Abstract
Background:
All established disease-modifying drugs for multiple sclerosis require parenteral administration, which can cause difficulties for some patients, sometimes leading to suboptimal adherence. A new electronic autoinjection device has been designed to address these issues.
Methods:
Patients with relapsing multiple sclerosis currently receiving subcutaneous or intramuscular interferon beta-1a, interferon beta-1b, or glatiramer acetate completed an online questionnaire (July 4–25, 2008) that surveyed current injection practices, experiences with current injection methods, and impressions and appeal of the new device.
Results:
In total, 422 patients completed the survey, of whom 44% used autoinjectors, 43% prefilled syringes, and 13% syringes and vials; overall, 66% currently self-injected. Physical and psychological barriers to self-injection included difficulty with injections, needle phobia, and concerns over correct injection technique. Only 40% of respondents were “very satisfied” with their current injection method. The new electronic autoinjector was rated as “very appealing” by 65% of patients. The benefits of the new device included the ability to customize injection settings and to review dosing history.
Conclusion:
New technologies may help patients overcome physical and psychological barriers to self-injection. The combination of a reliable and flexible autoinjection device with dose-monitoring technology may improve communication between health care professionals and patients, and improve treatment adherence.
Background
Multiple sclerosis is a chronic degenerative disease of the central nervous system, and is the most common disabling neurological condition affecting young adults.Citation1,Citation2 Because multiple sclerosis is currently incurable, patients require long-term treatment that delays or stops the progression of irreversible disability. Currently, the first-line therapies for relapsing forms of multiple sclerosis are interferon (IFN) beta-1a 44 μg or 22 μg administered subcutaneously three times weekly (Rebif®, Merck Serono S.A., Geneva, Switzerland); IFN beta-1a 30 μg administered intramuscularly once weekly (Avonex®, Biogen Idec Inc, Cambridge, MA); IFN beta-1b 250 μg administered subcutaneously every other day (Betaferon®/Betaseron®, Bayer Schering Pharma AG, Berlin, Germany; Extavia®, Novartis AG, Basel, Switzerland); and glatiramer acetate 20 mg administered subcutaneously daily (Copaxone®, Teva Pharmaceutical Industries Ltd, Petah Tikva, Israel). All these first-line disease-modifying drugs for multiple sclerosis require parenteral self-administration, by either subcutaneous or intramuscular injection.Citation3
Adherence to multiple sclerosis treatment is important to ensure optimal clinical outcomes.Citation4,Citation5 However, the debilitating nature of the disease itself, together with the need for long-term, frequent, parenteral drug administration, means that adherence is a challenge for many patients. A recent multicenter observational study of 2648 patients reported that 25% of 2566 patients for whom data were available (643 patients) were nonadherent (defined as missing at least one disease-modifying drug injection over a four-week period).Citation6 Among these nonadherent patients, 32% reported injection-related reasons for their nonadherence.
There are limited prospective data on factors that influence adherence to disease-modifying drugs.Citation7 However, contributing factors to nonadherence that have been identified include problems with injecting, perceived lack of efficacy, and adverse events.Citation5,Citation7,Citation8 Patients who are required to self-inject often react with fear, avoidance, anxiety, or disgust, with some asking family members to administer the treatment to avoid self-injection.Citation5 However, dependence on others for injection may also reduce treatment adherence.Citation5 Other injection-related barriers to adherence include injection site reactions and injection pain.Citation7 In an observational study that surveyed patients via online questionnaires, factors directly related to the injection of therapy accounted for 32% of the reasons given for missing injections, including “tired of shots”, “skin reactions”, and “pain at injection site”.Citation7 Additionally, many patients with multiple sclerosis have reduced manual dexterity, which can make the correct self-injection procedure physically problematic.
In response to these potential problems with injecting, various injection technologies have been introduced, which are designed to improve convenience and the safety and reliability of injections, and to reduce pain and anxiety.Citation8 These technological developments include thinner needles, prefilled syringes for manual injection, and autoinjection devices (or “autoinjectors”). Autoinjectors automatically insert the needle and deliver a controlled dose, and are available for all first-line disease-modifying drugs used for multiple sclerosis with the exception of intramuscular IFN beta-1a, for which an injection device is available, but the injection process remains partially manual. Autoinjectors have been shown to provide a number of benefits, including a reduced risk of injection site reactions, reduced discomfort, and greater ease of use compared with manual syringe injections.Citation9–Citation11
A recent cross-sectional, questionnaire-based survey of 3006 patients with multiple sclerosis examined patients’ reasons for using or for being reluctant to use an autoinjector for the subcutaneous administration of IFN beta or glatiramer acetate.Citation12 The reasons most commonly cited by patients for using an autoinjector were “certainty that the injection was being carried out correctly” (47.1% of patients) and the ability to administer injections at sites on the body that were previously inaccessible (41.1%). A major reason for not using an injection device, cited by 34.4% of patients, was that patients wanted control of the injection, including control of the speed and depth of needle insertion, as well as the duration of the injection. The survey also found that treatment adherence was greater among patients who always used an autoinjector (79% stated that they never missed an injection) than in those who used a prefilled syringe (71% stated that they never missed an injection). However, over 20% of patients who always used an autoinjector still reported that they sometimes missed an injection.
Adherence to treatment in multiple sclerosis thus remains suboptimal, indicating that patients may still have unmet needs. In the survey by Bayas et al,Citation12 patients were also asked to identify the characteristics that an ideal autoinjector should have. The most frequently cited characteristics included the ability to carry out the injection simply and swiftly in just a few steps, drug release only with skin contact, prevention of accidental activation, and the opportunity to adjust needle insertion speed, as well as injection depth, speed, and duration. Advances in delivery technology towards this ideal profile may, therefore, offer greater patient convenience and increase adherence.Citation8
A new electronic, multidose autoinjector (RebiSmart™, Merck Serono S.A., Geneva, Switzerland) has been developed for administering subcutaneous IFN beta-1a.Citation13,Citation14 This device offers a number of innovative features designed to improve the convenience and comfort of subcutaneous IFN beta-1a administration. Patients using this device have the facility to improve injection comfort by adjusting the needle insertion speed, needle depth, injection speed, and injection time (the time between the end of injection and needle retraction). In addition, interactive onscreen instructions, together with clear visual and audible signals, provide guidance for correct use. The device also features a dosing log that records the date, time, and dosage of every injection. This function may help patients to avoid missing doses, and thus potentially improve adherence.
Here we report the results of an online survey of patients diagnosed with relapsing multiple sclerosis and currently receiving treatment with subcutaneous IFN beta-1a, intramuscular IFN beta-1a, subcutaneous IFN beta-1b, or glatiramer acetate. The aims of the survey were to investigate patients’ injection practices in the normal clinical setting, to evaluate their experiences with current injection methods, and to assess their reactions to the new electronic autoinjector.
Methods
Eligible patients in Canada, France, Germany, Italy, Spain, and the US were recruited from an online panel of patients and completed an online, self-administered questionnaire during July 4–25, 2008. Patients were eligible if they were aged 18–65 years, had a diagnosis of relapsing multiple sclerosis or had experienced a demyelinating event suggestive of multiple sclerosis, were currently receiving IFN beta or glatiramer acetate, had been injecting treatment for at least two months prior to participating in the survey, and were not affiliated with any medical marketing, market research, or pharmaceutical company.
Patients were shown a series of slides describing the new electronic, multidose autoinjector, its key features, and the injection procedure, including insertion and removal of the drug cartridge and needle. The questionnaire was designed to collect information on current injection practices, patients’ attitudes to and experience with current treatment, and impressions and appeal of the new electronic autoinjector (). The questionnaire consisted mainly of multiple-choice questions and took approximately 30 minutes to complete. Three of the questions asked patients to indicate their attitudes on a scale of 0–10, ranging from “not at all” (score of 0) to “very comfortable/satisfied/appealing” (score of 10). For question 9 (satisfaction with the patient’s current injection method), a score of 0–2 was categorized as “unsatisfied”, 3–7 as “moderately satisfied”, and 8–10 as “very satisfied”. The responses to questions 1 and 10 were also categorized using these score ranges.
Table 1 Survey questionnaire
Results
Demographics, patient clinical characteristics, and current therapy
In total, 422 patients completed the survey in Canada (n = 35, 8%), France (n = 35, 8%), Spain (n = 45, 11%), Germany (n = 50, 12%), Italy (n = 47, 11%), and the US (n = 210, 50%). Patient demographic and clinical characteristics are presented in . Respondents had been receiving injected therapy for a mean of 4.2 years. The most common current therapy was subcutaneous IFN beta-1a, which was being received by 189 respondents (45%). A further 85 respondents (20%) were currently receiving treatment with intramuscular IFN beta-1a, 60 (14%) with subcutaneous IFN beta-1b, and 88 (21%) with glatiramer acetate.
Table 2 Patient demographic and clinical characteristics
Current injection habits
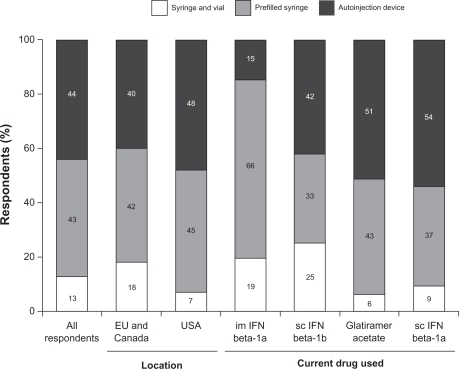
Overall, a similar proportion of respondents used prefilled syringes (43%) and autoinjectors (44%), while a smaller percentage (13%) used syringes and vials (). However, when analyzed by current drug used, the percentage of respondents using autoinjection devices was smaller among patients taking intramuscular IFN beta-1a (15%) than among patients taking subcutaneous IFN beta-1a (54%), IFN beta-1b (42%), or glatiramer acetate (51%, ).
Figure 1 Current method of injection administration.
Approximately two-thirds (66%) of respondents self-injected, while 19% had someone else administer the injection. The remaining 15% of patients sometimes self-injected and sometimes had someone else inject their medication. Rates of self-injection were similar among patients treated with subcutaneous IFN beta-1a (71%), IFN beta-1b (65%), or glatiramer acetate (70%), but lower among those receiving intramuscular IFN beta-1a (51%). Patients reported a number of physical and psychological reasons for not currently self-administering injections (). The most common reason was physical difficulty with injecting (57% of respondents), whereas pain on injection was cited by only 10% of respondents. The most common psychological reasons for not self-injecting included dislike of looking at needles (39%), dislike of the thought of injecting (37%), and a lack of confidence in their ability to inject correctly (32%). When patients were asked to rate their satisfaction with their current injection method on a scale of 0–10 (0 = not at all satisfied, 10 = very satisfied), the mean score was 6.7 (median 7.0, range 0–10). Overall, 40% of respondents were “very satisfied” (score of 8–10) with their current injection method (), with a lower percentage among users of vials and syringes (23%) than among users of prefilled syringes (39%) or autoinjectors (46%).
Figure 2 Reasons given for not self-injecting. Respondents could give more than one reason (values total more than 100%). Physical (total) score is the percentage of respondents selecting one or both of the two physical reasons; psychological (total) score is the percentage of patients selecting one or more of the psychological reasons.
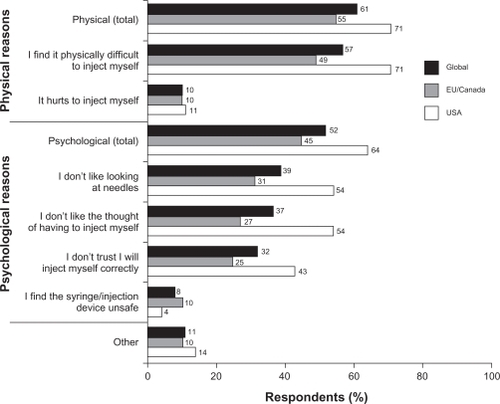
Table 3 Satisfaction with current injection method
Attitudes to technology
Most respondents were very comfortable in general with new technologies. On a scale of 0–10 (0 = not at all comfortable, 10 = very comfortable), the mean score was 8.0 (median 8.0, range 0–10), and 69% of patients scored between 8 and 10; 29% were somewhat comfortable (score of 3–7), and only 2% were uncomfortable (score of 0–2). Approximately one-third (35%) of respondents described themselves as early adopters of new technologies, while 57% described themselves as cautious adopters, and only 8% as late adopters.
Reactions to the new electronic autoinjection device
When patients were asked how appealing the new electronic autoinjection device was compared with their current method on a scale of 0–10 (0 = not at all appealing, 10 = very appealing), the overall mean score was 7.7 (median 8.0, range 0–10). Approximately two-thirds (65%) of patients found the new device “very appealing” (score of 8–10), with a higher proportion among current users of subcutaneous IFN beta-1a (73%) and IFN beta-1b (70%) than among users of intramuscular IFN beta-1a (54%) and glatiramer acetate (56%) favoring the new device. Overall, the new device was rated as moderately appealing (score of 3–7) by 30% of patients, and not at all appealing (score of 0–2) by 5%.
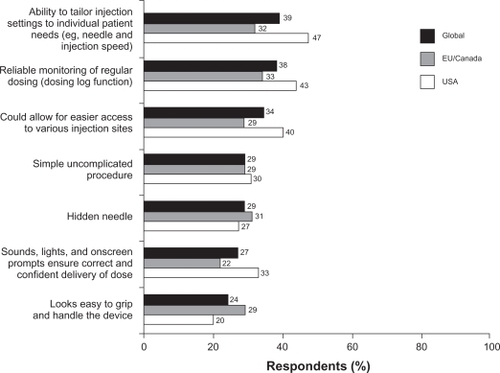
Questions 11 and 12 presented the patients with lists of several potential benefits and drawbacks of the new device. Overall, 96% of respondents identified a benefit that would encourage them to ask their nurse or doctor about the new device. The most appealing benefits were the ability to tailor injection settings (such as speed of injection and depth of needle insertion) to individual needs (cited by 39% of respondents), and the dosing log that allows reliable monitoring of regular dosing (cited by 38%, ). Overall, 23% of respondents considered that the device had no drawbacks. The most frequently stated drawback was fear that the device might malfunction, which was cited by 37% of respondents.
Discussion
This survey of patients with relapsing multiple sclerosis indicates that patterns of self-injection practice and satisfaction with current injection methods are broadly similar across the two groups in the US and the European Union/Canada. Auto-injection devices and prefilled syringes were used by most respondents, but the use of autoinjectors was considerably less common among patients treated with intramuscular IFN beta-1a than in patients receiving other disease-modifying drugs, which reflects the lack of a fully automated injection device for use with this treatment.
A third of respondents reported that they relied on others (at least part of the time) to administer injections. The percentage of patients who self-injected among those receiving medications for which an autoinjector is available (ie, subcutaneous IFN beta-1a, IFN beta-1b, and glatiramer acetate) was 65%–71%. In contrast, the percentage of patients receiving intramuscular IFN beta-1a who self-injected was 51%. While it should be noted that the routes of administration are different in these two cases and there may be differences in clinical characteristics between these populations, these data could suggest that self-injection might be more acceptable if an autoinjector is available than if the injection must be performed manually.
The percentage of patients who were either unsatisfied or only moderately satisfied with their current method of administration ranged from 54% among patients using an autoinjection device to 60% among patients using a prefilled syringe and 78% among those using vials and syringes. These findings indicate that even among patients already using an autoinjection device, there is scope for improvements in autoinjector design that may increase patient satisfaction with their injection method. It should be noted that 9% of subcutaneous IFN beta-1a patients reported using a syringe and vial although this drug was not available in such a delivery format at the time of the survey.
Results from this multinational survey indicate that patients reacted favorably to the new electronic autoinjection device. The design improvements that were found to be most appealing to patients were the dosing log and the ability to tailor injection settings to suit individual needs. The new electronic autoinjector appraised in this survey has also been evaluated in another study, which assessed ease of use and suitability in 106 patients with multiple sclerosis who had the opportunity to use the device.Citation13 Most patients considered the device suitable for self-injection, with 95% of patients rating it as “easy” or “very easy” to use, and over 80% of patients rating individual features, such as dose history, confirmation of end of injection, audible and visual signals, and display of last injection time and date, as “useful” or “very useful”. Additional international user trials will further validate the new device in patients with relapsing-remitting multiple sclerosis.Citation14
It should be acknowledged that patient characteristics outside the scope of this analysis, such as patient age and length of time previously injecting therapy, could influence patient responses to these questions. The online format of this survey may have selected for patients who are more comfortable with technology. This survey confirms the findings of previous studies that have found that barriers to self-injection include both physical factors, such as injection pain, and psychological factors, including anxiety about the use of needles.Citation5,Citation8 However, this survey also found that over two-thirds of respondents were “very comfortable” with new technologies. These results suggest that new technologies such as those incorporated in the new electronic autoinjection device could potentially help large numbers of patients to, at least partially, overcome some of the physical and psychological barriers to self-injection. In particular, the ability to adjust speed and depth of needle insertion and injection speed and time may allow patient comfort to be maximized. Concealment of the needle may also reduce needle-related anxiety. Evidence from the field of diabetes suggests that use of an automatic injection device that conceals the needle during the entire injection process is associated with significantly reduced pain perception.Citation15 Furthermore, by providing reassurance regarding correct injection technique and improving the comfort of the injection, this new autoinjector may improve adherence. Importantly, the dosing log incorporated into the device provides an objective means by which to assess adherence to subcutaneous IFN beta-1a treatment and may facilitate discussion between patients and health care professionals, which may, in turn, improve adherence and treatment decision-making.
Acknowledgements
The authors thank Double Helix Development for data collection and analysis. The authors also thank Adam McGechan for ACUMED (supported by Merck Serono S.A. – Geneva, Switzerland) for assisting the authors with preparation of the first draft and Reza Sayeed for Caudex Medical (supported by Merck Serono S.A. – Geneva, Switzerland) for collation of comments and revisions from the authors and for the preparation of subsequent drafts.
Disclosure
This study was supported by Merck Serono S.A. – Geneva, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. All authors are salaried employees of Merck Serono S.A. – Geneva, Switzerland.
References
- FordHLGerryEJohnsonMWilliamsRA prospective study of the incidence, prevalence and mortality of multiple sclerosis in LeedsJ Neurol200224926026511993523
- SlokaJSPryse-PhillipsWEStefanelliMIncidence and prevalence of multiple sclerosis in Newfoundland and LabradorCan J Neurol Sci200532374215825544
- IngleseMMultiple sclerosis: New insights and trendsAm J Neuroradiol20062795495716687523
- Al-SabbaghABennetRKozmaCDicksonMMeleticheDMedication gaps in disease-modifying therapy for multiple sclerosis are associated with an increased risk of relapse: Findings from a national managed care databaseJ Neurol.2008255Suppl 2S79
- CostelloKKennedyPScanzilloJRecognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long termMedscape J Med20081022519008986
- DevonshireVLapierreYMacdonellRThe Global Adherence Project (GAP): A multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosisEur J Neurol2010614 [Epub ahead of print]
- TreadawayKCutterGSalterAFactors that influence adherence with disease-modifying therapy in MSJ Neurol200925656857619444532
- LugaresiAAddressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivationExpert Opin Drug Deliv20096995100219637982
- BrochetBLemaireGBeddiafAReduction of injection site reactions in multiple sclerosis (MS) patients newly started on interferon beta 1b therapy with two different devicesRev Neurol (Paris)2006162735740 French.16840982
- LugaresiADurastantiVGasperiniCSafety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: The CoSa studyClin Neuropharmacol20083116717218520983
- MikolDLopez-BresnahanMTaraskiewiczSChangPRangnowJA randomized, multicentre, open-label, parallel-group trial of the tolerability of interferon beta-1a (Rebif) administered by autoinjection or manual injection in relapsing-remitting multiple sclerosisMult Scler20051158559116193898
- BayasAJappGFuldaUKallmannBInjection devices in the basic therapy of multiple sclerosis. Survey among neurologists, MS nurses and patientsNervenheilkunde2010295762 [German].
- DevonshireVArbizuTBorreBPatient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: An international, single-arm, multicentre, Phase IIIb studyBMC Neurol2010102820433746
- ExellSVerdunEDriebergenRMigliaccioMDevelopment of an electronic injection device for subcutaneous interferon beta-1a administration in patients with relapsing multiple sclerosisJ Neurol Sci.2009285SupplS203
- DiglasJFeinbockCIrsiglerKReduced pain perception with Pen Mate™, an automatic needle insertion device for use with an insulin penPractical Diabetes1999163941