Abstract
Lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH) will usually affect older men, of whom 50% over the age 60 years and almost 90% in their nineties will be bothered enough by their symptoms that they request some type of treatment. However, symptomatic bother may also affect men in their forties with a prevalence rate of almost 18%. The International Prostate Symptom Score (IPSS) has become the most widely used and best validated questionnaire to allow the patient to quantify the severity of his LUTS/BPH symptoms. This score has become the cornerstone in demonstrating the “rate of symptom response” for the patient who has been exposed to any type BPH management. Question 8 on the IPSS score is what is defined as the “Quality of Life” question or what is also termed the “Bothersome Index.” The score out of 6 as declared by the patient will reflect the degree of concern that the patient is feeling about his symptoms and the reduction of the score after treatment is a statement of their improved quality of life. There are 2 families of accepted medical therapy to treat the symptoms of BPH and potentially prevent the most worrisome long-term sequelae of progression of BPH: urinary retention or the need for surgery. When defining the impact of the main types of medical therapy, the alpha blockers have been termed the “openers” and the 5 alpha-reductase inhibitors are described as the “shrinkers.” Since they each offer a different mechanism of effect, the concept of combination therapy was raised and trialed many times over recent years. The final aspect of any medical therapy is the patient’s satisfaction with the treatment and the side effects. In the CombAT (Combination of Avodart and Tamsulosin) trial a new assessment was developed and tested called the Patient’s Perception of Study Medication (PPSM) which told the investigators if the patients, given free choice, would choose to take that combination of medication to treat their problem and stay on the medication.
Introduction
For over 20 years men have been treated medically for the symptoms of what was always thought to arise from benign prostatic hyperplasia (BPH). Today we understand that the symptoms are more correctly termed lower urinary tract symptoms (LUTS). The bladder has 2 main functions, storage and voiding (emptying). The commonest cause for the man over the age of 50 years for LUTS is indeed BPH.
Many years ago, Barry et al developed what was to become the most widely accepted and utilized validated questionnaire to measure the severity of LUTS symptoms. The International Prostate Symptom Score (IPSS) is a quantification of the severity of symptoms as it is self-reported by the patient.Citation1 This has become the gold standard in establishing the baseline and then follow-up score after any type (medical, natural, or surgical) of intervention for the management of BPH. Each question of the first 7 could be classified as either a “voiding” or a “storage” question.
A score of:
0 is defined as NO symptoms
≤7 is defined as MILD symptoms
≥8 and ≤19 is defined as MODERATE symptoms
≥19 and ≤35 is defined as SEVERE symptoms.
Barry also proved in clinical trials that a minimum of a 3-point improvement on the symptom scale was necessary for the patient to perceive the clinical improvement.
Question 8 on the IPSS score is the Quality of Life question or what is more aptly termed the “Bothersome Index.”
This key question is “If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?” A score of 0 equates to “delighted” and a score of 6 equates to “terrible.”
Again this is a self-reported declaration of the patient’s perceived bother from his symptoms after considering his ability to tolerate the present degree of symptoms for the rest of his life.
I also define question 8 as the “motivational index.” The higher the bothersome index, the more motivated the patient will be to accept the “risk/benefit” ratio discussion about the benefits of the proposed therapy balanced against the potential side effects of the medication. Kirby et al also determined that the 3/6 is the cutpoint for the minimum number that the patient will report, which will indicate that he is willing to accept treatment for his symptoms.Citation11
Another questionnaire used to measure quality of life (QoL) for BPH patients is called the BPH Impact Index (BII), which
Assesses overall impact of BPH on a patient’s general sense of well-being
Measures aspects of physical discomfort, worry, and bother, all of which can be affected by BPH and its symptoms
BPH Impact Index:
Over the past month how much physical discomfort did any urinary problems cause you?
Over the past month, how much did you worry about your health because of any urinary problems?
Overall, how bothersome has any trouble with urination been during the past month?
Over the past month, how much of the time has any urinary problem kept you from doing the kind of things you would usually do?
Total score from 0 to 13 (higher score = worse BPH-related health status).Citation2
It has been shown that BPH, if untreated, may progress. There are certain risk factors that will predict the progression of BPH/LUTS. Progression of BPH can have a dramatic impact on the patients well-being ().
Physiology
There are two medical approaches to the management of BPH, which work through the different receptors within the bladder and the prostate.
The alpha blockers block the smooth muscle receptors at the bladder neck, in the bladder, and within the prostate, thereby relaxing the tension that “opens” the bladder neck allowing for easier, stronger flow and a more complete emptying, which means less frequency, urgency, and nocturia. The response is very quick, in hours to a few days, but the long-term response is relatively short, where most men need an adjustment in dosage or movement to another therapy by 4 years. As was seen in the Medical Therapy of Prostate Symptoms (MTOPS) trial at 5.5 years, the alpha blocker doxozasin was able to provide better symptom response than the monotherapy of finasteride or placebo.Citation8 However, alpha blockers do not prevent the progression of BPH, which usually culminates in urinary retention and/or the need for surgery.Citation9
The 5 alpha reductase (5ARI) inhibitors work through a different mechanism. They prevent the conversion of testosterone to dihydrotestosterone (DHT). It is the DHT, when bound to the RNA in the cells of the prostate, that stimulate growth of the cells and glands within the prostate. After 3–6 months of therapy the cells and prostate that has been deprived of DHT will shrink. There are 2 5ARI iso-enzymes, type 1 and type 2. Finasteride will inhibit only the type 2 receptors whereas dutasteride will inhibit both type 1 and type 2. This extra inhibition results in a greater reduction of DHT of almost 95% versus 71%.Citation10 We do not yet know the exact minimum DHT threshold suppression to achieve maximum shrinkage of the gland through maximum and prolonged androgen receptor blockade within the prostate. The 5ARIs achieve a slow onset of response as demonstrated by the expected 6-month drop in the prostate-specific antigen (PSA), volume reduction of the gland, and the prevention of progression.
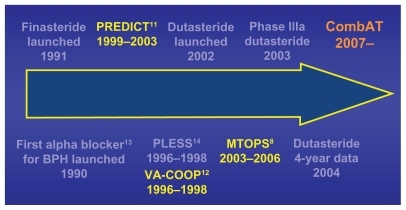
History of combination therapy
From the launch of the first alpha blocker and 5ARI in the early 1990s the concept of combination therapy was entertained (). The hypothesis was that the synergistic effect of the two drugs would provide the early onset of symptom response from the alpha blocker and the long-term durability of symptom response and prevention of progression from the 5ARI. The concern was in the lack of knowledge as to whether there would also be synergy in the complications or side effects of the 2 drug families as well.
There were numerous trials, but the results of the early combination trials using finasteride and the non-selective alpha blockers (Predict:Citation11 European – doxazosin and finasteride; Va-coop:Citation12 American – terazosin and finasteride) were a disaster. The results showed that the finasteride monotherapy arm was no better than placebo when measuring symptom response.
In retrospect the faults were that the studies were too short (only 1 year) and the prostate volumes may have been too small (<30 mL) to reap the benefits of the 5ARI finasteride.
Efficacy of long-term combination therapy
The first long-term combination trial, MTOPS, which was performed only in the United States on 3047 men with moderate symptoms of BPH and a PSA < 4, lasted for 5.5 years and compared monotherapy of doxozasin, finasteride, or placebo to the combination of doxozasin and finasteride.Citation8
The results were very impressive in that they demonstrated a 67% risk reduction in progression of disease at 4.5 mean years of follow-up in the combination arm compared with placebo. There was an insignificant difference in the response in any of the arms compared with placebo at 1 year.
The same cohort also demonstrated a 66% risk reduction in developing acute urinary retention (AUR) or the need for surgery in the combination arm compared with placebo at study end.
An analysis of the response when the patients were stratified by prostate volume revealed that a volume of 30 mL was the minimum necessary to derive a clinically significant response in the 5ARI arm whether monotherapy or combination compared with placebo.Citation15
QoL was a secondary endpoint in MTOPS (assessed using the Medical Outcomes Study Short Form-36 Health Survey instrument). However, no QoL outcomes for combination therapy have been published from MTOPS.
Dutasteride monotherapy trials had been performed and reported in 2004 in which the long-term effects of dutatsteride were compared with placebo. The results of the 4-year extended monotherapy trial showed an 80% better symptom response, 56% PSA reduction, 27% volume reduction, and a 70% risk reduction in either AUR or the need for surgery.Citation16
Tamsulosin had previously been recognized as a uroselective alpha blocker, which provided the maximum therapeutic effect while causing far less risk of cardiovascular side effects such as orthostatic hypotension, as was found in the nonselective alpha blockers like doxazosin.Citation17
It had already been shown that dutasteride was very effective compared with placebo in men with BPH, especially in those with large prostates (>30 mL). As well, as previously stated the risk factors for BPH progression as determined at time of diagnosis were age > 50 years, prostate volume > 30 mL, and moderate symptoms on the IPSS (≥7).
Another recent consideration is the fact that it has been shown that the PSA in BPH can be a surrogate predictor of the prostate volume. One can virtually guarantee that if the PSA is >1.5 ng/mL, regardless of the age of the patient the actual prostate volume will be at least 30 mL.Citation18
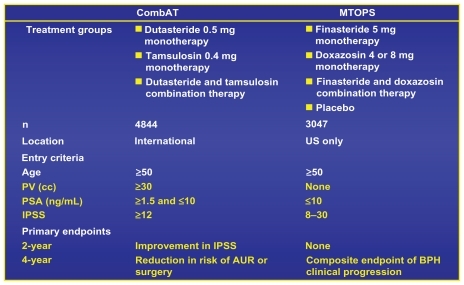
Consequently, the Combination of Avodart and Tamsulosin (CombAT) trial had different inclusion criteria from MTOPS, which were recognized as defining a more “at risk” population for BPH progression.Citation20 The average prostate volume in all 3 arms at the baseline was 55 mL and the average PSA was 4.0.
Based on all of these considerations no ethical review board in any of the 32 countries felt comfortable in requesting a placebo arm for the 4 years of the trial knowing that this population, without treatment, had a great chance of progressing, developing AUR, or needing surgery by the end of the trial. Therefore, only 3 arms were required: Monotherapy of Tamsulosin or Dutatsteride, compared with the combination arm of Dutatsteride and Tamsulosin.
There were other significant differences as well ().
Figure 3 Comparison of baseline demographics and study designs of MTOPS and CombAT.Citation19
Note: Reprinted with permission from Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57(1):123–131.Citation19 Copyright © 2010 Elsevier.
The protocol was designed such that there was both a 2-year and 4-year analysis. The primary endpoint at the 2-year mark was the change in the IPSS responders’ scores from baseline comparing combination to the active treatment of tamsulosin (MTOPS compared with placebo).
There were many secondary endpoints, which included percent with a ≥25% change in IPSS, change in prostate volume, Qmax (peak uroflow rate) improvement in mL/second, BII, QoL as defined by question 8 in the IPSS as well as a newly developed assessment called the Patients Perception of Study Medication (PPSM).
In every endpoint measured at 2 years there was a significant improvement in the combination arm compared with either monotherapy arm, with a consistently and statistically better response than the tamsulosin arm.Citation22
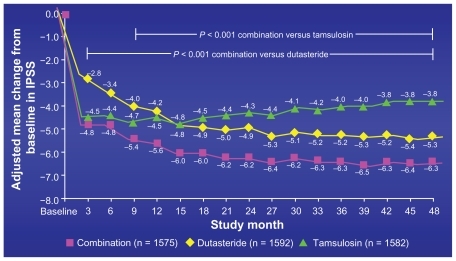
This was the first combination trial ever where the response as measured by the IPSS score was better in the 5ARI or combination arm than the alpha blocker. Until this study, it had always been reported when measuring symptom response, especially up to 4 years, that the alpha blocker would outperform the 5ARI. In MTOPS, when assessing IPSS response, doxozasin always achieved a lower IPSS score than finasteride even out to 5.5 years. In this study the crossover point where dutasteride monotherapy outperformed tamsulosin monotherapy occurred at 15 months and never reverted.Citation19 Combination therapy surpassed tamsulosin in IPSS response as early as 3 monthsCitation22 ().
Figure 4 Mean change International Prostate Symptom Score (IPSS) from baseline: primary endpoint in 4-year CombAT trial.
Acceptance
QoL improvement with combination therapy
The QoL responses were also very significant. It is the patient’s feeling of improvement that will encourage him to continue in the study. Barry et al had previously determined that a 1.1 point improvement in question 8 or a 2-point improvement in the BII would be considered as a “marked” (slight, moderate, marked) clinical improvement and definitely perceived by the patient. Again the combination arm achieved both a clinically significant question 8 response compared with tamsulosin as early as 3 months (−1.4 vs −1.1) as well as the “marked” BII response (−2.1 vs −1.5) by the end of 2 years.Citation23
Two other significant clinical responses were seen by the end of the 2 years. The first was that there was a 55% reduction from the baseline PSA in the dutasteride or combination arm and a 12.5% increase from the baseline PSA in the tamsulosin arm. Also there was a 28% volume reduction in the dutasteride/tamsulosin combination arm, with no change or a slight increase from the baseline volume in the alpha blocker arm.
At 4 years the endpoints were more inclusive and the results even more significant (). It is important to remember that these patients had average baseline volumes of 55 mL (proven by transrectal ultrasound measurements), average PSAs of 4.0, average IPSS score of 16.7 (almost in the “severe” range). Also all responses that were achieved were compared with the active treatment arm of tamsulosin, unlike MTOPS where the combination arm response was compared with placebo.
Figure 5 Four-year primary and secondary CombAT endpoints.Citation21

The most important consideration for a man who is at risk for BPH progression is the possibility that he will develop AUR or need surgery to correct the sequelae of his profound BPH.
The 4-year primary composite endpoint of AUR or the need for surgery achieved significant resultsCitation20 ().
Figure 6 CombAT 4-year composite primary endpoint – time to first AUR or BPH surgery.
Abbreviations: AUR, acute urinary retention; BPH, benign prostatic hyperplasia; CI, confidence interval; RRR, relative risk reduction.
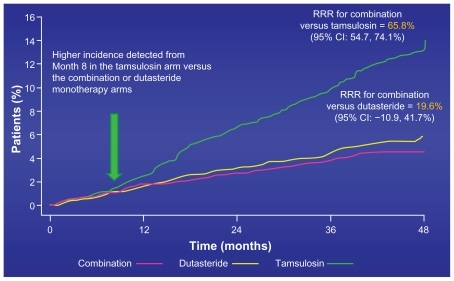
The patients in the combination arm of dutasteride and tamsulosin enjoyed a 66% risk reduction for developing these devastating problems compared with those in the active treatment tamsulosin arm. In the MTOPS trial there was a 67% risk reduction in these same endpoints, but in that case it was the combination of finasteride and doxozasin compared with placebo.Citation8
All of the other secondary 4-year endpoints of clinical progression also had a statistically better response in the combination arm than either monotherapy armCitation19 ().
Figure 7 CombAT 4-year secondary endpoints – clinical progression.
Abbreviations: AUR, acute urinary retention; BPH, benign prostatic hyperplasia; IPSS, International Prostate Symptom Score; ITT, intention-to-treat; UTI, urinary tract infection.
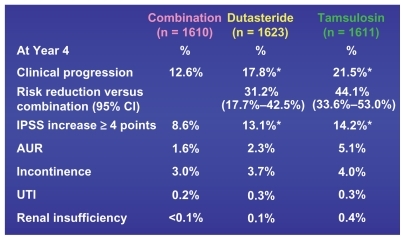
The 4-year response as measured by the BII or question 8 was maintained and actually increased compared with the 2-year reported response. BII reached a −2.2 points, whereas question 8 achieved a −1.6 points, both of which were statistically significant compared with tamsulosin.Citation24
PPSM
The new parameter that was developed and validated specifically for this study was the PPSM (). This was a questionnaire that tested the patient’s appreciation of the study medication and their ultimate desire, after weighing the risks (side effects actually experienced) against the clinical benefits achieved, would they voluntarily choose and be compliant in taking that medicine.
Figure 8 Description of Patient’s Perception of Study Medicine Questionnaire (PPSM).Citation14

The absolute improved response in the combination arm compared with either dutasteride or tamsulosin was statistically significant (−7.0 vs −5.5 vs −4.1 out of a maximum of 25, respectively).Citation19
Even more important was the overall acceptance of the combination therapy compared with the monotherapy arms ().
Safety and side effects
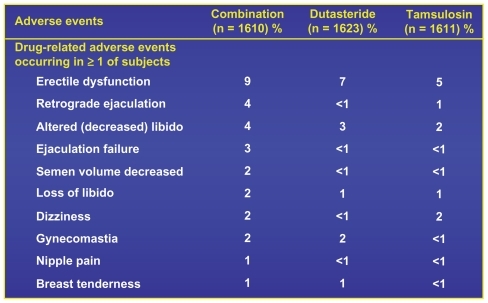
It was shown that the combination was very effective. The question about whether the incidence of side effects as reported in either monotherapy arm would be less than, additive, or greater in the combination arm was reported.
The only side effect for which the total in the combination arm was greater than the sum of the reported incidences in either monotherapy arm was in the complaint related to ejaculatory dysfunction. In this area the combination of the decreased volume associated with the 5 ARI, when added to either the retrograde ejaculation or diminished release from the seminal vesicles, was experienced and reported by the patients more in the combination arm than in the 2 monotherapy arms combinedCitation19 ().
Figure 10 CombAT 4-year incidence of drug-related adverse events.
As was reflected in the PPSM, there was no significant difference in the “drug related adverse events leading to study withdrawal” rates in the 3 arms when comparing combination with dutasteride with tamsulosin. The reported rates were 6%, 4%, and 4% respectively out of > 1600 patients in each arm.
It is also important to be able to inform the patients that drug-related adverse events diminished over time, the incidence being about 12% in the first year, but only 2% in the fourth year. Therefore, it is important to encourage the patients to stay on the drugs, because if they do not get side effects in the first year, the chances of experiencing side effects later on is greatly diminished.
Initiation and/or withdrawal of combination therapy
If there is concern about the economics or potential long-term side effects of combination therapy, there is information to suggest that in 77% of patients who received combination (dutasteride and tamsulosin) therapy for 6 months followed by alpha blocker withdrawal, felt the same or better 6 months after stopping the alpha blocker. If their original IPSS was >20, this favored response was reached in only 56% of the men.Citation25
It is also important to consider that if combination therapy is going to be started, it should be initiated early (adding 5ARI to alpha blocker) or simultaneously. For every month delay in adding the 5ARI, if a patient starts with alpha blocker monotherapy, a decrease in response of about 2% as measured at the end of 1 year.Citation21
Conclusion
CombAT has clearly demonstrated that for the man with an enlarged prostate (>30 mL) and moderate symptom complaints, the combination of dutasteride and tamsulosin compared with monotherapy will provide the most effective and most durable long-term benefits. This was demonstrated in all parameters including symptom response, lack of progression, and the development of AUR or the need for surgery. Moreover, the combination is safe with very few significant side effects or adverse events. Finally, if given the choice a greater number of patients would choose and continue to regularly take the combination therapy over either monotherapy.
Disclosure
The author has been an investigator, medical advisory board member, speaker and publisher for 5ARI’s and Alpha Blockers produced by Merck, GSK, Boeringer, Abbott and Sanofi pharmaceuticals.
References
- BarryMJFowlerFLO’LearyMPThe American Urological Association Symptom Index for benign prostatic hyperplasiaJ Urol19921485154915571279218
- BarryMJWillifordWOChangWBenign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients?J Urol19951545177017747563343
- AndersonJBRoehrbornCGSchalkenJAEmbertonMThe progression of benign prostatic hyperplasia: examining the evidence and determining the riskEur Urol200139439039911306876
- MadersbacherSMarszalekMLacknerJBergerPSchatzlGThe long-term outcome of medical therapy for BPHEur Urol20075161522153317416456
- MarksLSRoehrbornCGAndrioleGLPrevention of benign prostatic hyperplasia diseaseJ Urol20061764 Pt 11299130616952616
- DjavanBWaldertMGhawidelCMarbergerMBenign prostatic hyperplasia progression and its impact on treatmentCurr Opin Urol2004141455015091050
- RoehrbornCGDefinition of at-risk patients: baseline variablesBJU Int200697Suppl 2711 discussion 21–2216507046
- McConnellJDRoehrbornCGBautistaOMThe long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasiaN Engl J Med2003349252387239814681504
- OelkeMBachmannADescazeaudMEAU Guidelines on Conservative Treatment of Non-neurogenic Male LUTSArnhem, The NetherlandsEuropean Association of Urology2010 Available from: http://www.uroweb.org/gls/pdf/BPH%202010.pdfAccessed June 24, 2011
- ClarkRVHermannDJCunninghamGRWilsonTHMorrillBBHobbsSMarked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitorJ Clin Endocrinol Metab2004892179218415126539
- KirbyRSRoehrbornCBoylePEfficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trialUrology200361111912612559281
- LeporHWillifordWOBarryMJThe efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study GroupN Engl J Med199633585335398684407
- LeporHNonoperative management of benign prostatic hyperplasiaJ Urol1989141128312892470925
- McConnellJDBruskewitzRWalshPThe effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasiaN Engl J Med19983385575639475762
- KaplanSAMcConnellJDRoehrbornCGCombination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 ml or greaterJ Urol2006175121722 discussion 220–22116406915
- DebruyneFBarkinJvan ErpsPEfficacy and safety of long- term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasiaEur Urol200446448849515363566
- AbramsPSchulmanCCVaageSTamsulosin, a selective alpha 1c-adrenoceptor antagonist: a randomized, controlled trial in patients with benign prostatic “obstruction” (symptomatic BPH). The European Tamsulosin Study GroupBr J Urol19957633253367551841
- RoehrbornCBoylePGouldALWaldstreicherJSerum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasiaUrology199953358158910096388
- RoehrbornCGSiamiPBarkinJThe effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT studyEur Urol201057112313119825505
- SiamiPRoehrbornCGBarkinJCombination therapy with dutasteride and tamsulosin in men with moderate-to-severe benign prostatic hyperplasia and prostate enlargement: the CombAT (Combination of Avodart and Tamsulosin) trial rationale and study designContemp Clin Trials200728677077917761460
- NaslundMEaddyMTHogueSLKruepEJShahMBImpact of delaying 5-alpha reductase inhibitor therapy in men on alpha-blocker therapy to treat BPH: assessment of acute urinary retention and prostate-related surgeryCurr Med Res Opin200925112663266919757985
- RoehrbornCSiamiPBarkinJThe effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT studyJ Urol20091792616621 discussion 62118082216
- BarkinJRoehrbornCSiamiPEffect of dutasteride, tamsulosin and the combination on patient-reported quality of life and treatment satisfaction in men with moderate-to-severe benign prostatic hyperplasia: 2-year data from the CombAT trialBJU Int2008103791992619239460
- MontorsiFHenkelTGeboersAEffect of dutasteride, tamsulosin and the combination on patient-reported quality of life and treatment satisfaction in men with moderate-to-severe benign prostatic hyperplasia: 4-year data from the CombAT studyInt J Clin Pract20106481042105120487046
- BarkinJGuimarãesMJacobiGPushkarDTaylorSvan Vierssen TripOBAlpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasterideEur Urol200344446146614499682