Abstract
Objectives
This review summarizes factors relevant for adherence to phosphate-control strategies in dialysis patients, and discusses interventions to overcome related challenges.
Methods
A literature search including the terms “phosphorus”, “phosphorus control”, “hemo-dialysis”, “phosphate binder medications”, “phosphorus diet”, “adherence”, and “nonadherence” was undertaken using PubMed, PsycInfo, CINAHL, and Embase.
Results
Hyperphosphatemia is associated with cardiovascular and all-cause mortality in dialysis patients. Management of hyperphosphatemia depends on phosphate binder medication therapy, a low-phosphorus diet, and dialysis. Phosphate binder therapy is associated with a survival benefit. Dietary restriction is complex because of the need to maintain adequate protein intake and, alone, is insufficient for phosphorus control. Similarly, conventional hemodialysis alone is insufficient for phosphorus control due to the kinetics of dialytic phosphorus removal. Thus, all three treatment approaches are important contributors, with dietary restriction and dialysis as adjuncts to the requisite phosphate binder therapy. Phosphate-control adherence rates are suboptimal and are influenced directly by patient, provider, and phosphorus-control strategy-related factors. Psychosocial factors have been implicated as influential “drivers” of adherence behaviors in dialysis patients, and factors based on self-motivation associate directly with adherence behavior. Higher-risk subgroups of nonadherent patients include younger dialysis patients and non-whites. Provider attitudes may be important – yet unaddressed – determinants of adherence behaviors of dialysis patients.
Conclusion
Adherence to phosphate binders, low-phosphorus diet, and dialysis prescription is suboptimal. Multicomponent strategies that concurrently address therapy-related factors such as side effects, patient factors targeting self-motivation, and provider factors to improve attitudes and delivery of culturally sensitive care show the most promise for long-term control of phosphorus levels. Moreover, it will be important to identify patients at highest risk for lack of control, and for programs to be ready to deliver flexible person-centered strategies through training and dedicated resources to align with the needs of all patients.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Plain language summary
Management of end-stage renal disease (ESRD) is complicated by hyperphosphatemia. This is the accumulation of phosphorus in the body due to the inability of dialysis patients to excrete phosphorus. This increases the risk of spontaneous bone fractures from abnormal mineral metabolism and risk of death from cardiovascular disease. Management of hyperphosphatemia depends on three approaches: use of medications known as phosphate binders, dietary phosphorus restriction, and removal of phosphorus through dialysis. Adherence to each of these approaches is a challenge for dialysis patients due to medication- or dialysis-treatment-associated burden, complexity of the diet, as well as patient-specific factors. Patient factors associated with phosphate-control adherence behaviors include age, gender, and race. In preliminary research, psychosocial autonomy-centered or self-motivation patient factors contribute to phosphorus-control adherence, and suggest that aligning with a person’s value system may be the key to optimizing medication, diet, and dialysis care. Existing strategies to improve phosphate control include educational and behavioral interventions delivered by multidisciplinary dialysis providers. Emerging research implicates that dialysis providers have varying attitudes and poor perceptions of their support of self-motivation in dialysis patients for adherence to prescribed phosphate binder medication therapy. Improvement in phosphorus-control adherence will require enhanced provider-level training strategies integrated into existing patient-level interventions, with a focused effort to identify patients at high risk of nonadherence who may benefit from more personalized solutions.
Introduction
Hyperphosphatemia is common in end-stage renal disease (ESRD) because of impaired renal phosphate excretion.Citation1 It is a critical component of mineral and bone disease (MBD) that increases the risk of fractures and osteoporosis,Citation2 and is associated with greater cardiovascular and all-cause mortality in dialysis patients.Citation3 Hyperphosphatemia may be effectively managed with phosphate binder medication therapy, dietary restriction, and dialysis prescription.Citation1
Phosphate binder medication therapy is the cornerstone of therapeutic management in hyperphosphatemia,Citation4 and it has been associated with survival benefits. The existing evidence, although robust, is from observational studies,Citation5 and this could be a possible opportunity for a pragmatic trial design in the future.Citation6 Optimization of phosphate binder use by patients with ESRD to achieve target serum phosphorus levels toward the normal range of 3.5–5.5 mg/dL is of utmost importance to minimize morbidity and mortality risks.Citation7,Citation8 However, it is estimated that up to 74% of ESRD patients are noncompliant to phosphate binder medication therapy.Citation9,Citation10 Challenges to adherence include 1) medication-related factors such as high pill counts, complex adjustable schedules, adverse side effects, and financial burden;Citation11 2) patient-specific factors such as limited knowledge about the importance of taking binder medications;Citation11,Citation12 3) recurrent hospitalizations disrupting the usual daily approaches to binder medications, and concomitant comorbidities such as diabetes and hypertension compounding medication complexity and overall burden;Citation12 and 4) provider-level factors related to educational and emotional support for patients.Citation11
High dietary phosphorus intake and increased dietary phosphorus-to-protein ratio have been associated with mortality in ESRD.Citation11 A low-phosphorus diet is insufficient to control the serum phosphorus level in the well-nourished ESRD patient, and has not been associated with improved survival.Citation13 Dietary phosphorus restriction is complex because it is challenging to maintain the adequate protein intake needed in ESRD patients to prevent malnutrition and simultaneously restrict phosphorus intake. Perhaps even more importantly, many processed foods contain a significant amount of phosphate additives that are are often undisclosed and difficult for patients to identify.Citation11 In ESRD, the ideal daily phosphorus intake is 700 mg/day; however, the usual intake commonly averages 1,000–2,000 mg/day.Citation14 Approximately 60% of the phosphorus is absorbed,Citation15 which results in a significant daily excess of phosphorus. Adherence to a low-phosphorus diet could be as low as 43%,Citation16 and is influenced similarly by 1) diet-specific factors such as menu selections, impact of the diet on social outings, and acceptance of the diet by friends and family;Citation16 2) patient factors such as depression, limited self-efficacy, and poor coping skills;Citation16 and 3) provider factors including inadequate support,Citation16 infrequent contact with dietitians, and conflicting phosphorus diet advice from different health professionals.Citation17
Thrice-weekly conventional dialysis removes phosphorus in the range of 1,800–3,600 mg and, thus, does not provide enough clearance of the daily amount of ingested phosphorus to maintain balance.Citation18 This is due to the kinetics of phosphorus removal during hemodialysis, whereby serum phosphorus levels plateau after an initial drop within the first 2 hours of treatment, followed by a rebound, resulting in up to 40% rise in serum phosphorus levels after dialysis.Citation19 Dialysis treatments are complicated by nonadherence, and it is estimated that up to 35% of patients miss treatments entirely whereas another 32% shorten their treatment time.Citation20 Reasons for nonadherence to the prescribed dose of dialysis include treatment- and patient-related factors. Dialysis vintage and schedule assignment, both, are associated with treatment nonadherence.Citation21 Patient factors associated with dialysis treatment nonadherence include younger ageCitation21,Citation22 and non-white raceCitation23 as well as psychosocial factors including negatively perceived effects of kidney disease on daily life and lack of perceived control over future health.Citation22 Nonadherence to the dialysis procedure contributes to significant morbidity and increases mortality risk – in part, due to uncontrolled mineral bone disease.Citation9,Citation24
There are unique drivers of adherence behaviors for medications, diet, and dialysis in the effort to control phosphorus, but there are also common themes that can be leveraged to simultaneously optimize all approaches. This review discusses current perspectives and challenges contributing to low adherence to phosphate control, and examines effective and emerging strategies for patient-centered care with hemodialysis.
Phosphate-control methods in hemodialysis patients
Phosphate binder medication therapy
An overview of phosphate binder medications is presented in . Phosphate binders regulate calcium–phosphate homeostasis and mitigate the metabolic abnormalities resulting from hyperphosphatemia.Citation25 They prevent phosphate absorption from the gastrointestinal tract through varied mechanisms. These medications can be broadly classified into 1) calcium-based and 2) non-calcium-based phosphate binders.
Table 1 Overview of currently available phosphate binders
Calcium-based phosphate binders including calcium acetate, citrate, and carbonate dissociate in the gastrointestinal tract and bind phosphate to form insoluble precipitates. They are less expensive than the non-calcium binders,Citation10 but are associated with greater risk of vascular calcification due to a positive calcium balance.Citation26
Non-calcium-based binders include sevelamer, lanthanum, and iron-based binders (eg, ferric citrate and sucroferric oxyhydroxide).Citation26 Sevelamer is an anion exchange resin that exchanges chloride ions for phosphate ions whereas lanthanum binds phosphate through its trivalent cation. Both are associated with gastrointestinal side effects such as abdominal bloating, diarrhea, and constipation. Lanthanum has a low pill burden compared to sevelamer. Sevelamer carbonate is available as a powder for patients who may benefit from a different formulation;Citation11 however, patients often get tired of taking it and usually request a change of phosphate binder preparation.Citation27 The pill form of sevelamer is comparatively large in size and, given its accompanying high pill burden, it requires the ingestion of large quantities of water.Citation7
Iron-based phosphate binders include ferric citrate and sucroferric oxyhydroxide. Ferric citrate is partially absorbed and, therefore, is ideal for the management of hyperphosphatemia in patients who are also iron deficient; however, the citrate content increases the potential for aluminum absorption and possible toxicity.Citation28 Sucroferric oxyhydroxide is better suited for dialysis patients who do not require iron supplementation and has the added benefit of low pill burden.Citation28 Findings from a recent meta-analysis suggest that nicotinic acid, a major form of vitamin B3, may be a novel effective alternative or adjunct for lowering serum phosphorus concentrations in dialysis patients.Citation29 It lowers the absorption of phosphorus from the gastrointestinal tract, has unique antilipemic effects, and warrants further investigation of its long-term safety and efficacy.Citation29
Patient tolerance of different phosphate binders varies, and patient-reported reasons for discontinuation of these medications also vary by the type of binder.Citation30 Patients with ESRD may be nonadherent to phosphate binder therapy because of the misconception that nonadherence results in no immediate symptoms or risks.Citation10,Citation11 A systematic review of nonadherence to medications in hemodialysis patients described medication side effects, pill burden, large tablet size, unpalatable taste, medication regimen complexity, difficulty opening the medication container, and prescription refilling as key contributors to nonadherence.Citation12 These medication-based factors are particularly characteristic of phosphate binders and, therefore, represent targets for control strategies.
Epidemiology of phosphate binder adherence
Nonadherence to phosphate binders ranges from 13% to 99%, with an average of 53%.Citation12 This range is wide partly due to heterogeneity in the methods of characterizing non-adherence.Citation12 Current methods of assessing nonadherence to phosphate binders include 1) subjective measures, 2) objective measures, and 3) biochemical assessment of serum phosphorus levels. Estimated rates of nonadherence, assessed by subjective and objective measures as well as biochemical assessment of serum phosphorus levels, are 48%, 78%, and 29% respectively.Citation12
Subjective measures using validated scalesCitation31,Citation32 or non-validated scales or interviewsCitation33,Citation34 are the most widely used methods of nonadherence assessment.Citation12 Objective measures including pill count,Citation35 bottle-use devices such as Medication Event Monitoring System (MEMS) capsCitation36,Citation37 and medication possession ratioCitation38 are the least utilized methods of assessment.Citation12 The biochemical assessment of serum phosphorus levels is frequently conducted as a part of routine dialysis care, but is complicated by variable definitions of the upper limit of the acceptable range.Citation31,Citation39 Moreover, these assessment methods have been used in combination in the absence of universally agreed upon standards for assessment of phosphate binder adherence.Citation40
KDIGO guidelines for phosphate binder use
The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease – Mineral and Bone Disorder (CKD-MBD) was updated in 2017. The current recommendation for serum phosphorus control in dialysis patients is that decisions about phosphate-lowering treatment should be based on progressively or persistently elevated serum phosphate (Not graded), and elevated serum phosphorus levels should be lowered toward the normal range (3.5–5.5 mg/dL) (Grade 2C recommendation). However, implementation of this recommendation is challenging because of laboratory variability in the normal range of phosphorus and diurnal variations in serum phosphorus levels. Furthermore, the KDIGO guidelines recommend restricting the dose of calcium-based phosphate binders (Grade 2B recommendation).Citation41
Economics of phosphate binder use
In the USA, phosphate binder use for US dialysis patients and patients with non-dialysis chronic kidney disease enrolled in Medicare Part D accounted for more than USD 1.5 billion in 2015.Citation42 Phosphate binder-associated costs outweigh the costs associated with all other Part D-covered drugs for patients on dialysis.Citation42 As of 2013, it cost Medicare five times as much for sevelamer carbonate and lanthanum carbonate, compared to calcium acetate, to achieve the same degree of phosphate control in a patient.Citation42 When adjusted for the costs of binders, calcium binders have lower Medicare per member per month costs.Citation43 Data from a recent systematic review suggest that calcium acetate is the most cost-effective therapy for first-line use in dialysis patients, although these conclusions were limited due to the heterogeneity of study quality.Citation44
The enormous costs associated with the use of phosphate binders in dialysis patients in the absence of conclusive evidence of their impact on end points has been a source of controversy.Citation42 There is controversy over the best way to determine the most cost-effective phosphate binder therapy, and much of the debate is due to the quality of existing data. One approach could be to look at overall expenses. It has been suggested that sevelamer is associated with a lower risk of strokeCitation45 as well as reduced Medicare inpatient and total costs as compared with calcium-based binders, which makes it more cost effective overall.Citation43 This continues to be a source of debate and may contribute to mixed messaging to patients about risks/benefits of the various choices.
Factors affecting phosphate binder adherence
Medication factors
Multiple factors have been implicated in nonadherence to phosphate binders (). Medication-related factors responsible for nonadherence to phosphate binders are well studied, and the most commonly acknowledged is pill burden. Phosphate binders are often the single largest contributor to the excessive pill burden for dialysis patients, constituting half of their daily pill burden.Citation35 Dialysis patients take a mean 11 ± 4 medications, with a median daily pill intake of 19 (interquartile range: 12).Citation11 The total number of phosphate binders prescribedCitation46 and total pill burden for other chronic conditions are associated with nonadherence to phosphate binders.Citation35,Citation47,Citation43 The frequency of dosing of phosphate binders with all food intake, including meals, beverages, and snacks, increases its complexity and worsens adherence.Citation12,Citation47 Unfortunately, nonadherence leads to poorer phosphate control and results in an increase in the number of prescribed tablets.Citation10 In addition to pill burden, the form, taste, and side effects – as discussed earlier – are also associated with nonadherence to these medications.Citation46
Table 2 Factors associated with nonadherence and summary of relevant associations (N=38)
Patient factors
Patient-related factors associated with phosphate binder non-adherenceCitation12 include 1) sociodemographic and 2) psychosocial variables. Younger ageCitation10,Citation12,Citation31–Citation33,Citation46–Citation49 has been most consistently linked to phosphate binder nonadherence. Perhaps, younger people are prioritizing other activities over their healthCitation50 or, alternatively, they may be more willing to report nonadherence than older patients.Citation10
Non-Caucasian raceCitation12,Citation23,Citation32,Citation36,Citation37,Citation49 has been associated with phosphate binder nonadherence (odds ratio [OR 0.76]; p < 0.05)Citation49 and may be confounded by lower socioeconomic status.Citation50 Other sociodemographic variables associated with phosphate binder nonadherence include lack of marital supportCitation12,Citation52,Citation53 (OR 1.21; p < 0.05)Citation49 and unemploymentCitation12,Citation52 (OR 1.21; p < 0.05),Citation49 although findings across studies are not consistent.
Psychosocial factors have been identified as the most influential and potentially modifiable determinants of phosphate binder nonadherence (). These include 1) patients’ health beliefs and 2) social support related to hyperphosphatemia treatment.Citation10 These health beliefs include concerns about the potential side effects of phosphate binders (OR = 3.17; 95% CI: 1.87–5.37; p < 0.001);Citation32 reduced beliefs in personal need for phosphate binder medications (OR = 0.34; 95% CI: 0.14–0.83; p < 0.05);Citation32 and low self-efficacy or perceived competence of taking phosphate binders (t (71) = 2.55, p < 0.02).Citation54 Knowledge about the purpose of phosphate binders has been found to be an important factor influencing adherence (r = 0.22; p < 0.05).Citation55 However, knowledge of treatment instructions does not correlate with adherence, suggesting that knowledge alone is insufficient to drive adherence to phosphate binders.Citation10
Figure 1 Psychosocial predictors of nonadherence to phosphate-binding medication assessed by two or more studies.
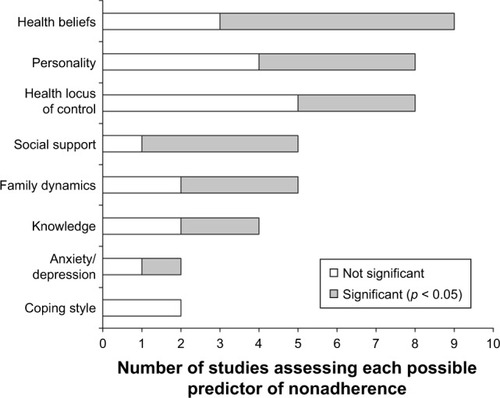
Whereas marital support may be associated with adherence, the support of other family and friends has not yet been demonstrated to have a significant impact on phosphate binder nonadherence.Citation55 Rather, the patient’s perception of illness and its disruption of their family life contributes significantly to phosphate binder nonadherence (r = −0.35; p < 0.05).Citation55
Depressive symptoms, furthermore, have been linked to nonadherence to phosphate binder therapy (OR = 1.11; 95% CI, 1.04–1.18; p = 0.001).Citation52,Citation56,Citation57 Factors such as forgetfulness, lack of interest, and monotony have been identified as contributing to nonadherence.Citation12 Intentional phosphate binder nonadherence behavior exists in dialysis patientsCitation58 and, in order to understand it, it is important to understand the patients’ personal values and level of motivation. This addresses the call for patient-centered care in dialysis management that aligns patients’ values to their therapy while taking into account their side-effect and tolerability profiles.Citation59
Emerging research has identified that novel motivation and autonomy-centered factors are associated with phosphate binder nonadherence. These psychosocial factors are based on the self-determination theory (SDT), which posits that autonomy is an essential factor for achieving durable positive change.Citation60 The SDT distinguishes between autonomous or self-motivated behavior and controlled behavior. It includes three unique psychosocial factors: autonomous regulation, autonomy support, and perceived competence.Citation60 Higher autonomous regulation of phosphate binder therapy or more positive attitudes toward phosphate binder use has been associated with phosphate binder adherence.Citation47,Citation61 Similarly, the perception of dialysis patients with regard to their providers’ support of autonomy for phosphate binder therapy and patient empowerment are associated with adherence to binders.Citation62 Moreover, perceived competence or self-efficacy has been associated with adherence to phosphate binder therapy.Citation63 These factors show great promise to better understand nonadherence as they are associated with self-reported phosphate binder adherence,Citation61,Citation62 and are potentially modifiable using patient-centered approaches, such as motivational interviewing.Citation60,Citation64
Racial differences in the relationship between these novel psychosocial factors, phosphate binder adherence, and phosphorus control suggest that they may be more important in non-whites. Non-white dialysis patients have a lower perception of provider support for phosphate binder adherence as compared to whites.Citation62 Furthermore, the association between autonomous regulation of phosphate binder therapy and serum phosphorus control is significant in non-whites (β 95% CI: −0.38 [−0.74 to −0.02]; p = 0.04) but not in whites (β 95% CI: 0.49 [−0.00 to 0.99]; p = 0.05).Citation61
Phosphate binder adherence in the elderly
Polypharmacy has been identified as a geriatric-related syndrome that is associated with medication nonadherence,Citation51 and it is exacerbated when the regimen includes phosphate binders. There are currently no guidelines for achieving a balance between phosphate adherence and health-related quality of life for the elderly, or others with predicted poor survival. Therefore, the overarching principle for phosphate-control adherence is the delivery of patient-centered care, with individualization of phosphorus-control regimens to optimize health-related quality of life in the elderly.
Provider factors
The World Health Organization (WHO) highlights provider factors as important determinants of patient adherence to prescribed medication and emphasizes that 1) “patients need to be supported by providers, not blamed”, 2) providers need to be able to assess adherence and factors that influence it, and 3) providers must be adequately trained in adherence management.Citation65 Provider factors relevant to phosphate binder nonadherence have not been fully investigated. Interview and focus-group data from hemodialysis patients suggested the presence of adversarial interactions between dialysis patients and their providers that impact their adherence.Citation66 In particular, dialysis providers do not 1) individualize their patients’ care, but rather, deliver “assembly line” treatment, 2) recognize patients’ knowledge based on their unique expertise on their bodies and experience gained from their chronic illness, or 3) engage in shared decision making.Citation66
Provider attitudes have been shown to correlate with clinical outcomes. For instance, facilities with providers that have more positive attitudes toward transplants have better wait-listing performance.Citation67 The phosphate binder prescription patterns of dialysis providers are highly variable, and some dialysis units prescribed phosphate binders for a significantly smaller proportion of their dialysis patients.Citation5 This suggests differences in the preferences of dialysis providers for, or attitudes toward, phosphate binders.Citation5 Patients who positively characterize their interactions with their dialysis providers have a lower odds for nonadherence to phosphate binders (OR = 0.52; 95% CI, 0.30–0.90).Citation33 Further, support by dialysis staff is associated with phosphate binder adherence (r = 0.20; p < 0.05).Citation55 Provider interventions show promise of a positive and sustained impact on medication adherence in dialysis patients.
Dialysis treatment and hospitalizations affect medication adherence
Longer duration on hemodialysis therapy of 5 years or more has been found to be the most consistent ESRD factor associated with phosphate binder nonadherence.Citation17,Citation47,Citation68 Perhaps, the longer duration on hemodialysis leads to more boredom and frustration over the need for continued adherence to this challenging medication regimen.Citation17 Another important consideration is the relatively frequent acute illnesses leading to hospitalization. This disrupts the rigorous routine of day-to-day phosphate binder medications and increases the perception of burden of therapy.Citation47 In general, hospitalizations also have an adverse impact on medication adherence due to errors in medication reconciliationCitation69 and patients’ limited understanding of the post-discharge treatment plan.Citation70,Citation71
Interventions to improve phosphate binder adherence
Patient education
Knowledge about the rationale for phosphate binders is associated with improved patient adherence to phosphate binder therapy.Citation33 Patients need to be effectively educated about the risk associated with phosphate binder nonadherence and, specifically, its association with increased morbidity and mortality.Citation14,Citation19,Citation72 Patient education about appropriate timing of dosing toward the end of each meal, as well as adjusting dosing to the phosphorus content of the food, is important to ensure binder efficacy.Citation14
Effective approaches for phosphate binder education utilize tools such as pamphlets, posters, websites, and videos.Citation14 However, the readability of many available patient-education materials remains a concern, with text written at above the ninth-grade levels and formatting that does not meet standards for optimal usability.Citation73,Citation74 Delivery of education occurs in all formats, including face-to-face individual consultations, group education, telephonic consultations, and practical demonstrationsCitation19 such as lobby days in dialysis units. Individual educational sessions for binder adherence have the benefit of providing personalized education, but are resource and time intensive. Education in small groups promotes interaction among dialysis patient peers, and has been shown to improve phosphate binder knowledge, as well as adherence, when facilitated by a dietitian.Citation19,Citation75
Incorporation of patient preferences
Dosing regimens can be simplified by reducing pill burden and adjusting phosphate binder prescriptions to accommodate the patient’s dialysis preferences.Citation11 For instance, some patients favor calcium acetate gel caps over the tablets because of ease of swallowingCitation76 whereas others have a preference for lanthanum because it requires fewer tablets.Citation77 Adopting an individualized strategy that takes into account patient preferences with regard to phosphate binders resulted in significant improvement in intentional nonadherence, phosphorus control, and even costs related to phosphate binder use.Citation27 This strategy empowers patients to request a change in binder type if they have had problems with the prescribed phosphate binders. It is recommended to offer alternate options to patients who object to a particular dosing method.Citation14
Patient empowerment techniques
Counseling interventions that incorporate a cognitive or behavioral component could be most effective for improving phosphate binder adherence.Citation19,Citation78 Cognitive behavioral interventions are psychological strategies that focus on the association between thoughts, feelings, and emotions and assist patients in identifying and modifying negative thoughts, feelings, and behaviors to facilitate coping. They may include education or relaxation training delivered in different settings and formats. Motivational interviewing – an autonomy-promoting style of communication – has been shown, in a small study, to improve phosphate binder adherence and phosphorus control.Citation79 This style of communication effectively engages patients to focus on a behavioral change; resolve ambivalence; and make plans that are specific, measurable, action-based, realistic, and time-based (). Motivational interviewing uses strategies such as open-ended questions, affirmations, reflections, and summaries.Citation80 Similarly, self-affirmation – which involves reflection on one’s personal values in order to reduce resistance to health-risk information – has been successfully used to improve adherence.Citation81 These patient empowerment techniques address the most influential factors of phosphate binder adherence, including beliefs and attitudes.Citation10
Table 3 Motivational interviewing
Other potential novel approaches for patient empowerment in improving medication adherence include the use of electronic monitoring devices. These can be used to remind patients to take their medications at prescribed times and may be helpful in empowering patients to improve phosphate binder medication adherence.Citation82 The Phosphate Education Program (PEP) is a novel program that incorporates patient empowerment by teaching patients how to estimate the phosphorus content of their food and adjust their phosphate binder therapy using a prescribed binder/unit ratio.Citation83 This program equips patients for “eye-estimating” the phosphorus content of various foods to guide these real-time adjustments. It assigns similar phosphorus units to similar whole food groups whereby 1 phosphorus unit is equivalent to 100 mg per serving.Citation84 Informed by similar approaches in diabetes management, this approach seems promising; however, additional complexities of dietary recommendations for phosphorus management must be acknowledged.
Dietary approaches to phosphorus control
Epidemiology of adherence to low-phosphorus diet
An integrative review of studies on adherence to the renal diet reports a wide variation in dietary adherence.Citation16 This is related to differences in how dietary adherence was measured – ranging from subjective approaches involving self-reported adherence to indirect approaches using serum phosphorus levels or a combination of approaches. Adherence to a low-phosphorus diet from 15 studies of 12,571 ESRD patients ranges from 43% to 84% and the majority of these studies measured low phosphate dietary adherence using serum phosphorus levels.Citation16 Interestingly, one study that measured the rates of low phosphate dietary adherence using two different methods reported a self-reported adherence rate of 33% as compared to an adherence rate of 44% when using serum phosphorus levels.Citation85
Types of dietary phosphorus
Dietary phosphorus is obtained from three different sources: 1) organic phosphorus in plant foods; 2) organic phosphorus in animal protein; and 3) inorganic phosphorus from additives in processed food.Citation14 The phosphorus content in plant foods has only 20%–40% bioavailability whereas the phosphorus content in animal protein has 40%–60% bioavailability.Citation14 In sharp contrast, the phosphorus content from food additives has 100% bioavailability and has the most impact on hyperphosphatemia.Citation14
Phosphorus additives
The “hidden” phosphorus content from phosphate additives found in processed foodsCitation86,Citation87 increases the complexity of dietary phosphorus management. The presence of unlabeled phosphorus content in many foods, in addition to the wide array of foods high in natural phosphorus content, contributes to nonadherence to a low-phosphorus diet. More recently, emphasis has been placed on the reduction of phosphate additives by avoidance of processed, high-phosphorus protein sources.Citation14
Balancing the protein-to-phosphorus ratio
Dietary phosphorus restriction is complex because of the delicate balance between ensuring adequate protein intake and simultaneously restricting phosphorus intake.Citation11,Citation14 Achieving this balance is a high priority because higher protein intake (up to 1.4 g/kg/day) has been linked to increased survival in dialysis patients, regardless of a simultaneous increase in serum phosphorus levels.Citation88 Yet, higher levels of dietary phosphorus intake and higher dietary phosphorus-to-protein ratios increase the 5-year mortality rates in hemodialysis.Citation89 Interestingly, prior research has not yet demonstrated a survival benefit as a result of prescribed dietary phosphorus restriction.Citation13 This may be explained, in part, because dietary phosphorus restriction is insufficient to reduce serum phosphorus load.Citation90 A daily low-phosphorus diet includes approximately 371 mg of absorbed phosphorus each day. Therefore, phosphorus control inherently requires strategies in addition to dietary restriction.Citation11,Citation14
Factors affecting adherence to low-phosphorus diet
presents factors affecting adherence to a low-phosphorus diet. A recent integrative review of dietary adherence in dialysis, including adherence to low-phosphorus diet, provides a detailed overview of contributory factors.Citation16 Longer dialysis vintage has been associated with nonadherence to a low-phosphorus diet,Citation16,Citation17,Citation49 perhaps due to the burden of managing such complex dietary recommendations for an extended time.Citation16 Poor dietary knowledge has been linked to nonadherence to phosphorus-restricted diet.Citation17,Citation91,Citation92 Furthermore, dialysis patients have acknowledged that the diet is challenging to incorporate into social occasions and dietary advice, preferably from renal dietitians or nephrologists, is of utmost importance.Citation93
Table 4 Factors associated with dietary adherence in adults with ESRD on hemodialysis categorized according to WHO criteria
Patient factors associated with nonadherence to low-phosphorus diet include age, gender, race, and education level.Citation16 Younger age (r = 0.19; p < 0.05) and male gender are more likely to predict dietary nonadherence (r = 0.25; p < 0.05). Nonwhites have been found to have more dietary nonadherence,Citation85,Citation92 and this may be driven by lower socioeconomic status. Employment status has been associated with dietary nonadherence in dialysis patients (r = −0.36; p < 0.01), and this may be because it is challenging to juggle the demands of the diet with the rigors of employment. Lower education level has been consistently associated with dietary nonadherence.Citation17,Citation94–Citation98
Several psychosocial factors have been consistently associated with dietary nonadherence in dialysis. Negative beliefs and attitudes were strongly linked to dietary nonadherence.Citation92,Citation96 Moreover, patients with lower self-efficacy or depressive symptoms experienced dietary nonadherence.Citation17,Citation85,Citation99,Citation100 Poor coping skills have correlated with nonadherence to a low-phosphorus diet.Citation68 Negative peer pressure or lack of acceptance of the prescribed diet by family or friendsCitation93 worsen dietary nonadherence.
Poor interaction between patients and dialysis providers is associated with dietary nonadherence,Citation16 and conflicting dietary advice from different dialysis clinicians is also associated with nonadherence.Citation17 Limited dietary education and support from renal dietitiansCitation49,Citation91,Citation101 was associated with dietary nonadherence, and this has largely been attributed to suboptimal staffing ratios.Citation49,Citation91
Interventions to improve adherence to low-phosphorus diet
Patient education
Effective education on dietary phosphorus restriction should include specific recommendations of foods with minimal inorganic phosphorus content, foods without phosphorus additives, low phosphorus-to-protein ratios, and adequate protein content ().Citation14 Patients need to understand that plant foods, animal-derived foods, and food additives have a range of low to high phosphorus bioavailability.Citation14 Examples of food options that have the lowest phosphorus-to-protein ratio include non-dairy products and animal foods with high protein content such as egg whites.Citation89 These food selections can effectively lower serum phosphorus level while simultaneously increasing the albumin level.Citation102 In addition, education should include cooking methods that preserve protein content while reducing phosphorus content (eg, boiling chicken) to promote the low-phosphorus diet.Citation14,Citation103
Table 5 Strategies to improve control of dietary phosphorus intake and adherence to phosphate binders in ESRD
Food fatigue or getting tired of eating the same allowed food has been identified as a larger problem than food intolerance or allergies in dietary management of chronic kidney disease, including ESRD.Citation14 Food fatigue can be ameliorated by diversifying the diet to include additional low-phosphorus, high-protein food options such as poultry.Citation14 Phosphate binder medication therapy, when taken effectively, also reduces food fatigue by permitting the patient’s preferred mainstream foods while controlling their serum phosphorus levels.Citation14
Ideal patient education tools include information estimating the phosphorus content of food with glossaries of additives to guide the interpretation of food labels; comprehensive labeling of phosphorus additives; and use of a “traffic light” scheme to classify foods based on low, intermediate, or high phosphorus content.Citation104 Educational interventions to improve phosphorus control through dietary restriction have demonstrated improvements in patient knowledge, adherence to the low-phosphorus diet, as well as serum phosphorus levels.Citation19,Citation91,Citation105 More recent educational initiatives, such as the Phosphate Education Program described earlier, empower patients to tailor the phosphorus content of food to their phosphate binder use per meal, leading to improved control of hyperphosphatemia.Citation14,Citation83,Citation84
Dietitian-led interventions have been successful.Citation75,Citation106,Citation107 Systematically delivered nursing instruction on low-phosphorus diet using a nursing instruction pamphlet, pictures, and reminder cards has also been shown to improve adherence, reduce serum phosphorus levels, and improve pruritus.Citation108 Although comprehensive low-phosphorus dietary education, developed and delivered by dialysis nurses and physicians, was effective in improving serum phosphorus levels,Citation109 dietitian involvement has been found to be more effective.Citation109
Behavioral interventions
Behavioral interventions to improve dietary phosphate adherence also commonly employ counseling delivered by dietitians.Citation19 Some interventions have been grounded in theoretical frameworks such as self-regulation theoryCitation91 and self-efficacy theory.Citation110 Individualized self-management dietary counseling – especially in combination with patient education – improves patient knowledge, dietary adherence, and serum phosphorus level.Citation91 Use of a phosphate management protocol incorporating dietary counseling as well as patient education and pharmacotherapy delivered by a dialysis dietitian and a dialysis pharmacist, respectively, has led to greater improvement in serum phosphorus control compared to usual care.Citation111 A motivational interviewing pilot study, focusing on dietary, medication, and dialysis attendance, demonstrated a positive impact on serum phosphorus control.Citation79
Other potential strategies
Patients have expressed frustration about insufficient psychosocial support and information from providers that affects their self-care.Citation112 Provider communication skills as well as the provider–patient relationship and interactions may have an impact on adherence to the prescribed low-phosphorus diet.Citation16 Providers need to recognize that patients have limited self-efficacyCitation17,Citation85 and suboptimal attitudesCitation92,Citation93 that contribute to poor adherence to a low-phosphorus diet and are potential modifiable targets. Staffing ratios in the dialysis unit has been linked to adherence metrics and need to be optimized. For instance, a ratio of no more than 60 dialysis patients per dietitian with monthly consultations has been shown to be more effective in improving phosphate binder adherence and serum phosphate control.Citation14 Provider-level strategies may be an important opportunity to complement ongoing patient-focused interventions to improve dietary adherence in dialysis patients, and all members of the multidisciplinary team should be equipped to deliver phosphate binder adherence education and counseling.Citation14
Dialysis
Conventional 4-hour thrice-weekly hemodialysis is limited in its ability to lower the phosphorus levels associated with the average dietary intake of phosphorus.Citation19,Citation113 Phosphorus removal through conventional hemodialysis occurs primarily during the first half of treatment, after which the serum phosphorus levels either plateau or even increase again (by up to 30%–40%) due to a rebound effect.Citation114 The daily phosphorus intake of dialysis patients can average 1,500 mg/day or 10,500 mg/week and, if 50% of that is absorbed, the phosphorus excess for removal by dialysis could be greater than 5,000 mg.Citation15 However, conventional hemodialysis removes an average of 1,800–3,600 mg of phosphorus per week. Therefore, conventional hemodialysis alone is usually insufficient for phosphorus control.Citation19 Optimal dialytic clearance of phosphorus is dependent on slow flow rates in addition to a longer dialysis time. Daily or extended nocturnal hemodialysis leads to greater phosphorus clearance compared with conventional thrice-weekly hemodialysis sessions.Citation115
Dialysis treatment nonadherence
Dialysis treatment nonadherence is a significant problem.Citation49 As much as 35% of patients miss treatments entirely whereas another 32% shorten their treatment time.Citation20 In addition to its direct impact on hyperphosphatemia management, dialysis treatment nonadherence has been linked to increased hospitalizationsCitation116,Citation117 and mortality.Citation23,Citation49 This high rate of treatment nonadherence has persisted and is linked to age,Citation23,Citation36 gender,Citation118 marital status,Citation118 ethnicity,Citation23,Citation119 and educationCitation118 as well as comorbidities and logistical barriers such as holidays that alter the dialysis unit scheduling.Citation116 As with other chronic diseases requiring self-management, autonomy-centered psychosocial factors may be important, modifiable determinants of dialysis adherence.Citation120 Deliberate multidisciplinary strategies to increase patient engagement in dialysisCitation59,Citation121,Citation122 is increasingly recognized as a high-value opportunity to impact adherence behaviors and outcomes.
Conclusion
Phosphate-control adherence is a fundamental component of care in dialysis, and adherence rates to phosphate binder therapy, low-phosphorus diet, and dialysis attendance remain suboptimal. Factors responsible for nonadherence include those related to the therapy (eg, medications, diet, dialysis); patient-specific factors including demographic, clinical, and psychosocial determinants, and provider-level factors. Psychosocial factors are the most influential determinants of adherence because they can be effectively modified using strategies that incorporate cognitive behavioral interventions to change negative beliefs, attitudes, and behaviors across treatment approaches to optimize phosphorus control. Provider-level factors are critical determinants of phosphate-control adherence in dialysis patients. Thus, provider–patient relationships must be enhanced by ensuring positive provider attitudes, adequate staffing ratio, and improved staff effectiveness by role clarification and training. All dialysis providers must be skilled in the delivery of culturally sensitive, patient-centered care using a novel combination of effective strategies and protocols. Optimal phosphate-control adherence rates will require multilevel interventions that recognize and address the preferences and unique attitudes of dialysis patients, enhance their self-regulation behaviors, and empower them to achieve sustained phosphate binder adherence.
Acknowledgments
The authors acknowledge the assistance of Heather Laferriere, MLIS, Library Liaison for Health Sciences at the Vanderbilt University Eskind Biomedical library for her support with detailed literature review from the selected databases.
Disclosure
Dr Umeukeje is supported by a BIRCWH K12 award (K12HDO43483-17 – PI: Katherine Hartmann). Dr Cavanaugh is supported by an NIH R01DK03935-01A1. Dr Mixon is supported by the Geriatric Research Education and Clinical Center (GRECC) at the VA Tennessee Valley Healthcare System. The authors report no other conflicts of interest in this work.
References
- MalbertiFHyperphosphataemia: treatment optionsDrugs201373767368823625273
- SlatopolskyEGonzalezEMartinKPathogenesis and treatment of renal osteodystrophyBlood Purif2003214–531832612944733
- TentoriFBlayneyMJAlbertJMMortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS)Am J Kidney Dis200852351953018514987
- KettelerMBiggarPHUse of phosphate binders in chronic kidney diseaseCurr Opin Nephrol Hypertens201322441342023736841
- LopesAATongLThummaJPhosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional statusAm J Kidney Dis20126019010122385781
- de BoerIHKovesdyCPNavaneethanSDAmerican Society of Nephrology Chronic Kidney Disease Advisory GroupPragmatic clinical trials in CKD: opportunities and challengesJ Am Soc Nephrol201627102948295427283497
- ArenasMDMalekTAlvarez-UdeFGilMTMoledousAReig-FerrerACaptores del fósforo: preferencias de los pacientes en hemodiálisis y su repercusión sobre el cumplimiento del tratamiento y el control del fósforo. [Phosphorus binders: preferences of patients on haemodialysis and its impact on treatment compliance and phosphorus control]Nefrologia2010305522530 Spanish [with English abstract]20613851
- IsakovaTNickolasTLDenburgMKDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD)Am J Kidney Dis201770673775128941764
- SchmidHHartmannBSchifflHAdherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literatureEur J Med Res200914518519019541573
- KaramanidouCClatworthyJWeinmanJHorneRA systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal diseaseBMC Nephrol20089218237373
- CovicARastogiAHyperphosphatemia in patients with ESRD: assessing the current evidence linking outcomes with treatment adherenceBMC Nephrol201314115323865421
- GhimireSCastelinoRLLioufasNMPetersonGMZaidiSTNonadherence to medication therapy in haemodialysis patients: a systematic reviewPLoS One20151012e014411926636968
- LynchKELynchRCurhanGCBrunelliSMPrescribed dietary phosphate restriction and survival among hemodialysis patientsClin J Am Soc Nephrol20116362062921148246
- Kalantar-ZadehKPatient education for phosphorus management in chronic kidney diseasePatient Prefer Adherence2013737939023667310
- WaheedAAPedrazaFLenzOIsakovaTPhosphate control in end-stage renal disease: barriers and opportunitiesNephrol Dial Transplant201328122961296823901051
- LambertKMullanJMansfieldKAn integrative review of the methodology and findings regarding dietary adherence in end stage kidney diseaseBMC Nephrol20171831829061163
- ChanYMZalilahMSHiiSZDeterminants of compliance behaviours among patients undergoing hemodialysis in MalaysiaPLoS One201278e4136222870215
- HouSHZhaoJEllmanCFCalcium and phosphorus fluxes during hemodialysis with low calcium dialysateAm J Kidney Dis19911822172241867178
- MilaziMBonnerADouglasCEffectiveness of educational or behavioral interventions on adherence to phosphate control in adults receiving hemodialysis: a systematic reviewJBI Database System Rev Implement Rep20171549711010
- DenhaerynckKManhaeveDDobbelsFGarzoniDNolteCDe GeestSPrevalence and consequences of nonadherence to hemodialysis regimensAm J Crit Care2007163222235 quiz 23617460313
- TohmeFMorMKPena-PolancoJPredictors and outcomes of non-adherence in patients receiving maintenance hemodialysisInt Urol Nephrol20174981471147928455663
- KutnerNGZhangRMcClellanWMColeSAPsychosocial predictors of non-compliance in haemodialysis and peritoneal dialysis patientsNephrol Dial Transplant2002171939911773470
- LeggatJEJrOrzolSMHulbert-ShearonTENoncompliance in hemodialysis: predictors and survival analysisAm J Kidney Dis19983211391459669435
- O’BrienMECompliance behavior and long-term maintenance dialysisAm J Kidney Dis19901532092142106260
- SekerciogluNThabaneLDiaz MartinezJPComparative effectiveness of phosphate binders in patients with chronic kidney disease: a systematic review and network meta-analysisPLoS One2016116e015689127276077
- LocatelliFDel VecchioLVioloLPontorieroGPhosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profilesExpert Opin Drug Saf201413555156124702470
- Arenas JiménezMDParra MoncasiEÁlvarez-Ude CoteraFA strategy based on patient preference improves the adherence and outcomes of phosphate binders in hemodialysisClin Nephrol2017881111
- NegriALUrena TorresPAIron-based phosphate binders: do they offer advantages over currently available phosphate binders?Clin Kidney J20158216116725815172
- LiuXYangRDaiBZhangHWangJMaNNicotinic acid and related compounds: a meta-analysis of their use for hyperphosphatemia in dialysis patientsMedicine (Baltimore)20189712e011729561409
- WangSAnumEARamakrishnanKAlfieriTBraunhoferPNewsomeBReasons for phosphate binder discontinuation vary by binder typeJ Ren Nutr201424210510924462496
- WilemanVFarringtonKWellstedDAlmondMDavenportAChilcotJMedication beliefs are associated with phosphate binder non-adherence in hyperphosphatemic haemodialysis patientsBr J Health Psychol201520356357825209368
- ChaterAMParhamRRileySHutchisonAJHorneRProfiling patient attitudes to phosphate binding medication: a route to personalising treatment and adherence supportPsychol Health201429121407142025012529
- MartinsMTSilvaLFKraycheteAPotentially modifiable factors associated with non-adherence to phosphate binder use in patients on hemodialysisBMC Nephrol20131420824090377
- LindbergMPrützKGLindbergPWikströmBInterdialytic weight gain and ultrafiltration rate in hemodialysis: lessons about fluid adherence from a national registry of clinical practiceHemodial Int200913218118819432692
- ChiuYWTeitelbaumIMisraMde LeonEMAdzizeTMehrotraRPill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patientsClin J Am Soc Nephrol2009461089109619423571
- CurtinRBSvarstadBLAndressDKellerTSackstederPDifferences in older versus younger hemodialysis patients’ noncompliance with oral medicationsGeriatr Nephrol Urol19977135449422438
- CurtinRBSvarstadBLKellerTHHemodialysis patients’ noncompliance with oral medicationsANNA J1999263307316 discussion 317, 33510633602
- ParkHRascatiKLLawsonKABarnerJCRichardsKMMaloneDCAdherence and persistence to prescribed medication therapy among Medicare part D beneficiaries on dialysis: comparisons of benefit type and benefit phaseJ Manag Care Spec Pharm201420886287625062080
- Dolores ArenasMPerez-GarciaRBennounaMEstudio COMQUELFOSImprovement of therapeutic compliance in haemodialysis patients with poor phosphorus control and adherence to treatment with binders: COMQUELFOS studyNefrologia201333219620323364580
- GhimireSPetersonGMCastelinoRLJoseMDZaidiSTRMedication regimen complexity and adherence in haemodialysis patients: an exploratory studyAm J Nephrol201643531832427166159
- KettelerMBlockGAEvenepoelPDiagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder: Synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline updateAnn Intern Med2018168642243029459980
- St PeterWLWaznyLDWeinhandlEDPhosphate-binder use in US dialysis patients: prevalence, costs, evidence, and policiesAm J Kidney Dis201871224625329195858
- St PeterWLFanQWeinhandlELiuJEconomic evaluation of sevelamer versus calcium-based phosphate binders in hemodialysis patients: a secondary analysis using centers for Medicare & Medicaid services dataClin J Am Soc Nephrol20094121954196119833904
- RizkRHiligsmannMKaravetianMEversSMEconomic evaluations of interventions to manage hyperphosphataemia in adult haemodialysis patients: a systematic reviewNephrology (Carlton)201621317818726246269
- PanichiVRosatiADi GiorgioAA pharmacoeconomic analysis of phosphate binders cost-effectiveness in the RISCAVID studyBlood Purif2015391–317418025765293
- ArenasMDMalekTGilMTMoledousAAlvarez-UdeFReig-FerrerAChallenge of phosphorus control in hemodialysis patients: a problem of adherence?J Nephrol201023552553420119931
- NeriLMartiniAAndreucciVEGallieniMReyLABrancaccioDMigliorDialisi Study GroupRegimen complexity and prescription adherence in dialysis patientsAm J Nephrol2011341717621677429
- WilemanVChilcotJNortonSHughesLWellstedDFarringtonKChoosing not to take phosphate binders: the role of dialysis patients’ medication beliefsNephron Clin Pract20111193c205c21321832846
- SaranRBragg-GreshamJLRaynerHCNonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPSKidney Int200364125426212787417
- JinJSklarGEMin Sen OhVChuen LiSFactors affecting therapeutic compliance: a review from the patient’s perspectiveTher Clin Risk Manag20084126928618728716
- ChaoCTHuangJWCOGENT (COhort of GEriatric Nephrology in NTUH) study groupGeriatric syndromes are potential determinants of the medication adherence status in prevalent dialysis patientsPeerJ20164e212227326380
- TheofilouPMedication adherence in Greek hemodialysis patients: the contribution of depression and health cognitionsInt J Behav Med201320231131822407452
- AlkatheriAMAlyousifSMAlshabanahNMedication adherence among adult patients on hemodialysisSaudi J Kidney Dis Transpl201425476276824969185
- ChristensenAJWiebeJSBenotschEGLawtonWJPerceived health competence, health locus of control, and patient adherence in renal dialysisCognit Ther Res1996204411421
- CummingsKMBeckerMHKirschtJPLevinNWPsychosocial factors affecting adherence to medical regiments in a group of hemodialysis patientsMed Care19822065675807109740
- OssarehSTabrizianSZebarjadiMJoodatRSPrevalence of depression in maintenance hemodialysis patients and its correlation with adherence to medicationsIran J Kidney Dis20148646747425362222
- Rosenthal AsherDVer HalenNCukorDDepression and nonadherence predict mortality in hemodialysis treated end-stage renal disease patientsHemodial Int201216338739322469200
- PatelPAntoniouSPopatRUnintentional non-adherence to phosphate bindersEur J Hosp Pharm20152211822
- CavanaughKLPrioritizing patient-centered care implementation and research for patients with kidney diseaseSemin Dial201528213114025470535
- DeciELRyanRMSelf-determination theory in health care and its relations to motivational interviewing: a few commentsInt J Behav Nutr Phys Act201292422385839
- UmeukejeEMMerighiJRBrowneTSelf-motivation Is associated with phosphorus control in end-stage renal diseaseJ Ren Nutr201525543343925912398
- UmeukejeEMMerighiJRBrowneTHealth care providers’ support of patients’ autonomy, phosphate medication adherence, race and gender in end stage renal diseaseJ Behav Med20163961104111427167227
- UmeukejeEPerceived competence is related to phosphorus control in end stage renal diseaseIDDK Network of Minority Research Investigators Conference20–22 April 2016Bethesda, MA
- MillerWRRollnickSMeeting in the middle: motivational interviewing and self-determination theoryInt Behav Nutr Phys Act2012925
- SabateEAdherence to Long-term Therapies: Evidence for ActionGenevaWorld Health Organization2003
- AllenDWainwrightMHutchinsonT‘Non-compliance’ as illness management: hemodialysis patients’ descriptions of adversarial patient–clinician interactionsSoc Sci Med201173112913421665340
- GanderJBrowneTPlantingaLDialysis facility transplant philosophy and access to kidney transplantation in the SoutheastAm J Nephrol201541650451126278585
- O’ConnorSMJardineAGMillarKThe prediction of self-care behaviors in end-stage renal disease patients using Leventhal’s Self-Regulatory ModelJ Psychosom Res200865219120018655865
- OmoriDMPotykRPKroenkeKThe adverse effects of hospitalization on drug regimensArch Intern Med19911518156215641872660
- CalkinsDRDavisRBReileyPPatient-physician communication at hospital discharge and patients’ understanding of the postdischarge treatment planArch Intern Med19971579102610309140275
- ZiaeianBAraujoKLVan NessPHHorwitzLIMedication reconciliation accuracy and patient understanding of intended medication changes on hospital dischargeJ Gen Intern Med201227111513152022798200
- de AraujoLPFigueiredoAEd’AvilaDOAvaliação de programa de ensino-aprendizagem sobre metabolismo de cálcio e fósforo para pacientes em hemodiálise. [Evaluation of an educational program on calcium and phosphorus metabolism for patients on hemodialysis]Rev Esc Enferm USP2010444928932 Portuguese [with English abstract]21337773
- TuotDSCavanaughKLEvaluating the merits of CKD patient educational materials: readability is necessary but not sufficientAm J Kidney Dis201565681481626003608
- TuotDSDavisEVelasquezABanerjeeTPoweNRAssessment of printed patient-educational materials for chronic kidney diseaseAm J Nephrol201338318419423970127
- ReddyVSymesFSethiNDietitian-led education program to improve phosphate control in a single-center hemodialysis populationJ Ren Nutr200919431432019539185
- KaplanMRStashenkoCLBledsoeALMcGowanJA preference study: calcium acetate tablets versus gelcaps in hemodialysis patientsNephrol Nurs J200229436336512224369
- MehrotraRMartinKJFishbaneSSpragueSMZeigSAngerMHigher strength lanthanum carbonate provides serum phosphorus control with a low tablet burden and is preferred by patients and physicians: a multicenter studyClin J Am Soc Nephrol2008351437144518579668
- MattesonMLRussellCInterventions to improve hemodialysis adherence: a systematic review of randomized-controlled trialsHemodial Int201014437038220796047
- RussellCLCronkNJHerronMMotivational interviewing in dialysis adherence study (MIDAS)Nephrol Nurs J201138322923621877456
- LevenskyERForcehimesAO’DonohueWTBeitzKMotivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendationsAm J Nurs2007107105058 quiz 58–59
- WilemanVFarringtonKChilcotJEvidence that self-affirmation improves phosphate control in hemodialysis patients: a pilot cluster randomized controlled trialAnn Behav Med201448227528124532394
- Obrero ChurilloTMedication reminder systems: an adjunct technique in improving phosphate binder adherenceJ Ren Nutr2012221e3e9
- KuhlmannMKManagement of hyperphosphatemiaHemodial Int200610433834517014508
- KuhlmannMKHoechstSLandthalerIPatient empowerment in the management of hyperphosphatemiaInt J Artif Organs200730111008101318067103
- ElliottJOOrtmanCAlmaaniSLeeYHJordanKUnderstanding the associations between modifying factors, individual health beliefs, and hemodialysis patients’ adherence to a low-phosphorus dietJ Ren Nutr201525211112025282006
- UribarriJPhosphorus additives in food and their effect in dialysis patientsClin J Am Soc Nephrol2009481290129219608709
- BeniniOD’AlessandroCGianfaldoniDCupistiAExtra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patientsJ Ren Nutr201121430330821055967
- ShinabergerCSGreenlandSKoppleJDIs controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease?Am J Clin Nutr20088861511151819064510
- NooriNKalantar-ZadehKKovesdyCPBrossRBennerDKoppleJDAssociation of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patientsClin J Am Soc Nephrol20105468369220185606
- MartinKJGonzalezEAPrevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat?Clin J Am Soc Nephrol20116244044621292848
- KaravetianMGhaddarSNutritional education for the management of osteodystrophy (nemo) in patients on haemodialysis: a randomised controlled trialJ Ren Care20133911930
- ThomasLKSargentRGMichelsPCRichterDLValoisRFMooreCGIdentification of the factors associated with compliance to therapeutic diets in older adults with end stage renal diseaseJ Ren Nutr2001112808911295028
- HollingdaleRSuttonDHartKFacilitating dietary change in renal disease: investigating patients’ perspectivesJ Ren Care200834313614218786080
- KaraBCaglarKKilicSNonadherence with diet and fluid restrictions and perceived social support in patients receiving hemodialysisJ Nurs Scholarsh200739324324817760797
- KuglerCVlaminckHHaverichAMaesBNonadherence with diet and fluid restrictions among adults having hemodialysisJ Nurs Scholarsh2005371252915813583
- MellonLReganDCurtisRFactors influencing adherence among Irish haemodialysis patientsPatient Educ Couns2013921889323481215
- FordJCPopeJFHuntAEGeraldBThe effect of diet education on the laboratory values and knowledge of hemodialysis patients with hyperphosphatemiaJ Ren Nutr2004141364414740329
- ParkKAChoi-KwonSSimYMKimSBComparison of dietary compliance and dietary knowledge between older and younger Korean hemodialysis patientsJ Ren Nutr200818541542318721736
- ZrinyiMJuhaszMBallaJDietary self-efficacy: determinant of compliance behaviours and biochemical outcomes in haemodialysis patientsNephrol Dial Transplant20031891869187312937237
- KhalilAAFrazierSKLennieTASawayaBPDepressive symptoms and dietary adherence in patients with end-stage renal diseaseJ Ren Care2011371303921288315
- Morales LópezCBurrowesJDGizisFBrommageDDietary adherence in Hispanic patients receiving hemodialysisJ Ren Nutr200717213814717321954
- TaylorLMKalantar-ZadehKMarkewichTDietary egg whites for phosphorus control in maintenance haemodialysis patients: a pilot studyJ Ren Care2011371162421288313
- CupistiAD’AlessandroCBaldiRBarsottiGDietary habits and counseling focused on phosphate intake in hemodialysis patients with hyperphosphatemiaJ Ren Nutr200414422022515483782
- RitzEHahnKKettelerMKuhlmannMKMannJPhosphate additives in food – a health riskDtsch Arztebl Int20121094495522334826
- BrogdonRMA self-care educational intervention to improve knowledge of dietary phosphorus control in patients requiring hemodialysis: a pilot studyNephrol Nurs J2013404313318 quiz 31924175440
- MayneTJBennerDRickettsKResults of a pilot program to improve phosphorus outcomes in hemodialysis patientsJ Ren Nutr201222547247922056148
- CampbellKLAshSZabelRMcFarlaneCJuffsPBauerJDImplementation of standardized nutrition guidelines by renal dietitians is associated with improved nutrition statusJ Ren Nutr200919213614419218040
- ChengTYTarngDCLiaoYMLinPCEffects of systematic nursing instruction on a low-phosphorus diet, serum phosphorus level and pruritus of patients on haemodialysisJ Clin Nurs2017263–448549427381648
- TsaiWCYangJYLuanCCAdditional benefit of dietitian involvement in dialysis staffs-led diet education on uncontrolled hyperphosphatemia in hemodialysis patientsClin Exp Nephrol201620581582126658792
- YunKSChoiJYEffects of dietary program based on self-efficacy theory on dietary adherence, physical indices and quality of life for hemodialysis patientsJ Korean Acad Nurs2016464598609 Korean [with English abstract]27615049
- YokumDGlassGCheungCFCunninghamJFanSMaddenAMEvaluation of a phosphate management protocol to achieve optimum serum phosphate levels in hemodialysis patientsJ Ren Nutr200818652152918940656
- TongASainsburyPChadbanSPatients’ experiences and perspectives of living with CKDAm J Kidney Dis200953468970019216015
- Lorenzo SellaresVTorres RamírezAManagement of hyperphosphataemia in dialysis patients: role of phosphate binders in the elderlyDrugs Aging200421315316514979734
- LeypoldtJKKinetics of beta2-microglobulin and phosphate during hemodialysis: effects of treatment frequency and durationSemin Dial200518540140816191181
- WalshMMannsBJKlarenbachSTonelliMHemmelgarnBCulletonBThe effects of nocturnal compared with conventional hemodialysis on mineral metabolism: a randomized-controlled trialHemodial Int201014217418120041960
- ChanKEThadhaniRIMadduxFWAdherence barriers to chronic dialysis in the United StatesJ Am Soc Nephrol201425112642264824762400
- VaiciunieneRKuzminskisVZiginskieneESkarupskieneIBumblyteIAAdherence to treatment and hospitalization risk in hemodialysis patientsJ Nephrol201225567267821983989
- KuglerCMaedingIRussellCLNon-adherence in patients on chronic hemodialysis: an international comparison studyJ Nephrol201124336637520954134
- ChristensenAJBenotschEGWiebeJSLawtonWJCoping with treatment-related stress: effects on patient adherence in hemodialysisJ Consult Clin Psychol19956334544597608358
- Clark-CutaiaMNRenDHoffmanLABurkeLESevickMAAdherence to hemodialysis dietary sodium recommendations: influence of patient characteristics, self-efficacy, and perceived barriersJ Ren Nutr2014242929924462498
- BearRAStockieSPatient engagement and patient-centred care in the management of advanced chronic kidney disease and chronic kidney failureCan J Kidney Health Dis201412425780613
- O’HareAMArmisteadNSchragWLDiamondLMossAHPatient-centered care: an opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD programClin J Am Soc Nephrol20149122189219425035275
- Agondi RdeFGallaniMCRodriguesRCCornelioMERelationship between beliefs regarding a low salt diet in chronic renal failure patients on dialysisJ Ren Nutr201121216016820537916
- AhrariSMoshkiMBahramiMThe relationship between social support and adherence of dietary and fluids restrictions among hemodialysis patients in IranJ Caring Sci201431111925276744
- DowellSAWelchJLUse of electronic self-monitoring for food and fluid intake: a pilot studyNephrol Nurs J200633327127716859199
- DuroseCLHoldsworthMWatsonVPrzygrodzkaFKnowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary complianceJ Am Diet Assoc20041041354114702581
- LeeSHMolassiotisADietary and fluid compliance in Chinese hemodialysis patientsInt J Nurs Stud200239769570412231026
- MolaisonEFYadrickMKStages of change and fluid intake in dialysis patientsPatient Educ Couns200349151212527147
- MokETamBStressors and coping methods among chronic haemodialysis patients in Hong KongJ Clin Nurs200110450351111822498
- PangSKIpWYChangAMPsychosocial correlates of fluid compliance among Chinese haemodialysis patientsJ Adv Nurs200135569169811529971
- PoduvalRDWolgemuthCFerrellJHammesMSHyperphosphatemia in dialysis patients: is there a role for focused counseling?J Ren Nutr200313321922312874747
- SagawaMOkaMChaboyerWThe utility of cognitive behavioural therapy on chronic haemodialysis patients’ fluid intake: a preliminary examinationInt J Nurs Stud200340436737312667513
- SharpJWildMRGumleyAIDeighanCJA cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: a randomized controlled trialAm J Kidney Dis20054561046105715957134
- TsaySLSelf-efficacy training for patients with end-stage renal diseaseJ Adv Nurs200343437037512887355
- WelchJLHemodialysis patient beliefs by stage of fluid adherenceRes Nurs Health20012410511211353458
- YokoyamaYSuzukamoYHottaODialysis staff encouragement and fluid control adherence in patients on hemodialysisNephrol Nurs J200936328929719588696
