Abstract
Background
Despite a movement toward patient-centered outcomes, best practices on how to gather and refine patients’ perspectives on research endpoints are limited. Advanced care planning (ACP) is inherently patient centered and would benefit from patient prioritization of endpoints for ACP-related tools and studies.
Objective
This investigation sought to prioritize patient-centered endpoints for the content and evaluation of an ACP video being developed for patients undergoing major surgery. We also sought to highlight an approach using complementary engagement and research strategies to document priorities and preferences of patients and other stakeholders.
Materials and methods
Endpoints identified from a previously published environmental scan were operationalized following rating by a caregiver co-investigator, refinement by a patient co-investigator, review by a stakeholder committee, and validation by patients and family members. Finalized endpoints were taken to a state fair where members of the public who indicated that they or a loved one had undergone major surgery prioritized their most relevant endpoints and provided comments.
Results
Of the initial 50 ACP endpoints identified from the review, 12 endpoints were selected for public prioritization. At the state fair, 359 individuals prioritized the endpoints, the highest ranking of which was having a meaningful conversation with a physician before surgery (57%).
Conclusion
Using a novel combination of engagement and research techniques provided the opportunity to understand which endpoints were most important to patients and families and fostered framework development clarifying the differential contributions of engagement and research. Results from this study ultimately changed the content and evaluation of the ACP video.
Introduction
An endpoint in clinical research is defined as “an event or outcome that can be measured objectively to determine whether the intervention studied is beneficial”.Citation1 Decision makers now recognize that patients should guide study designCitation2,Citation3 and be engaged in endpoint selection.Citation4–Citation10 This is important as patients may choose different endpoints than researchers and clinicians,Citation11,Citation12 given their different experiences and knowledge.Citation13,Citation14 The goal should be to include patients (and caregivers when appropriate) throughout all stages of inquiry to avoid token participation.Citation10
Patient-centered evaluation requires that patients are involved in all aspects of research, including the selection of endpoints.Citation15,Citation16 Including patients in endpoint selection remains somewhat limited,Citation9 and best practices are not established.Citation17,Citation18 Endpoint selection can occur through prioritization mechanisms such as multi-criteria decision analysis, utility eliciting, and rating/ranking methods. Ranking methods have been used to identify endpoints that patients and their family members prefer.Citation8,Citation12 Patients can be involved in endpoint selection through both research and engagement activities.Citation19 Using a combined research and engagement approach satisfies a moral rationale for patients to participate in processes that inform their care.Citation20
Our study focuses on promoting advance care planning (ACP) in high-risk surgery. ACP offers individuals the opportunity to clarify health care goals, concerns, and wishes in preparation for situations where they may be unable to make their own decisions.Citation21 The Patient-Centered Outcomes Research Institute contracted this team to develop and evaluate a patient-centered, video-based ACP tool for patients and family members preparing for major surgery.
The aim of this research was to identify patient- and caregiver-preferred ACP endpoints for the video tool being developed. It was hypothesized that patients and their families would prefer an anxiety and depression endpoint, as previous studies highlight the presence of significant anxiety and depression in preoperative and in seriously and critically ill patients.Citation22,Citation23 Although anxiety and depression are often used in ACP given their responsivity,Citation24,Citation25 it was unknown if patients and their loved ones would want an ACP video to focus on alleviating anxiety and depression.
We also sought to highlight the difference between the objectives and outcomes of patient, caregiver, and stakeholder engagement strategies as compared with research approaches when documenting the priorities and preferences of patients and other stakeholders. More specifically, the purpose of engagement activities within this study was to identify ACP endpoints of value and to refine these endpoints so that they were appropriate for an ACP video tool in the surgical setting. The purpose of research activities was to validate the endpoints identified through engagement and to isolate which endpoint was the most meaningful to patients and their loved ones.
Materials and methods
The research team developed a framework comparing facets of engagement and research generally () and specifically in the current study (). To develop this framework, the research team first identified five major domains (communication, ethical obligations, data collection, data analysis, and dissemination) that were integral components to both research and engagement activities. The research team then disaggregated each domain into two salient components, called “process factors”, which were common to both research and engagement. Emphasis is placed on this framework throughout the “Materials and methods” section to highlight how the shared domains and process factors are operationalized differently depending on the approach. The data collection and data analysis domains were of primary interest in the current investigation, given the overarching aim to identify an ACP endpoint based on input from diverse data sources. In the data collection domain, process factors include participant pool, ie, who takes part, and activities, ie, the structure of the participation process. In the data analysis domain, process factors include the level of inference, ie, individual vs group, as well as results interpretation, ie, the approach to “make sense” of the data.
Table 1 Conceptual comparative framework of engagement and traditional research
Table 2 Application of the comparative framework in the current study
Engagement approaches
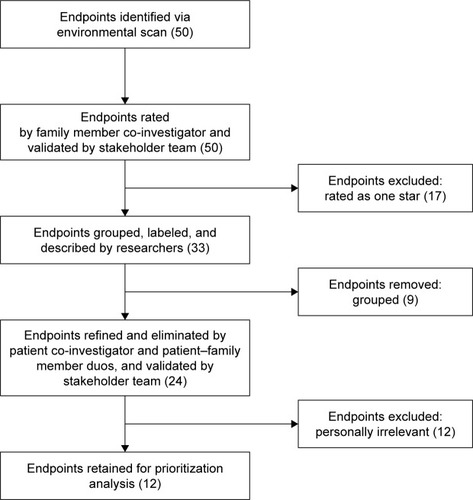
In order to identify and refine endpoints, a deliberately selected caregiver co-investigator (JM) reviewed 50 endpoints previously identified by a systematic review.Citation24 The caregiver co-investigator had more than three decades of experience as both an informal patient advocate for her loved ones and as a formal caregiver-consultant on perioperative ACP research studies (, data collection, participant pool). The endpoints were presented in groups of fourCitation12 to the caregiver co-investigator, who rated the personal meaningfulness of the endpoints using the following criteria: one-star, unimportant; two-stars, low importance; three-stars, important; four-stars, very important (, data analysis, results interpretation). Star-rating systems are used to rate item quality in the consumer marketCitation26,Citation27 and in health care systems.Citation28 Given that patient participation extends beyond “having a patient sit at the table”,Citation29 the caregiver rated endpoints prior to endpoint deliberation by a larger stakeholder team to ensure that her opinions were represented.Citation19 Following a deliberative process, the stakeholder team (composed of palliative care clinicians, surgeons, health services researchers, and the patient and caregiver co-investigators) reflected upon its own experiences in order to contextualize each endpoint’s rating, merit, and weakness. Through group discussion (, communication, information exchange), the stakeholder team collectively agreed to omit all endpoints that were not supported by the caregiver co-investigator as a first culling (, data collection, activities).
Research team members (RAA, JFPB) grouped, labeled, and wrote descriptions for the remaining endpoints. Redundant endpoints were grouped together, and multifaceted endpoints were disaggregated into endpoints that were actionable in aim and scope. A deliberately selected patient co-investigator who had undergone major surgery (CP) reviewed the refined endpoints and suggested edits based on personal experienceCitation12 (, ethical obligations, privacy). The complete stakeholder team reviewed the updated endpoints and patient co-investigator edits. The stakeholder team participated in the same deliberative process of reflection, and ultimately culled the endpoints in a manner consistent with patient co-investigator feedback.
All engagement activities were considered non-human subjects research by the Johns Hopkins School of Medicine Institutional Review Board (, ethical obligations, ethical review).
Research approaches
Consistent with good Patient-Centered Outcomes Research (PCOR) practices,Citation9 cognitive interviews were conducted with two sets of patient–family member dyads recruited from surgical clinics to validate engagement-identified endpoints (, data collection, activities). The dyads were informed that the research team was developing an ACP video tool and that the endpoints they would examine were possible endpoints for this video tool. The patients and their family members discussed their thoughts on the content and understandability of the endpoints presented to them. Based on this feedback, the dyads were asked to recommend improvements to the endpoints. Research team members (JFPB, ALRS, RAA) reviewed and finalized the revised endpoints and immediately presented them to the stakeholder team, completing the engagement process (, dissemination, target audience).
The project team rented a booth at the Maryland State Fair to elicit broader input on a meaningful endpoint for the preoperative ACP tool (, data collection, participant pool). State fairs, which typically have agricultural exhibitions and carnival/amusement activities, are gaining traction as research sites.Citation30 A sign at the booth invited individuals who had undergone major surgery, or their caregivers, to participate in a survey (, communication, information exchange). Participants viewed storyboards outlining the proposed content and scope of the ACP video under developmentCitation31 and were asked to anonymously (, ethical obligations, privacy) prioritize the 5 out of 12 most important endpoints for the ACP video being developed. Twelve total cards, each featuring an endpoint and its description, were presented to participants in the same order of three sets of four cards (, data collection; ).Citation12 Participants were able to seek clarification about the endpoints and the ranking activity.
Table 3 Potential endpoint labels and descriptions
Endpoint prioritization was summarized by frequency. Two-sample t-Tests in Stata softwareCitation32 assessed preference heterogeneity by sex (male vs female), age (<60 vs ≥60 years), and whether or not the surgery was for cancer (, data analysis, results interpretation). Patient and caregiver preferences were analyzed collectively as the ACP tool was intended for both audiences (, data analysis, level of inference). Participants also completed an open-ended prompt providing advice for those preparing for surgery. The 12 endpoints presented at the state fair acted as components of the analytic framework for the open-ended advice. Comments were reviewed by members of the research team with the participant’s prioritized endpoint in mind. Comments were also reviewed for any suggestions of general information to feature in the ACP video. All comments were compiled and reviewed by one research team member (ALRS), and decisions regarding incorporating comments into the video were made by two additional research team members (RAA, JFPB).
The study team received written informed consent from all participants in the cognitive interviews. Surveying at the state fair was approved as a human subject research activity exempt from full review by the Johns Hopkins School of Medicine Institutional Review Board as it was anonymous and did not contain sensitive information (, ethical obligations, ethical review). State fair participants provided implied consent to participate; the first page of the survey activity outlined that participation was voluntary and that by completing the survey, participants were providing consent.
Results
Engagement approaches
The caregiver co-investigator rated 27 of the total 50 endpoints as one star, 10 as two star, 11 as three star, and 12 as four star (). After review, the stakeholder team ultimately agreed that the 27 endpoints rated as one star by the caregiver co-investigator were not, based on their experience, meaningful endpoints to patients and families. The stakeholder team ultimately elected to discard the endpoints rated as one star (). The remaining 33 endpoints were compacted into 24 endpoints with descriptions (). The patient co-investigator identified 12 of the 24 endpoints as being personally relevant and recommended refined labels and explanations for endpoints. The stakeholder team agreed to retain only the 12 relevant endpoints.
Table 4 Endpoint ratings for two, three, and four star endpoints as determined by caregiver co-investigator
Research approaches
The patient–family member dyads agreed that the 12 endpoints were personally meaningful to them, and the stakeholder team subsequently finalized the following endpoints: anxiety and depression, comfort watching the video, decision making, decision maker conversations, decision maker designation, helpfulness of the video, leeway preference, perceived benefits, physician discussion, identification of values, telling others about decision makers, and well-being. Endpoint descriptions were intended to serve as the basis for how each endpoint might be operationalized in a video evaluation study and are provided in .
Over 11 days at the state fair, 359 people completed the survey (). Most participants were female (63%), aged >50 years (77%), had at least some college education (79%), were Caucasians (81%), and not reporting on experiences from cancer surgery (74%). Older participants more highly prioritized naming an alternate decision maker (p=0.01; ) and identifying what makes life worth living (p=0.01; ). No differences were observed by sex or by cancer surgery.
Table 5 Demographic characteristics of participants at the Maryland State Fair, N=359
Table 6 Frequency of endpoint selection by participants at the Maryland State Fair in 2014
The most valued endpoint was having a discussion with a physician about treatment wishes and goals prior to surgery (57%; 95% CI: 52–62; ). In participants’ advice to others preparing for surgery, they described the importance of this discussion. One participant urged people preparing for surgery to “have open conversations with people included in your care (especially surgery)”. Another participant advised to “never be afraid to ask (the) doctor questions”. Only 13% of participants prioritized the anxiety and depression endpoint within their top five (95% CI: 10–17; ). Although some advice reflected value in the anxiety and depression endpoint, such as to “have a positive attitude”, another participant explicitly recommended removing this endpoint.
Discussion
Patients’ and caregivers’ preferences for endpoints can be elicited through engagement and research approaches. The most highly prioritized endpoint for the ACP video was having a meaningful discussion with a physician rather than the hypothesized anxiety and depression endpoint. Differences between older and younger participants’ preferences for ACP have been previously observed,Citation33,Citation34 but it is notable that preference differences by age were not observed for the prioritized endpoint of physician discussion. That the oft-used anxiety and depression endpoint was not the most valued endpoint contributes to a growing body of literature demonstrating that professionals and patients may have different endpoint preferences,Citation12,Citation13 further validating the need to include patient perspectives early in the research process.
A meaningful conversation between a patient and a surgeon prior to surgery was not initially hypothesized as a meaningful endpoint by the research team, given the highly biomedical nature of surgical care. Physician discussion is a relevant endpoint as communication skills with patients (inherent for good ACP conversations) are positively associated with patient satisfaction,Citation35 and patients often prefer doctors who participate in communication-based shared decision making.Citation36 Studies specifically highlight potential challenges for ACP communication in the preoperative setting as surgeons and patients may enact an implicit “covenant of care” during the consent process in which patients transfer decision making about life-sustaining perioperative treatments to the surgeon.Citation37,Citation38 Patient–surgeon communication is of particular importance, given the complexity of conversations that must occur in advance of surgery.Citation39 Communication is a well-documented facet of patient-centered care;Citation40,Citation41 however, surgeons often do not explore emotional concerns of patients.Citation42 Given this evidence, it is significant that results support that patients and family members place a high value on a preoperative ACP video tool that fosters meaningful patient–physician discussions about treatment wishes and goals, suggesting that many surgery patients and family members may be ready to talk about ACP.
Patient-centered research does not stop at the identification of endpoints; rather, it actively incorporates the perspectives of patients and family members into the research process. Patient prioritization of meaningful physician discussion over reducing anxiety and depression, therefore, resulted in a substantial redesign of both the ACP video and its evaluation trial. The team negotiated with its funder to change the primary outcome of the video’s evaluation from anxiety and depression to having a meaningful discussion. Meaningfulness of the discussion was operationalized by the measure “patient-centered nature of the patient–surgeon conversation” in the resulting video evaluation trial, procedures for which have been previously described.Citation43 This change in outcome also required that language be added to the video to encourage open communication between the patient and surgeon.Citation43
In the practice-oriented context of the current study, using both engagement and research approaches in endpoint selection provided an innovative means of identifying and prioritizing endpoints. The conceptual framework presented in this paper adds knowledge to the current literature by depicting how engagement and research compare across various domains. This conceptual model may serve as guidance for future studies, considering the value and purpose of engagement and/or research to identify the endpoints for PCOR.
Interpretation of the study must consider its limitations. First, although the endpoints were initially identified through an environmental scan, all relevant endpoints may not have been included for consideration. Second, although the patient-centered nature of the intervention is the strength of the study, the patient and caregiver-driven selection of endpoints may have looked different had different patients and caregivers been included in the selection process. Although reproducibility and replication are highly important in research, it is important to highlight that engagement purposively involves “insiders” because of their unique experiences. Engagement is a pragmatic approach for eliciting patient and caregiver input into formative stages of larger research studies. The largest limitation of the study pertains to the generalizability of the research findings at the state fair. Even though a large number of people attending completed the survey, this self-selected convenience sample may not be representative of all patients preparing for major surgery, and future studies using a dual engagement–research approach should strive to maximize generalizability during endpoint prioritization. It is noteworthy that the results from the state fair are certainly more generalizable than those that would have emerged from engagement activities alone and that the full generalizability of these findings will be further evaluated during the trial of the developed ACP intervention.Citation43
From its inception, researchers on this project planned to use a novel patient-centered method to identify, refine, and select endpoints in the absence of best practices on how to do so. Using a combined research and engagement approach represents a commitment to the hypothesis that gathering patient perspectives makes research more relevant to patient and family member decisions and is more likely to lead to the outcomes that matter most to them.Citation7
Acknowledgments
We would like to thank all patients and caregivers who participated in the varying stages of this project, as well as the stakeholder team which helped to refine endpoints. Thanks to Thomas Lynch for his contributions to this project. We are very grateful to our anonymous peer reviewers for providing thoughtful insight that helped us to strengthen the paper. This paper is dedicated to Carolyn Pastorini.
Research reported in this article was funded through a Patient-Centered Outcomes Research Institute (PCORI) award (CD-12-11-4362). The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the PCORI, its board of governors, or methodology committee.
Disclosure
JFPB acknowledges support from the PCORI Methods Program Award (ME-1303-5946). The authors report no other conflicts of interest in this work.
References
- NCI Dictionary of Cancer Terms2017 Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=346519Accessed July 31, 2017
- Institute of Medicine Committee on Quality of Health Care in AmericaCrossing the Quality Chasm: A New Health System for the 21st CenturyWashington, DCNational Academies Press2001
- PittsPJThe patient voice: at the intersection of a US regulatory revolutionPatient20169537337727581685
- Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidanceHealth Qual Outcomes2006479
- Agency for Healthcare Research and QualityNational Healthcare Quality ReportRockville, MDAgency for Healthcare Research and Quality2006
- BastianHSpeaking up for ourselves. The evolution of consumer advocacy in health careInt J Technol Assess Health Care19981413239509791
- HansenHThe patientKristensenFSigmundHThe Health Technology Assessment HandbookCopenhagenDanish Centre for Health Technology Assessment2007104114
- JanssenIMGerhardusASchröer-GüntherMAScheiblerFA descriptive review on methods to prioritize outcomes in a health care contextHealth Expect20151861873189325156207
- Methodology Committee of the Patient-Centered Outcomes Research Institute (PCORI)Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspectiveJAMA2012307151636164022511692
- TerrySFPatrick-LakeBHearing voices: FDA seeks advice from patientsSci Transl Med20157313313ed12
- PyneJMLabbateCRanking of outcome domains for use in real-time outcomes feedback laboratory by patients with schizophreniaJ Nerv Ment Dis2008196433633918414130
- KinterETSchmedingARudolphIBridgesJFIdentifying patient-relevant endpoints among individuals with schizophrenia: an application of patient-centered health technology assessmentInt J Technol Assess Health Care20092501354119126249
- EntwistleVARenfrewMJYearleySForresterJLamontTLay perspectives: advantages for health researchBMJ199831671294634669492683
- Caron-FlintermanJFBroerseJEBundersJFThe experiential knowledge of patients: a new resource for biomedical research?Soc Sci Med200560112575258415814182
- MooneyGWhat else do we want from our health services?Soc Sci Med19943921511548066493
- BridgesJFJonesCPatient-based health technology assessment: a vision of the futureInt J Technol Assess Health Care2007231303517234014
- FrankLBaschESelbyJVPatient-Centered Outcomes ResearchIThe PCORI perspective on patient-centered outcomes researchJAMA2014312151513151425167382
- StewartRJCairdJOliverKOliverSPatients’ and clinicians’ research prioritiesHealth Expect201114443944821176014
- FaceyKBoivinAGraciaJPatients’ perspectives in health technology assessment: a route to robust evidence and fair deliberationInt J Technol Assess Health Care201026333434020584364
- Caron-FlintermanJFA New Voice in Science: Patient Participation in Decision-Making on Biomedical ResearchAmsterdamVrije Universiteit2005
- SilveiraMJKimSYLangaKMAdvance directives and outcomes of surrogate decision making before deathN Engl J Med2010362131211121820357283
- HellstadiusYLagergrenJZylstraJPrevalence and predictors of anxiety and depression among esophageal cancer patients prior to surgeryDis Esophagus20152981128113426542282
- LautretteADarmonMMegarbaneBA communication strategy and brochure for relatives of patients dying in the ICUN Engl J Med2007356546947817267907
- AslaksonRASchusterALReardonJPromoting perioperative advance care planning: a systematic review of advance care planning decision aidsJ Comp Effect Res201546615650
- AslaksonRASchusterALMillerJWeissMVolandesAEBridgesJFAn environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocolPatient20147220721724469597
- HamlinRMcNeillLDoes the Australasian “Health Star Rating” front of pack nutritional label system work?Nutrients201686327
- NathanRYaktineALichtensteinAHWartellaEAFront-of-Package Nutrition Rating Systems and Symbols: Promoting Healthier ChoicesWashington, DCNational Academies Press2012
- First Release of the Overall Hospital Quality Star Rating on Hospital Compare2016 Available from: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2016-Fact-sheets-items/2016-07-27.htmlAccessed December 2017
- LomasJCulyerTMccutcheonCLawSTetroeJFinal Report-Conceptualizing and Combining Evidence for Health System Guidance2005
- D2D: The Driven to Discover Research Facility2017 Available from: http://d2d.umn.edu/Accessed July 31, 2017
- AslaksonRASchusterALLynchTJDeveloping the storyline for an advance care planning video for surgery patients: patient-centered outcomes research engagement from stakeholder summit to state fairJ Palliat Med Epub2017817
- Stata Statistical Software: Release 12 [computer program]College Station, TXStataCorp LP2011
- RatcliffeJLancsarEFlintTDoes one size fit all? Assessing the preferences of older and younger people for attributes of quality of lifeQual Life Res201726229930927553968
- van WijmenMPPasmanHRWWiddershovenGAOnwuteaka-PhilipsenBDContinuing or forgoing treatment at the end of life? Preferences of the general public and people with an advance directiveJ Med Ethics201541859960625182697
- TakHRuhnkeGWShihYCThe association between patient-centered attributes of care and patient satisfactionPatient20158218719725011683
- WileyJWestbrookMGreenfieldJRDayROBraithwaiteJShared decision-making: the perspectives of young adults with type 1 diabetes mellitusPatient Prefer Adherence2014842324729690
- SchwarzeMLBradleyCTBraselKJSurgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapyCrit Care Med201038384384820048678
- SchwarzeMLRedmannAJAlexanderGCBraselKJSurgeons expect patients to buy-in to postoperative life support preoperatively: results of a national surveyCrit Care Med20134111823222269
- Clarence BraddockPLHIIIFeldmanJJBereknyeiSFrankelRMLevinsonW“Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgeryJ Bone Joint Surg Am2008909183018762641
- EpsteinRThe science of patient-centered careJ Fam Pract200049980581011032204
- EpsteinRMFranksPFiscellaKMeasuring patient-centered communication in patient-physician consultations: theoretical and practical issuesSoc Sci Med20056171516152816005784
- LevinsonWHudakPTriccoACA systematic review of surgeon–patient communication: strengths and opportunities for improvementPatient Educ Counl2013931317
- AslaksonRAIsenbergSRCrossnohereNLUtilising advance care planning videos to empower perioperative cancer patients and families: a study protocol of a randomised controlled trialBMJ Open201775e016257