Abstract
Background
Understanding perception of risks and benefits is essential for informed patient choices regarding medical care. The primary aim of this study was to evaluate the perception of risks and benefits of 9 drug classes during pregnancy and associations with women’s characteristics.
Methods
Questionnaires were distributed to pregnant women who attended a Dutch Obstetric Care facility (first- and second-line care). Mean perceived risk and benefit scores were computed for 9 different drug classes (paracetamol, antacids, antibiotics, antifungal medication, drugs against nausea and vomiting, histamine-2 receptor antagonists/proton pump inhibitors, antidepressants, nonsteroidal anti-inflammatory drugs, and sedatives/anxiolytics). For each participant, we computed weighted risk and benefit sum scores with principal component analysis. In addition, major concerns regarding medication use were evaluated.
Results
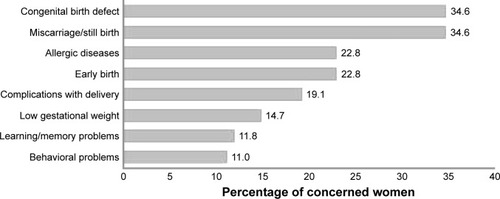
The questionnaire was completed by 136 women (response rate 77%). Pregnant women were most concerned about having a child with a birth defect (35%), a miscarriage (35%), or their child developing an allergic disease (23%), respectively, as a result of drug use. The majority of studied drug classes were perceived relatively low in risk and high in benefit. Higher risk scores were reported if women were in their first trimesters of pregnancy (p=0.007). Lower benefit scores were reported if women were single (p=0.014), smoking (p=0.028), nulliparous (p=0.006), or did not have a family history of birth defects (p=0.005).
Conclusion
Pregnant women’s concerns regarding potential drug adverse effects were not only focused on congenital birth defects but also included a wider range of adverse outcomes. This study showed that most of the studied drug classes were perceived relatively low in risk and high in benefit.
Background
The majority of pregnant women use medication during pregnancy.Citation1 Despite increasing availability of information about teratogenic risks, medication use during pregnancy still causes uncertainty and concern among pregnant women and their health care providers.Citation2,Citation3
Recent risk perception studies observed that women tend to overestimate the magnitude of teratogenic risks.Citation2–Citation15 Although it is difficult to estimate the real risk of medication use during pregnancy, unrealistic perception of risk among pregnant women may lead to poor adherence, discontinuation of treatment, and even abortion of otherwise wanted and healthy infants.Citation9,Citation14 Counseling enables a more balanced decision on the use of medication during pregnancy.Citation8,Citation11,Citation13 However, the manner in which information is presented can make a substantial difference to people’s responses. For example, providing pregnant women with positively framed information will lower risk perceptions significantly.Citation8 In addition, women’s perception of the benefits of medication use may have a major influence on the acceptance of risks.
In previous risk perception studies, risk was often presented as the probability of having a child with a congenital malformation.Citation4,Citation12 Although congenital malformations are severe adverse effects, medication use during pregnancy has been associated with a broader spectrum of disorders than congenital malformations alone.Citation16–Citation18 The study of Petersen et alCitation15 presented the risk of medication use as a harmful effect for the fetus. It is likely that many women may have interpreted harmful effects broadly and considered factors such as congenital anomalies, still birth, preterm birth, low birth weight, growth retardation of the fetus, and developmental delays in totality. However, it remains unknown what the major concerns are among pregnant women regarding medication use.
The primary aim of this study is to evaluate the perception of risks and benefits of medication use during pregnancy and associations with women’s sociodemographic characteristics. In addition, we evaluated the major concerns among pregnant women regarding medication use.
Methods
Study design, setting, and study population
This study was based on data from a survey of pregnant women who attended an obstetric care facility (Medical Center Leeuwarden) in the Netherlands (both first- and second-line care). Yearly about 1,900 pregnant women (1.1% of all Dutch women) are cared for in this large (800 beds) teaching hospital in the northern part of the Netherlands. Researchers asked all pregnant women who were visiting the obstetric care facility for a consultation between May 1, 2013 and June 30, 2013 to participate in the study. Questionnaires were distributed by the researchers to all women willing to participate, regardless of their health status or antenatal care needs. Inclusion criteria for participation in the study were that women had to be pregnant and had to understand the Dutch language. Women who could not understand Dutch were excluded.
Self-reported questionnaire
In this study, an anonymous self-reported questionnaire was developed and used. The questionnaire consisted of 4 parts to collect data on 1) general characteristics, 2) medication use during pregnancy and information sources used, 3) major concerns related to medication use, and 4) perceived risks and benefits of medication use during pregnancy. In Dutch health care, first-line care is easily accessible and patients can contact first-line care providers without a referral. Second-line care is specialist care in hospitals where pregnant women need a referral from a general practitioner (GP) or a midwife.
A group of 10 pregnant women were asked to pretest a tested version of the questionnaire. They were interviewed to confirm if the questionnaire was clear to them and if they had comments or suggestions to state in the text box given at the end of the questionnaire. No specific comments were returned, so we concluded that the test version was good and no further changes were required. Since waiting times were often extensive, questionnaires could easily be completed by the participants.
Part 1 – general characteristics
Sociodemographic variables included in the questionnaire were age, education, marital status, duration of the pregnancy, gravidity, parity, status of employment, and attending first or second-line care facility. Lifestyle variables that were examined were the use of alcohol and smoking during pregnancy.
Part 2 – medication use during pregnancy and information sources used
Respondents were asked whether and which medication they used with and without a prescription during pregnancy. Classes of drugs were presented, including examples of generic and trade names. Presented drug classes were paracetamol, nonsteroidal anti-inflammatory drugs (NSAIDs), vitamins other than folic acid and vitamin D, drugs against a cold, acid-suppressive medication, antibiotics, antifungal medication, drugs against nausea and vomiting (NVP), sedatives/anxiolytics, anti-asthma medication, anti-diabetes medication, medication against high blood pressure, and homeopathic medication.Citation19
Folic acid and vitamin D are vitamins which are advised in the Netherlands during pregnancy.Citation20 Therefore, women who only used these vitamins were categorized as non-medication users. When prenatal vitamins were reported, we considered them as multivitamins designed for pregnant women containing more and/or other vitamins than folic acid and vitamin D.
Women were asked if they obtained information about medication before and during pregnancy. Commonly used sources of information were listed: Internet, gynecologist, GP, pharmacist, midwife, package information leaflets (PIL), friends and family, books, or others.
Part 3 – measurements of concerns
Women were asked to rate their level of concern about having a miscarriage, preterm birth, complicated delivery, child with a birth defect, or a child with a low gestational age as a result of medication use during pregnancy. In addition, women were asked to rate their level of concern about having a child with memory/learning problems, behavioral problems, or an allergic disease. These events are not evident at birth, but are associated with medication use during pregnancy in the literature.Citation16–Citation18,Citation21 Women could rate their level of concern with a score from 1 to 5 on a 5-point scale ().Citation21
Table 1 Questions and scales on which concerns related to medication use, probability, severity, and benefits were rated
Part 4 – perceived risks and benefits of medication use during pregnancy
Different studies on risk perception proposed that risk is dependent on the expectancy of the probability of an event and the beliefs about the potential harm.Citation12,Citation22–Citation27 In this study, women were asked to rate the probability and severity of an event occurring due to exposure to several drug classes (paracetamol, antacids, antibiotics, antifungal medication, drugs against NVP, histamine-2 receptor antagonists/proton pump inhibitors, antidepressants, NSAIDs, sedatives/anxiolytics) on a 7-point Likert scale ().Citation27 For each drug class, examples of generic (domperidone, metoclopramide, ibuprofen, diclofenac, naproxen, nitrofurantoin, amoxicillin, cimetidine, pantoprazole, omeprazole, citalopram, paroxetine, amitriptyline, temazepam, oxazepam, diazepam, miconazole and clotrimazole) or trade names (Motilium, Primperan, Rennie, Gaviscon, Furabid, Cipramil, Seroxat, Canesten) were given. Risk scores were obtained by multiplication of the measures of perceived probability and severity. A square root extraction was performed to make the risk scores comparable with the benefit scores.Citation23
To get information on the perceived benefits of medication during pregnancy, women were asked to rate the benefits of the drug classes on a scale from 1 to 7 ().
Statistical analysis
Percentages were computed for the respondents who were concerned or very concerned (scored 4 or 5 on the concern scale) about having a miscarriage, preterm birth, complicated delivery, child with a birth defect, or a child with a low gestational age as a result of medication use during pregnancy. Means and standard deviations (SDs) were computed for the perceived risk (probability times severity score) and benefit scores of the different drug classes. We also calculated Pearson’s correlation coefficients between risk and benefit scores for each separate drug class.
Separate principal component analyses (PCA) were performed on the 9 different drugs on the risk scale and benefit scale, respectively. PCA was used to construct weighted risk and benefit sum scores using the loadings of the first principal component as weights.Citation28,Citation29 With this construction, the weighted sum scores represent unbiased estimates of standardized values on a latent variable (eg, “overall perceived benefit”). The loadings are used as weights because the size of each loading indicates the degree to which a variable represents an aspect of the underlying latent variable. Given the relatively high proportion of variance explained by each first principal component, we take their respective latent variables to represent “overall” risk and benefit, respectively. The first principal component on the risk scale explained 58.5% of the variance, whereas the first principal component of the benefit scale explained 53.4% of the variance. Barlett’s test of sphericity and the Kaiser–Meyer–Olkin (KMO) test were performed to test if the number of significant correlations was sufficient to perform PCA.Citation28 Bartlett’s test of sphericity was significant (p<0.001) for the 3 scales. KMO statistic showed that a factor analysis was appropriate for the risk and benefit scales (0.833 and 0.859, respectively).Citation29
Linear regression models were used to examine associations between maternal age, education level, and the weighted risk and benefit sum score. Student’s t-test was performed to assess differences in the weighted risk and benefit sum score for the different general characteristics, medication use, and acquirement of information. All analyses were performed using the SPSS software for Windows (version 21; IBM Corporation, Armonk, NY, USA).
Ethical approval
The institutional review board of the Medical Center Leeuwarden stated that ethical approval and patient informed consent were not required, since the study involved a questionnaire only, was completely anonymous, and no patient data were used. However, the study was approved by the institutional review board of the Medical Center Leeuwarden.
Results
Response and general characteristics
During the 2 months when the questionnaire was distributed, 177 women were informed about the study. The questionnaire was returned by 136 women (77%). Data on general characteristics of the study population are provided in . The mean age of the respondents was 30.8 (SD ±5) years. Most of the respondents (55%) participated in the study while they were in their third trimester of pregnancy. Most of the respondents had been pregnant before (72%) and 30% of the respondents had had a previous miscarriage.
Table 2 General characteristics of study population (N=136)
Medication use
Most of the women (82.2%) had used medication during pregnancy (). Paracetamol was used most commonly during pregnancy (42%). Other commonly used drugs were vitamins (21%), acid-suppressive medication (25%), and antibiotics (10%). Of the respondents, 56 (48%) reported folic acid and/or vitamin D use during pregnancy. Information about medication was obtained by 76 women (67%). Most used information sources were the Internet, PIL, and GP.
Table 3 Reported medication use during pregnancy (N=136)
Concerns
Pregnant women were most concerned (score 4 or 5 on the concern score) about having a child with a congenital birth defect (35%), having a miscarriage (35%), or having a child with an increased risk of an allergic disease (23%), respectively, as a result of their drug use. The different concerns pregnant women had regarding medication use during pregnancy are presented in .
Perceptions of risk and benefits
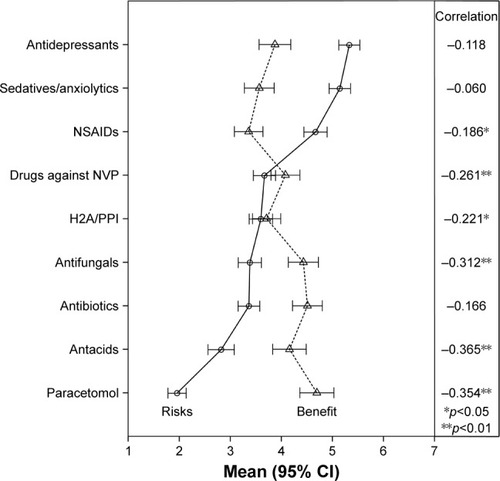
Highest risk scores were reported for antidepressants, sedatives/anxiolytics, and NSAIDs, respectively. Highest benefit scores were reported for antibiotics, antifungal medication, and antacids. Mean risk and benefit scores, including Pear-son correlation coefficients, are presented in . Risks and benefit scores were significantly inversely correlated for most of the drug classes. Almost half of the drug classes were perceived as low in risk (mean score <3.5) and high in benefit (mean score >3.5). In addition, shows that the spread of mean values of the different drugs on the risk scale is higher (from 1.9 to 5.3) than the spread of mean values of the different drugs on the benefit scale (from 3.3 to 4.7).
Figure 2 Means of the perceived risk and perceived benefits of different medication groups during pregnancy.
Analysis of the weighted sum risk score showed that women in the first 2 trimesters of pregnancy perceived significantly higher risks of medication use (p=0.007). The remaining characteristics, namely, education, maternal age, employed in health care sector, nulliparity, previous miscarriages, smoking during pregnancy, and use of medication showed no statistically significant differences in weighted risk sum scores. Lower benefit scores of medication use were perceived if women were single (p=0.014), smoking during pregnancy (p=0.028), nulliparous (p=0.006), or did not have a family history of birth defects (p=0.005).
Discussion
Main findings
Women were most concerned that medication use during pregnancy could result in a child with a congenital birth defect, a miscarriage, or a child with an allergic disease. Most of the drug classes were perceived relatively low in risk and high in benefit. Only antidepressants, sedatives/anxiolytics, and NSAIDs were perceived as high-risk medication. Women had higher risk perception scores during early trimesters of pregnancy. Lower benefit scores were perceived if women were single, nulliparous, smoking during pregnancy, or had no family history of birth defects.
Strengths and limitations
Our study is the first to investigate the perceived risk–benefit balance with regard to medication use during pregnancy. Another major strength of our study is the method of inclusion. In previous risk perception studies, women were invited to participate in the studies when visiting websites containing pregnancy-related information.Citation12,Citation15 In our opinion, this could have led to a selection bias and the inclusion of more concerned women. Since women were included independent of their information seeking behavior in the current study, the influence of this type of selection bias will likely be less. The women included in the study were representative of the general Dutch population of pregnant women with respect to age (age of the general pregnant population was 31 years vs 31 years in this study population) and education level (high education of 40% in the general population vs 42% in the study population).Citation30,Citation31 Further, all participants were asked to fill out the questionnaire while they were pregnant. With this approach, the outcome of the pregnancy could not have influenced their risk perceptions. It should, however, be noted that most women completed the questionnaire while they were in their third trimester. In the third trimester, the likelihood of an abortion is low and congenital anomalies already may have been detected via ultrasound. In addition, recall bias regarding medication use will be minimal when asked during pregnancy.Citation32 Another strength of our study is the questionnaire design, because information about medication use was obtained with both open and closed questions. It has been reported that prevalence estimates are higher when questions include indications or specific drugs when compared to open-ended questions.Citation33 The use of the weighted risk and benefit sum scores was another advantage of this study. The weighted sum scores represent unbiased estimates of standardized values on a latent variable (eg, “overall perceived benefit”).
Apart from these strengths, potential limitations need to be discussed. First, we included all pregnant women who were attending first and second-line care facilities, regardless of their health status or antenatal care, to reduce the risk of sampling bias. Although every pregnant woman living in the Netherlands can undergo a prenatal screening, the majority of our study population consisted of women who came for a routine follow-up with an obstetrician. We could, therefore, not exclude the possibility that the study population differed from the general birthing population. Nevertheless, as stated above, the distribution of age and level of education was similar between the study population and the general birthing population in the Netherlands.Citation30,Citation31
Second, all women understood the Dutch language; however, the extent to which they understood the questions that were asked was not assessed.
Third, though the response rate was rather high for a questionnaire study (77%) and associations were significant in most evaluations, the total number of participants was low. Due to the low statistical power, we could not perform comparisons between women who were prescribed specific drugs and women who were not. Such analyses would show how actual users balance the risks and benefits of a specific drug and would represent the potential noncompliance in that population.
Fourth, we compared risks and benefits for drug classes instead of specific drugs within the drug classes. In relation to the measurement of concerns, questions were asked about concerns for “any medication” rather than concerns related to a specific medication. This made our questions more comprehensible by pregnant women; however, since there may be variation within and between each drug class, findings cannot be interpreted for specific drugs. We did not provide the option of “do not know” when asked for the perception of risks and benefits. The number of unfilled responses regarding risks and benefits was low and ranged from 4 (paracetamol) to 9 (drugs against NVP) for questions about risks and ranged from 3 (paracetamol) to 6 (antibiotics) for the questions about benefits. It is also possible that participants listed the mid-value if they did not know the medication group described. It is unclear to what extent this influenced the results.
Fifth, interpretations were given only for scores 1 and 7 of the 7-point Likert scales. It is not correct to infer that the intensity of feeling between 1 and 7 is equivalent to the intensity of feeling between other consecutive categories on the Likert scale. It could not be excluded that the differences in the perception of risks and benefits were due to interpersonal differences in interpretation of the values of the scale.
Sixth, the reliability and validity of the questionnaire were not assessed and it is unclear how this could have affected the results of this study.
Interpretation and comparison with literature
Women were most concerned that medication use during pregnancy could result in a child with a birth defect, a miscarriage, or a child who develops an allergic disease. Although different studies have investigated the different worries of pregnant women in general,Citation21 this is the first study that reports women’s concerns regarding medication use during pregnancy.
Different studies reported that risks associated with medication use were perceived higher than actual risks.Citation2–Citation15 However, it has been considered that the relativeness of risk perceptions is more interesting than the mean risk perceptions itself.Citation12 From the different drug classes, sedatives and antidepressants were perceived highest in risk and paracetamol and antacids were perceived lowest. This is in accordance with findings from a recent study in Norway.Citation12 In the current study, women were not asked to rate the absolute risks of the different drug classes and the results of this study cannot be compared with true risks. However, for the drug classes perceived highest in risk, teratogenicity has been described in the literature and the product information of several products from these classes.
Recent studies have shown that pregnant women overestimate the teratogenic risk of medication use.Citation2–Citation15 However, this study showed that women perceive benefits of medication use as well. When risks were balanced with benefits, most of the drugs were perceived relatively low in risk and high in benefit. Risks and benefits were significantly negatively correlated for most of the drugs. This confounding of risks and benefits in women’s minds may implicate that it is possible to change the risk perception by changing the perception of benefits of medication use.Citation33 The variance of perceived risks was higher than the variance of perceived benefits between the different drug classes (). That risk scores were rated with more extreme values than the benefit scores may indicate that women have more difficulty rating the benefits of medication use than the risks. Including more information about the benefits of medication use during counseling may play an important role in lowering risk perceptions among pregnant women.Citation34
Analyses of the weighted risk sum score showed that women perceived higher risks during the first 2 trimesters of pregnancy than women in the third trimester of pregnancy. In the first trimester, organogenesis takes place and risks on spontaneous abortion and congenital anomalies are highest. However, recently it has been stated that exposures during the third trimester can also have negative implications for the child for certain pediatric outcomes.Citation16,Citation17
Analyses of the weighted benefit sum score showed that women perceived lower benefit scores if they were single, smoking, or nulliparous. Since no earlier research focused on the potential benefits of medication use during pregnancy, we can only speculate about potential explanations. Single women lack the option to discuss their concerns and fears with a partner, which may contribute to a more cautious attitude toward medication use during pregnancy and lower perceived benefit scores. Nulliparous women may also have a more cautious attitude toward medication use during pregnancy, since they had never experienced a pregnancy before. In addition, a previous study performed in Norway found that nulliparous women perceived higher risks of medication use during pregnancy.Citation12 Women who smoked during pregnancy more often tend to have a low socioeconomic status. In addition, the vision of these women on a healthy pregnancy may be different, since they maintain an unhealthy lifestyle even when they are pregnant. They may therefore not fully comprehend the potential benefits of medication use during pregnancy. Moreover, none of the women indicated having consumed alcohol during pregnancy, which is unlikely. The influence of response bias cannot be ruled out and it is unclear how this could affect the results.
Women with a family history of birth defects perceived higher benefit scores of medication use during pregnancy. These women may have more knowledge about medication use during pregnancy, especially about the benefits. For example, they may be aware that folic acid supplementation may prevent certain birth defects.
Conclusion
This study shows that concerns regarding medication use during pregnancy are not restricted to having a child with a congenital birth defect only. Although pregnant women overestimate the teratogenic risk of medication use, most of the drugs were perceived relatively low in risk and high in benefit. Health care providers can take this into account when counseling pregnant women.
Acknowledgments
The authors wish to thank the Department of Obstetrics and Gynaecology of the Medical Centre Leeuwarden and the prenatal screening facility SICHT who contributed in the inclusion of pregnant women.
This study was financed by the Department of Pharmacy, University of Groningen. The funder had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the paper for publication. The corresponding author had full access to all data and the final responsibility for the decision to submit the study for publication.
This research was presented as an oral presentation at the International Congress of Behavioral Medicine from August 20, 2014 to August 23, 2014 in Groningen, the Netherlands and as a poster at the International Conference on Pharmacoepidemiology and Therapeutic Risk Management from October 24, 2014 to October 27, 2014 in Taipei, Taiwan.
Disclosure
All authors declare that they had support from the Department of Pharmacy, University of Groningen, for the submitted work; had no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; had no other relationships or activities that could appear to have influenced the submitted work; and report no conflicts of interest in this work.
References
- BakkerMKJentinkJVroomFVan Den BergPBDe WalleHEDe Jong-Van Den BergLTDrug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the NetherlandsBJOG200611355956816637899
- WidnesSFSchjottJEideGEGranasAGTeratogenic risk perception and confidence in use of medicines in pairs of pregnant women and general practitioners based on patient information leafletsDrug Saf20133648148923539202
- CsajkaCJaquetAWinterfeldUMeyerYEinarsonAPanchaudARisk perception by healthcare professionals related to drug use during pregnancy: a Swiss surveySwiss Med Wkly20147144w13936
- KorenGBologaMLongDFeldmanYShearNHPerception of teratogenic risk by pregnant women exposed to drugs and chemicals during the first trimesterAm J Obstet Gynecol19891605 Pt 1119011942729394
- KorenGPastuszakAPrevention of unnecessary pregnancy terminations by counselling women on drug, chemical, and radiation exposure during the first trimesterTeratology19904166576612353314
- MazzottaPMageeLAMaltepeCLifshitzANaviozYKorenGThe perception of teratogenic risk by women with nausea and vomiting of pregnancyReprod Toxicol199913431331910453916
- PoleMEinarsonAPairaudeauNEinarsonTKorenGDrug labeling and risk perceptions of teratogenicity: a survey of pregnant Canadian women and their health professionalsJ Clin Pharmacol200040657357710868307
- JasperJDGoelREinarsonAGalloMKorenGEffects of framing on teratogenic risk perception in pregnant womenLancet200135892891237123811675064
- SanzEGomez-LopezTMartinez-QuintasMJPerception of teratogenic risk of common medicinesEur J Obstet Gynecol Reprod Biol200195112713111267734
- KorenGLevichekZThe teratogenicity of drugs for nausea and vomiting of pregnancy: perceived versus true riskAm J Obstet Gynecol20021865 Suppl UnderstandingS248S25212011895
- BonariLKorenGEinarsonTRJasperJDTaddioAEinarsonAUse of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence based counseling and determinants of decision makingArch Womens Ment Health20058421422015959622
- NordengHYstromEEinarsonAPerception of risk regarding the use of medications and other exposures during pregnancyEur J Clin Pharmacol201066220719841915
- BehringerTRollmanBLHerbeck-BelnapBHouckPRMazumdarSSchwarzEBImpact of physician counseling and perception of teratogenic risks: a survey of 96 nonpregnant women with anxietyPrim Care Companion CNS Disord2011132 pii:PCC.10m01028
- WalfischASermerCMatokIEinarsonAKorenGPerception of teratogenic risk and the rated likelihood of pregnancy termination: association with maternal depressionCan J Psychiatry2011561276176722152645
- PetersenIMcCreaRLLupattelliAWomen’s perception of risks of adverse fetal pregnancy outcomes: a large-scale multinational surveyBMJ Open20155e007390
- BriggsGGFreemanRKYaffeSJDrugs in pregnancy and lactation: a reference guide to fetal and neonatal riskPhiladelphiaLippincott Williams & Wilkins2012
- MulderBSchuiling-VeningaCCBosJHde VriesTWHakEAcid-suppressive drug use in pregnancy and the toddler’s asthma risk: a crossover, case-control studyJ Allergy Clin Immunol201313261438144023992747
- TohSMitchellAALouikCWerlerMMChambersCDHernández-DíazSAntidepressant use during pregnancy and the risk of preterm delivery and fetal growth restrictionJ Clin Psychopharmacol200929655556019910720
- SchirmEMeijerWMTobiHDe Jong-van den BergLTWDrug use by pregnant women and comparable non-pregnant women in the Netherlands with reference to the Australian classification systemEur J Obstet Gynecol Reprod Biol200411418218815140513
- WINap Geneesmiddel Informatie en Wetenschapswinkel Groningen: Zelfzorgmedicijnen tijdens zwangerschap en borstvoeding;wat kan ik doen, wat mag ik slikken?Groningen2004
- PetersenJJPaulitschMAGuethlinCGensichenJJahnAA survey on worries of pregnant women – testing the German version of the Cambridge Worry ScaleBMC Public Health2009949020038294
- AustinJCRe-conceptualizing risk in genetic counseling: implications for clinical practiceJ Genet Counsel201019228234
- BrewerNTChapmanGBGibbonsFXGerrardMMcCaulKDWeinsteinNDMeta-analysis of the relationship between risk perception and health behavior: the example of vaccinationHealth Psychology20072613614517385964
- WeinsteinNDKwitelAMcCaulKDMagnanREGerrardMGibbonsFXRisk perceptions: assessment and relationship to influenza vaccinationHealth Psychology20072614615117385965
- ZwartOVeldhuijzenIKElamGPerceived threat, risk perception and efficacy beliefs related to SARS and other (emerging) infectious diseases: Results of an International surveyInt J Behav Med200916304019125335
- SlovicPPetersEGranaJBergerSDieckGSRisk perception of prescription drugs: results of a national surveyDrug Inform J200741181100
- BeyerARFasoloBPhillipsLDde GraeffPAHillegeHLRisk perception of prescription drugs: results of a survey among experts in the European regulatory networkMed Decis Making201333457959223478076
- HeamanMIGuptonALPsychometric testing of the perception of pregnancy risk questionnaireRes Nurs Health200932549350319606451
- CarrollSJPaquetCHowardNJAdamsRJTaylorAWDanielMValidation of continuous clinical indices of cardiometabolic risk in a cohort of Australian adultsBMC Cardiovasc Disord2014142724571233
- CBS (Centraal Bureau voor de Statistiek) Available from: http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=37422ned&D1=0,4-5,19,21,23-24,26,35,40,42,47&D2=0,10,20,30,40,50,60,(l-1)-l&VW=TAccessed October 4, 2013
- CBSBevolkingsprognose 2012–2060: veronderstellingen over de geboorte Available from: http://www.cbs.nl/NR/rdonlyres/3BA03CC0-F684-4481-946E-BB4338AF1083/0/20131002b15art.pdfAccessed October 4, 2013
- van GelderMMvan RooijIAde WalleHERoeleveldNBakkerMKMaternal recall of prescription medication use during pregnancy using a paper-based questionnaireDrug Saf2013361435423315295
- KlungelOHde BoerAPaesAHHeringsRMSeidellJCBakkerAInfluence of question structure on the recall of self-reported drug useJ Clin Epidemiol200053327327710760637
- AlhakamiASSlovicPA psychological study of the inverse relationship between perceived risk and perceived benefitRisk Anal1994146108510967846317