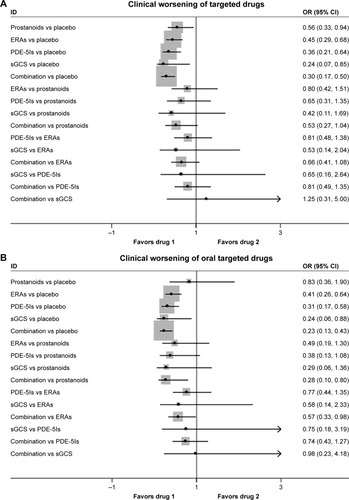
Oral ERAs, oral PDE-5Is, sGCS, and combination therapy were associated with reduced clinical worsening events than placebo, while oral prostanoids had similar events with placebo (18 studies, ), with a consistency model used (τ=0.33, χ2=4.87, P=0.561). Oral prostanoids, oral ERAs, oral PDE-5Is, and sGCS were comparable with each other. Moreover, combination therapy improved clinical worsening events more than oral prostanoids (OR: 0.28, 95% CI: 0.10, 0.80; P=0.017) and oral ERAs (OR: 0.57, 95% CI: 0.33, 0.98; P=0.044).
SAEs
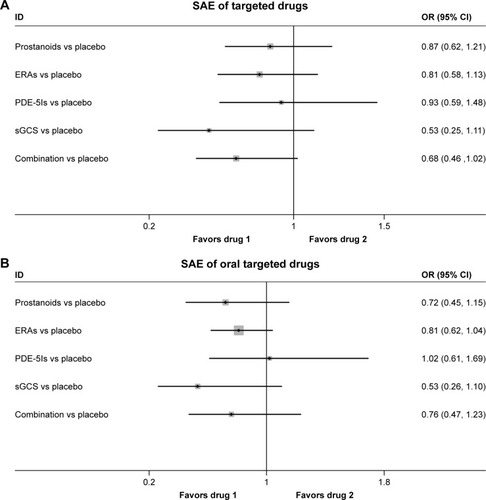
A total of 19 studies evaluated the safety of targeted drugs by assessing the SAEs. The incidences of SAEs were similar, and the differences were not statistically significant between targeted drugs and placebo, with a consistency model used, regardless of the forms of drug administration (τ=0.07, χ2=1.29, P=0.863; ; τ≈0, χ2=0.63, P=0.889; ).
Figure 6 Pooled OR and 95% CIs determined by network meta-analysis for SAEs of targeted drugs (A) or oral targeted drugs (B) for PAH.
Discussion
To the best of our best knowledge, this was the first network meta-analysis comparing the efficacy of different targeted drugs of PAH. The major findings of the present meta- analysis were as follows: 1) prostanoids, ERAs, PDE-5Is, sGCS, and combination therapy could improve 6MWD, mPAP, PVR, and clinical worsening events for patients with PAH, regardless of drug dosage forms; 2) nonoral targeted drugs for PAH patients, including intravenous, inhaled, and subcutaneous forms, were superior to their oral forms; 3) none of the targeted drugs could significantly reduce the risk of all-cause mortality of PAH patients; 4) PDE-5Is might be more superior to prostanoids in increasing 6MWD (primary end point), with a similar result with combination therapy; and 5) PAH patients might benefit mostly from the combination therapy and modestly from prostanoids.
PAH is a hemodynamic and pathophysiologic state, characterized by a progressive increase in pulmonary vascular resistance and loss of pulmonary arterial compliance, which is associated with dyspnea, decreased exercise tolerance, and right heart failure.Citation1 According to the classification of WHO, PAH could be idiopathic, heritable, or associated with connective tissue disease, congenital heart disease, portal hypertension, and others.Citation45 The pathophysiology of PAH is complex, but it involves vasoconstriction and/or vasodilation imbalance, thrombosis, cell proliferation, and remodeling of the pulmonary arterial walls, particularly the molecular mechanisms of endothelial dysfunction factors including nitric oxide, prostacyclin, and endothelin.Citation46 Treatment options for PAH have developed rapidly, due to the increasing knowledge of the pathophysiology of this disease, mainly including supportive therapy, psychosocial support, and targeted drug therapy. Classical compounds of targeted drugs cover prostacyclin analogs, ERAs, PDE-5Is, and sGCS. The importance of distinguishing PAH from the other types of pulmonary hypertension is that these specific drugs target the molecular pathways of PAH, which might not be effective in the other forms of pulmonary hypertension. For this reason, these targeted drugs in treating non-PAH pulmonary hypertension are not supported,Citation47 except for riociguat (sGCS), which is also approved for patients with chronic thromboembolic pulmonary hypertension. Furthermore, many RCTsCitation9–Citation11,Citation31 and meta-analysesCitation12–Citation14 have demonstrated that these four types of targeted drugs has been shown to improve hemodynamics, exercise capacity, and possible survival. However, which drug or therapy regimen could provide the best efficacy for PAH patients in these four targeted drugs and their combination therapies remains unanswered. So far, direct head-to-head comparisons of targeted drugs are lacking, and traditional meta-analyses do not allow adequate assessment of the comparative effectiveness of all therapy regimens. Therefore, a comprehensive network meta-analysis enrolling as many relevant studies as possible is warranted to guide clinical practice in the real world.
In the present network meta-analysis, we confirmed that all targeted drugs could improve 6MWD and hemodynamics compared to placebo, in accordance with previous studies,Citation13,Citation14 but there was no significant decreased risk of mortality found in these active treatments. Similarly, the meta-analysis by Zhang et alCitation14 and Lajoie et alCitation48 concluded that oral targeted drugs, and even combination therapy, were associated with no sufficient efficacy on survival in the short-term follow-up. Although another meta-analysis by Galiè et alCitation49 showed significant reduction in mortality in PAH patients with the targeted drugs, only one study by Brast et al with epoprostenolCitation21 provided favorable data in the total of 23 RCTs analyzed. Of note, none of the current studies of targeted drugs were designed to evaluate the mortality of PAH, and most studies showed no survival benefit, which might due to the small sample size and relative short duration. In addition, all studies included safety margins for patients on placebo, who were allowed to switch to active drug or other medications in case of deterioration. Future clinical trials, setting mortality as the primary end point with enough power, should be designed to clarify the real survival value of targeted drugs. Furthermore, we found that combination therapy improved 6MWD and reduced the risk of clinical worsening in PAH patients compared with monotherapy, especially prostanoids. The results were also consistent with previous studies.Citation50,Citation51 Overall, we concluded that monotargeted drugs and their combination regimens could improve multiple clinical and hemodynamic outcomes, but they might have no effect on mortality.
Several questions remain unanswered. First, we found none of clinical trials assessing mortality as a primary end point. Surrogate end points, including 6MWD, hemodynamics, and clinical worsening events, have played a critical role in PAH clinical trials because of disease rarity, small population, participant burden, cost, and short follow-up time.Citation52 However, it is still unclear whether these surrogate end points could represent the true response to targeted drugs for PAH patients. Second, many agents approved for PAH are always delivered in pill form, for stability and a convenient administration route. Although oral targeted drugs have an important position in the PAH management, they have several limitations and drawbacks, especially in patients in WHO IV category and with severe right heart failure. According to our study, non-oral-targeted drugs, including intravenous, inhaled, and subcutaneous drugs, have improved efficacy compared to oral forms. Third, numerous RCTs and meta-analyses have suggested that sequential combination therapy is effective, meanwhile the recent AMBITION (Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension) study showed upfront treatment with tadalafil and ambrisentan was superior to monotherapy.Citation30 Nevertheless, it is still unclear whether upfront combination therapy improves clinical outcomes compared with sequential add-on therapy in cases of unsatisfactory response to initial monotherapy, which is recommended by the guidelines.Citation1,Citation5 In addition, various treatment options, apart from targeted drugs, are also available for PAH, including atrial septostomy, pulmonary transplantation, and pulmonary artery denervation (PADN). PADN from single-center studies has been demonstrated to be safe and effective in improving 6MWD and hemodynamics and in reducing PAH-related events and death throughout the 1-year follow-up.Citation53,Citation54 Further multicenter RCTs are required to confirm the efficacy of PADN and to explore whether PADN could replace the targeted drugs, given the expensive fee and unclear long-term effect.
Limitations
There are several limitations in the present study. First, different studies used different clinical worsening events definition, which might be an important source of bias. Second, lack of other potential confounding factors, such as etiology of PAH, WHO function, background treatment, and withdrawal due to adverse effects, did not allow us to investigate the detailed impact of targeted drugs on clinical end points and the underlying mechanisms. Third, there was no publication bias evaluation in the present study, which did not affect the interpretation of the results. Fourth, the study about selexipag, a novel prostacyclin receptor agonist, was excluded in present meta-analysis, just because the publication date of GRIPHON studyCitation55 was later than the predetermined time period in our protocol. Furthermore, there was not enough data about PAH-related SAEs and treatment-related SAEs in most of the enrolled studies. As a result, we could not evaluate the SAEs caused by targeted drugs. Finally, the follow-up period in all enrolled studies was relatively different for comparison of targeted drugs.
Conclusion
In conclusion, this network meta-analysis suggests that all targeted drugs for PAH are associated with improved clinical outcomes, especially combination therapy. However, all these drugs seem to show less favorable effects on survival in the short-term follow-up, suggesting further clinical trials are required.
Author contributions
CSL had the original idea and designed the study. GXF and ZJJ performed the systematic literature search, study identification, data extraction, and quality assessment. JXM and GZ undertook the statistical analysis. WZM, LB, and MWX drafted the article. All authors revised and approved the final report. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The study is supported by the Jiangsu Provincial Special Program of Medical Science (BL2013001).
Supplementary materials
Table S1 General characteristics of the included studies
References
- RubinLJMendozaJHoodMTreatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trialAnn Intern Med19901124854912107780
- OlschewskiHSimonneauGGalieNInhaled iloprost for severe pulmonary hypertensionN Engl J Med200234732232912151469
- GalieNHumbertMVachieryJLEffects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trialJ Am Coll Cardiol2002391496150211985913
- GalieNOlschewskiHOudizRJAmbrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2Circulation20081173010301918506008
- BadeschDBTapsonVFMcGoonMDContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trialAnn Intern Med200013242543410733441
- BarstRJRubinLJLongWAA comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertensionN Engl J Med19963342963018532025
- BarstRJMcGoonMMcLaughlinVBeraprost therapy for pulmonary arterial hypertensionJ Am Coll Cardiol2003412119212512821234
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- HumbertMBarstRJRobbinsIMCombination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2Eur Respir J20042435335915358690
- GalieNBeghettiMGatzoulisMABosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled studyCirculation2006114485416801459
- ChannickRNSimonneauGSitbonOEffects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled studyLancet20013581119112311597664
- GalieNRubinLHoeperMTreatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trialLancet20083712093210018572079
- JingZCYuZXShenJYVardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled studyAm J Respir Crit Care Med20111831723172921471085
- TapsonVFTorresFKermeenFOral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trialChest20121421383139022628490
- TapsonVFJingZCXuKFOral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trialChest201314495295823669822
- JingZCParikhKPulidoTEfficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trialCirculation201312762463323307827
- HoeperMMLeuchteHHalankMCombining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertensionEur Respir J20062869169417012628
- McLaughlinVVGaineSPBarstRJEfficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertensionJ Cardiovasc Pharmacol20034129329912548091
- McLaughlinVVOudizRJFrostARandomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertensionAm J Respir Crit Care Med20061741257126316946127
- GhofraniHAGalieNGrimmingerFRiociguat for the treatment of pulmonary arterial hypertensionN Engl J Med201336933034023883378
- BarstRJOudizRJBeardsworthATadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertensionJ Heart Lung Transplant20113063264321256048
- WilkinsMRPaulGAStrangeJWSildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) studyAm J Respir Crit Care Med20051711292129715750042
- PulidoTAdzerikhoIChannickRNMacitentan and morbidity and mortality in pulmonary arterial hypertensionN Engl J Med201336980981823984728
- SimonneauGBarstRJGalieNContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trialAm J Respir Crit Care Med200216580080411897647
- SimonneauGRubinLJGalieNAddition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trialAnn Intern Med200814952153018936500
- GalieNGhofraniHATorbickiASildenafil citrate therapy for pulmonary arterial hypertensionN Engl J Med20053532148215716291984
- McLaughlinVVBenzaRLRubinLJAddition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trialJ Am Coll Cardiol2010551915192220430262
- HiremathJThanikachalamSParikhKExercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trialJ Heart Lung Transplant20102913714920022264
- GalieNBarberaJAFrostAEInitial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial HypertensionN Engl J Med201537383484426308684
- ZhuangYJiangBGaoHZhaoWRandomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertensionHypertens Res20143750751224646647
- GalieNMullerKScaliseAVGrunigEPATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertensionEur Respir J2015451314132225657022
- McLaughlinVChannickRNGhofraniHABosentan added to sildenafil therapy in patients with pulmonary arterial hypertensionEur Respir J20154640541326113687
Disclosure
The authors report no conflicts of interest in this work.
References
- GalieNHoeperMMHumbertMGuidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT)Eur Heart J2009302493253719713419
- WilkinsMRPaulGAStrangeJWSildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) studyAm J Respir Crit Care Med20051711292129715750042
- BenzaRLMillerDPBarstRJBadeschDBFrostAEMcGoonMDAn evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL RegistryChest201214244845622281797
- NazzarenoGalièMHVachieryJLGibbsS2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)Eur Respir J2015461855185626621899
- McLaughlinVVShahSJSouzaRHumbertMManagement of pulmonary arterial hypertensionJ Am Coll Cardiol2015651976199725953750
- OlschewskiHSimonneauGGalieNInhaled iloprost for severe pulmonary hypertensionN Engl J Med200234732232912151469
- McLaughlinVVGaineSPBarstRJEfficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertensionJ Cardiovasc Pharmacol20034129329912548091
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- GalieNGhofraniHATorbickiASildenafil citrate therapy for pulmonary arterial hypertensionN Engl J Med20053532148215716291984
- GhofraniHAGalieNGrimmingerFRiociguat for the treatment of pulmonary arterial hypertensionN Engl J Med201336933034023883378
- PulidoTAdzerikhoIChannickRNMacitentan and morbidity and mortality in pulmonary arterial hypertensionN Engl J Med201336980981823984728
- CoeytauxRRSchmitKMKraftBDComparative effectiveness and safety of drug therapy for pulmonary arterial hypertension: a systematic review and meta-analysisChest20141451055106324371842
- ZhengYGMaHHuECLiuGChenGXiongCMOral targeted therapies in the treatment of pulmonary arterial hypertension: a meta-analysis of clinical trialsPulm Pharmacol Ther20142924124925173912
- ZhangHDZhangRJiangXSunKWuDCJingZCEffects of oral treatments on clinical outcomes in pulmonary arterial hypertension: a systematic review and meta-analysisAm Heart J201517096103103.e1e1426093869
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials1996171128721797
- ChaimaniAHigginsJPMavridisDSpyridonosPSalantiGGraphical tools for network meta-analysis in STATAPLoS One20138e7665424098547
- WhiteIRBarrettJKJacksonDHigginsJPConsistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regressionRes Synth Methods2012311112526062085
- DiasSWeltonNJCaldwellDMAdesAEChecking consistency in mixed treatment comparison meta-analysisStat Med20102993294420213715
- HigginsJPJacksonDBarrettJKLuGAdesAEWhiteIRConsistency and inconsistency in network meta-analysis: concepts and models for multi-arm studiesRes Synth Methods201239811026062084
- RubinLJMendozaJHoodMTreatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trialAnn Intern Med19901124854912107780
- BarstRJRubinLJLongWAA comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertensionN Engl J Med19963342963018532025
- BadeschDBTapsonVFMcGoonMDContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trialAnn Intern Med200013242543410733441
- ChannickRNSimonneauGSitbonOEffects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled studyLancet20013581119112311597664
- GalieNHumbertMVachieryJLEffects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trialJ Am Coll Cardiol2002391496150211985913
- SimonneauGBarstRJGalieNContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trialAm J Respir Crit Care Med200216580080411897647
- BarstRJMcGoonMMcLaughlinVBeraprost therapy for pulmonary arterial hypertensionJ Am Coll Cardiol2003412119212512821234
- HumbertMBarstRJRobbinsIMCombination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2Eur Respir J20042435335915358690
- HoeperMMLeuchteHHalankMCombining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertensionEur Respir J20062869169417012628
- BarstRJOudizRJBeardsworthATadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertensionJ Heart Lung Transplant20113063264321256048
- GalieNBarberaJAFrostAEInitial use of ambrisentan plus tadalafil in pulmonary arterial hypertensionN Engl J Med201537383484426308684
- GalieNBeghettiMGatzoulisMABosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled studyCirculation2006114485416801459
- GalieNMullerKScaliseAVGrunigEPATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertensionEur Respir J2015451314132225657022
- GalieNOlschewskiHOudizRJAmbrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2Circulation20081173010301918506008
- GalieNRubinLHoeperMTreatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trialLancet20083712093210018572079
- HiremathJThanikachalamSParikhKExercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trialJ Heart Lung Transplant20102913714920022264
- JingZCParikhKPulidoTEfficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trialCirculation201312762463323307827
- JingZCYuZXShenJYVardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled studyAm J Respir Crit Care Med20111831723172921471085
- McLaughlinVChannickRNGhofraniHABosentan added to sildenafil therapy in patients with pulmonary arterial hypertensionEur Respir J20154640541326113687
- McLaughlinVVBenzaRLRubinLJAddition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trialJ Am Coll Cardiol2010551915192220430262
- McLaughlinVVOudizRJFrostARandomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertensionAm J Respir Crit Care Med20061741257126316946127
- SimonneauGRubinLJGalieNAddition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trialAnn Intern Med200814952153018936500
- TapsonVFJingZCXuKFOral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trialChest201314495295823669822
- TapsonVFTorresFKermeenFOral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trialChest20121421383139022628490
- ZhuangYJiangBGaoHZhaoWRandomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertensionHypertens Res20143750751224646647
- SimonneauGGatzoulisMAAdatiaIUpdated clinical classification of pulmonary hypertensionJ Am Coll Cardiol201362D34D4124355639
- TuderRMArcherSLDorfmullerPRelevant issues in the pathology and pathobiology of pulmonary hypertensionJ Am Coll Cardiol201362D4D1224355640
- TaichmanDBOrnelasJChungLPharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel reportChest201414644947524937180
- LajoieACLauziereGLegaJCCombination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysisLancet Respir Med2016429130526935844
- GalieNManesANegroLPalazziniMBacchi-ReggianiMLBranziAA meta-analysis of randomized controlled trials in pulmonary arterial hypertensionEur Heart J20093039440319155250
- ZhuBWangLSunLCaoRCombination therapy improves exercise capacity and reduces risk of clinical worsening in patients with pulmonary arterial hypertension: a meta-analysisJ Cardiovasc Pharmacol20126034234622691882
- BaiYSunLHuSWeiYCombination therapy in pulmonary arterial hypertension: a meta-analysisCardiology201112015716522212696
- ParikhKSRajagopalSArgesKUse of outcome measures in pulmonary hypertension clinical trialsAm Heart J2015170419429.e326385024
- ChenSLZhangFFXuJPulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension)J Am Coll Cardiol2013621092110023850902
- ChenSLZhangHXieDJHemodynamic, functional, and clinical responses to pulmonary artery denervation in patients with pulmonary arterial hypertension of different causes: phase II results from the Pulmonary Artery Denervation-1 studyCirc Cardiovasc Interv20158e00283726553699
- SitbonOChannickRChinKMSelexipag for the Treatment of Pulmonary Arterial HypertensionN Engl J Med2015373262522253326699168