Abstract
The pharmacological treatment for stable COPD is based on the use of inhaled bronchodilators (long-acting muscarinic receptor antagonists and long-acting beta-2 adrenoceptor agonists) and inhaled corticosteroids. The use of triple inhaled therapy is recommended to selected patients with COPD. Among the various inhaler combinations in triple therapy, a new combination by fluticasone furoate, umeclidinium, and vilanterol is available for COPD patients. Recently, a large clinical trial using this combination has been published, resulting in a reduction in exacerbation rate in COPD patients. Furthermore, this combination has demonstrated efficacy and safety, with a single administration a day, through a dry powder inhalator device, which has shown a good adherence and is a preference of the patient. This review focuses on the main characteristics of this inhaler combination evaluating the main clinical effects, the patients’ adherence, and the safety.
Introduction
COPD is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation due to alveolar and/or airway abnormalities, usually caused by significant exposure to harmful particles or gases.Citation1 COPD represents a major public health problem, being a disease with a prevalence of 10% in Europe.Citation2,Citation3 Epidemiological estimates related to this pathological condition also describe an expanding disease, which in recent decades has become the third leading cause of death in the world. The burden of the disease also depends on the presence of comorbidity and on the frequency of exacerbations. The latter is responsible for 50%–75% of the costs of the disease, especially if the patients then require hospitalization.Citation1 Therefore, the attention is increased toward prevention campaigns focused on risk factor control, an early diagnosis, and an optimal treatment for both stable disease and exacerbations. In fact, the objectives of drug therapy aim at obtaining an optimal control of the current pathology, acting primarily on the reduction of symptoms, on the improvement of patients’ performance status, and on the control of future risk, through a blockade of functional decline and prevention of exacerbations. These goals must be achieved by optimizing the therapy, in order to reduce the side effects, maximize the impact of treatment, and improve the compliance to the therapy. The latter represents a critical point in respiratory patients as it is approximately at 15%.Citation4 The choice of the device and the methods of administration also play a fundamental role.Citation1
A focal point in the treatment of COPD is represented by the patients’ adherence to the treatment,Citation5 which in turn depends on the perception of the disease, the safety of the therapy, and the reduction of their symptoms. Furthermore, an important point for the patients’ adherence to the treatment is the ease of use of the device or the number of the devices used for the therapy.
Several clinical trials take this into account and include methods of measuring patient satisfaction and the impact of therapy on quality of life.
This review will focus on the use of triple inhaled therapy with a particular focus on the once-a-day dry powder inhalator (DPI) fixed-dose combination of fluticasone furoate (FF), umeclidinium bromide (UMEC), and vilanterol trifenatate (VI).
The role of triple inhaled therapy in COPD
The inhaled therapy used in COPD consists in heterogeneous pharmacological classes that can act on the lungs by different mechanisms of action, determining a bronchodilation, and therefore the improvement of many functional parameters. In this way, they can have important repercussions on the patients’ state of health, on their performance status, and on the quality of life.Citation6 Moreover, through inhaled administration, it is possible to reduce the side effects given by systemic administration, even if these events cannot be completely eliminated. The categories of drugs referred to in this case are mainly antimuscarinic agents, beta-2 agonists, and corticosteroids.
Antimuscarinic agents act at the pulmonary level mainly by antagonizing the acetylcholine binding at the postsynaptic level on the M3 receptor and at the presynaptic level on the M2 receptor. They directly regulate the bronchial tone at the pulmonary level and at the systemic level, act on glands and epithelia, regulating the production and the clearance of mucus, the frequency of ciliary beating, and in general they can carry out a regulatory action on inflammation. The differences between the various agents belonging to this pharmacological category are related to the action selectivity, the time of action, and the speed of action.Citation7
Beta-2 agonists are an important pharmacological category for the treatment of bronchoconstriction. They act by binding to the beta-2 adrenergic receptor, which ultimately determines bronchodilation through the production of cAMP. Many molecules for this pharmacological category have been developed over the years, with some molecules focused on speed and duration of action, while others on selective receptor and, therefore, for efficacy and safety.Citation8 In addition to the bronchodilator effect, they may also have other functions at the pulmonary level, such as regulation of the inflammatory activity and inhibition of cholinergic action. This, therefore, is a synergistic effect with respect to antimuscarinic agents and a synergistic action also of glucocorticoids action.
Inhaled glucocorticoids (ICS) are the most important regulators of the inflammatory state of the airways. The mechanisms of action of glucocorticoids are very complex: they can regulate through the binding to specific receptors in gene transcription of many elements of inflammation, suppressing proinflammatory genes, and activating antiinflammatory genes instead. In this way, they can reduce the numbers of inflammatory cells at cellular level, including eosinophils, T-lymphocytes, mast cells, and dendritic cells.Citation9 These three classes of drugs act among them with synergic action by increasing the receptor expression and binding, by increasing the antiinflammatory effect, and by modulating the mediator release ().Citation10,Citation11
Figure 1 Schematic representation of synergic mechanisms of antimuscarinic agents, beta-2 agonists, and inhaled corticosteroids.
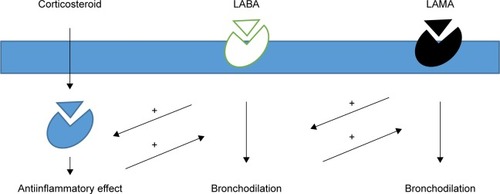
According to the GOLD guidelines, the most appropriate therapeutic choice for a COPD patient begins with the classification of his/her disease severity. The identification of subgroup to which the patient belongs is important because these specific recommendations can guide the physician to decide whether to start with an inhaled monotherapy or eventually move to a combination therapy of long acting beta agonist (LABA) and long acting muscarinic antagonist (LAMA).
In patients characterized by the most severe functional and symptomatic alteration and by the highest risk of exacerbations in groups D and C, the use of a triple combination LAMA, LABA, and ICS may be recommended.Citation1
The treatment of patients with COPD should be constructed based on patients’ spirometric values, symptoms, and perception of the patients’ disease, as well as on the frequency of exacerbations. Therefore, patients in stage A, less symptomatic, with a pathology of lesser severity and with less probability of exacerbations, should start the treatment with a single bronchodilator. In group B, similar to group A in terms of clinical–instrumental features, but with more pronounced symptoms, patients can benefit from a long-acting bronchodilator, and in case of persistence of symptoms, from the combination of two long-acting bronchodilators.Citation1
In the case of greater flow limitations and greater possibility of COPD exacerbations, it is possible to introduce an inhaled ICS therapy, either in combination with another bronchodilator if the patient is not very symptomatic, or in triple therapy if the patient is strongly symptomatic.
However, while the central role of bronchodilators in COPD is clearly established, controversy exists regarding the use of ICS in COPD.Citation12 In fact, the use of ICS may increase the risk of developing respiratory infections such as pneumonia. Furthermore, a retrospective general practice cohort study in the United Kingdom has shown that the rate of exacerbations requiring hospitalizations did not change, notwithstanding the increased number of prescriptions for LABA plus ICS combinations.Citation13
The identification of patients with an asthmatic component or the identification of elevated levels of eosinophils could in fact identify a group of patients more sensitive to this therapy. However, the use of a triple therapy is recommended only in patients of group C or D, which despite the combination therapy of LABA and LAMA still show severe symptoms or frequent exacerbations.Citation1
Clinical studies dedicated to the evaluation of the efficacy and safety of triple therapy have not reached an absolute agreement.
Many clinical studies have shown that the LABA/LAMA/ICS combination is able to demonstrate a significant improvement in the functional parameters of patients with moderate to severe COPD, compared with LAMA alone.Citation14–Citation18 These studies have shown an improvement in FEV1 and other physiological and symptomatological parameters, after the transition to triple therapy, such as the values of lung volumes or airway conductance, with statistically significant differences. The same studies did not give consistent data regarding exacerbations. In some cases,Citation14,Citation19,Citation20 the exacerbations seemed significantly reduced, while in other comparative studies no significant differences were observed.Citation16,Citation18,Citation21 These data could also be influenced by the criteria for defining and identifying the exacerbation, the difference in the inclusion criteria, and the duration of the follow-up. The effect on COPD exacerbations was studied on a Scottish cohort by Manoharan et al,Citation22 which demonstrated a reduction in exacerbations and related hospitalizations and a reduction in overall mortality when patients are treated with triple therapy or with ICS/LAMA combination compared with the ICS combination with LABA alone. In a deep analysis of the individual causes of death, only the triple therapy was recognized to be able to significantly reduce the cardiovascular mortality.Citation22
However, when triple therapy is prescribed in carefully selected patients, univocal data emerge: a statistically significant improvement in the quality of life, a lower use of the drug when needed, and an improvement in symptoms of dyspnea and pulmonary function when compared with LAMA alone.Citation14–Citation18 Less evidence is available in the literature on the comparison of the triple therapy to the therapy with LAMA/LABA, often with discordant results. A meta-analysis has shown that the combination indacaterol/tiotropium can have effects on lung function and quality of life overlapping the triple therapy.Citation23 A comparison study of the inhaled triple therapy versus the tiotropium/salmeterol combination demonstrates a risk of overlapping exacerbations between patients who continue and those who stop ICS, even if in the latter a decline in the spirometric parameters is observed in the final stages of the study.Citation24
The appropriateness of prescription is another point which has to be considered. In fact, the lack of a well-defined indication for triple inhaled therapy for COPD results in differences in real-life prescription patterns. Accordingly, Brusselle et al has shown in the UK, a prescription of triple therapy not always in accordance with the recommendations listed in the guidelines, observed patients with COPD GOLD A and B in treatment with triple therapy.Citation25 Di Marco et al has shown a poor prescriptive appropriateness even in Italy, also highlights a low demand for spirometry and specialist examinations by the general practitioner.Citation26 These data are not of little importance if we consider that this therapy is not without side effects, attributable in particular to the chronic use of corticosteroids.Citation25,Citation27–Citation29
The new combination of FF/umeclidinium/vilanterol inhaler
Among the various inhaler combinations in triple therapy, a new combination is given by FF/UMEC/VI.
FF is a potent corticosteroid with antiinflammatory action that acts by preserving epithelial integrity and reducing epithelial permeability in response to protease-induced cell damage or resulting from mechanical damaging stimuli.Citation18
UMEC acts for competitive antagonism on muscarinic receptors. It has a very strong bond with the M3 receptor comparable to that of tiotropium, with a speed of dissociation from the M3 receptor slower than that from the M2 receptor. The time required to reach the maximum concentration is between 5 and 15 minutes, ensuring a remarkable speed of action. This kinetics results in a long-lasting bronchodilation and, therefore, in the possibility of a single daily dose.Citation30
VI is a powerful and selective beta-2 agonist, which was developed in combination with FF in the treatment for asthma and with UMEC for COPD. It represents a good rapidity of action, since after its intake, it reaches its maximum concentration after about 10 minutes. It also has a minimal systemic absorption and therefore an optimal safety profile. VI is metabolized into compounds that continue to have an action, resulting in a long duration of action.Citation31 In some preclinical models, VI showed a significantly faster action of onset versus that of salmeterol and a longer duration of action than salmeterol and formoterol.Citation32
The efficacy of the combination between UMEC and VI has already been studied after the introduction of dual bronchodilation. In fact, many studies have shown the efficacy of this combinationCitation33–Citation35 and a good safety profile.Citation33 The UMEC/VI combination has shown a positive influence on the quality of life of the treated patients.Citation36
Symptoms and functional parameters
The combination of FF/UMEC/VI gave positive results when the effects were studied using two separate DPIs.Citation37 The same combination of FF/UMEC/VI gave positive results also when compared with other ICS + LABA combinations in improving lung function and health status, for the first time in the FULFIL study, in which the drugs are administered only once a day from the same device.Citation38
The FULFIL study was a 24-week, randomized, double-blind, double-dummy, parallel-group, multicenter study, which involved 1,810 patients with moderate or severe COPD and with a history of at least two or more moderate or one severe exacerbation (requiring hospitalization) in the previous 12 months after recruitment. The recruited patients also had a significant symptomatology, demonstrated by a COPD assessment test (CAT) score of ≥10 at the entrance to the study. The patients were divided into two groups, one of which received the triple FF/UMEC/VI therapy and the other with budesonide/formoterol. This study showed an improvement of FEV1 in the first arm compared with the ICS + LABA combination. In fact, an increase of 142 mL was observed at FEV1 at the end of 24 weeks in the study arm which included patients who had received the triple therapy, compared with a worsening of FEV1, of about 29 mL, in those who had received the ICS + LABA combination.Citation38
The FULFIL study also showed an improvement in the patients’ symptomatology, demonstrated by a reduction of 6.6 points in the result of the St George respiratory questionnaire (SGRQ) test at the end of the 24 weeks of observation in the first arm, and of 4.3 points in the second arm. The beneficial effects of triple therapy have also been evident in terms of reduced use of the drug when needed.
The advantages shown by the FF/UMEC/VI combination have also been confirmed by comparing FF/VI with UMEC using different devices. Bremner et al has shown, in a Phase III, multicenter, randomized, double-blind, parallel-group study, that the inhalation of the triple therapy with a single device is not inferior to the inhalation of the same therapy if administered in two different devices.Citation39
An important response to the use of FF/UMEC/VI combination has been given by the IMPACT trial, a 52-week Phase III, randomized, double-blind, three-arm, parallel-group, global multicenter study, which was completed in July 2017 and published in April 2018.Citation40
In this study, the primary outcome was to compare the rate of moderate and severe exacerbations between single-inhaler FF/UMEC/VI and single-inhaler FF/VI or UMEC/VI in >10,000 patients affected by severe-to-very severe COPD. The comparison of single-inhaler triple ICS/LABA/LAMA therapy versus single-inhaler dual LAMA/LABA therapy is of particular relevance, as the lack of similar studies represents an unmet need in pharmacological treatment of COPD.
Patients completed an electronic diary each morning to record their symptoms. The severity of an exacerbation was defined according to the treatment. The rate of moderate or severe exacerbations was significantly lower with the combination of FF, UMEC, and VI (0.91 per year) than with FF/VI (1.07 per year) or UMEC/VI (1.21 per year).Citation40
For the spirometric outcome of the mean change from baseline in trough FEV1, the difference between the triple therapy and the FF/VI groups was 97 mL (95% CI, 85–109; P<0.001), and the difference between the triple therapy and the UMEC/VI groups was 54 mL (95% CI, 39–69; P<0.001).
Quality of life
Several clinical trials have been performed to assess the patients’ general state of health and disease perception including the quality of life (HRQoL)Citation41,Citation42 using various questionnaires. One of the most complete questionnaires is the SGRQ, a questionnaire used for both asthma and COPD.Citation43 The SGRQ is the tool used to discriminate between patient differences and to assess changes in perception in the same patient over time. Another questionnaire, the CAT, consists in fact only eight questions, whose answer can be easily identified on a scale from 1 to 5, the higher the number, the more serious the condition of the patient in relation to that disorder.Citation44 This test was used in the FULFIL study to evaluate the patients’ symptoms at the entrance to the study: being required that the patients were symptomatic, one of the inclusion criteria provided a CAT score ≥10.
The FULFIL study demonstrated a statistically significant improvement in HRQoL. At the entrance to the study, the patients were selected according to CAT. Subsequently, the BDI (Basic Dyspnea Index) was administered as an interviewer-administered rating of severity of dyspnea at a single state. On subsequent visits, the patients’ health status was investigated through the SGRQ and the Transition Dyspnea Index, which measure changes in dyspnea severity from the baseline, as established by the BDI. The patients only carried out the CAT again at the final visit. At the end of the FULFIL study, an improvement in HRQoL was observed in both arms of the study at week 24, but the mean changes from baseline in the triple therapy group were statistically higher than those in the dual therapy group, if they evaluated the total scores of both the CAT and the SGRQ.Citation45 The SGRQ score, in fact, varies by 2.2 points in the group treated with triple therapy compared with the group treated with ICS + LABA and the CAT score shows a difference of 0.9 points in the improvement sense in the first group compared with the second at the end of the study.
The FULFIL study also demonstrated a statistically significant improvement in the triple therapy group compared with the arm of ICS + LABA in functional limitation and a socioeconomic benefit assessed by administering the health care resource utilization questionnaire, a tool designed to estimate the use of economic resources in the medical field, to patients.Citation46
The total cost of patients in triple treatment is greater at 24 weeks, but it was lower than the second arm if instead the observation is prolonged to 52 weeks. These socioeconomic evaluations should be reassessed in light of the fact that these findings were made in the FULFIL study only in the UK and that the economic benefit was demonstrated only at 52 weeks.Citation28
The effect of single-inhaler FF/UMEC/VI versus single-inhaler FF/VI or UMEC/VI in COPD on changes in SGRQ total score has also been a secondary outcome of the IMPACT trial.Citation40 In this study, the patients with triple therapy presented an improvement in mean change from baseline in the SGRQ total score when compared with the FF/VI and UMEC/VI groups.
Adherence to the treatment
Medication adherence in patients with COPD, like with all chronic disorders, is complex, although it is crucial for the best outcomes. Therapeutic adherence can be influenced by several factors relating to disease severity, the doctor–patient relationship, the socioeconomic factors, and the therapy itself. In particular, this includes the frequency of administration, the speed of action of the drug, and the manageability of the device.Citation47–Citation49
It is evident that a simpler therapeutic scheme will guarantee a better adherence of the patient, especially in COPD patients, which more frequently affect elder patients, often suffering from other comorbidities and therefore assuming numerous drugs. In these patients, it is suggested to simplify the treatment. Indeed, many studies have shown that patients are more compliant with once daily administration than treatment regimens twice or three times a day.Citation50,Citation51 In a large retrospective study (55,076 COPD patients), medication adherence strongly correlated with dosing frequency.Citation52 Furthermore, patients using multiple inhalers experienced more exacerbations.Citation53
In COPD patients, the poor adherence to the treatment may depend on the difficulty or insufficient training to the device.Citation54,Citation55 The choice of the most appropriate device also depends on the patient’s clinical condition. The devices currently in use are varied. In particular, there are metered-dose inhaler (MDI) devices on one side and DPI devices on the other. The first are spray or MDI aerosols predosed in pressurized cans, characterized by a high delivery speed, which increases the oropharyngeal deposition and therefore the frequency of local side effects and provide a good coordination of the patient to properly perform the inhalation. DPI devices are powder dispensers, which do not require coordination by the patient, do not contain propellants, and have reproducibility of the delivered dose. The disadvantage is represented by the need to apply an inhalation effort sufficient to inhale the powder from the device and the possibility that it will trigger the cough. DPI devices can be monodose or multidose. Next to these, soft mist inhaler and ultrasonic or compressed air nebulizers can be added. Although some studies have shown greater adherence to treatment with MDIs compared with DPIs,Citation56 other studies demonstrate greater efficacy of administration in DPI devices or an equal effectiveness between the two types of devices.
Ramadan and Sarkis have shown that ~70% of patients using DPI perform therapy correctly compared with about 40% of those using MDI.Citation57 In this study, ~81.4% of MDI users identify the coordination between container pressure and inhalation as the most difficult passage. These results are probably explained by the less complex administration (no coordination is needed) with DPI. The discrepancy between the various studies is an expression that there is no ideal inhaler, but we must choose the most appropriate device to the characteristics of the patient to be treated and the drug to be administered. All the devices present advantages and disadvantages. The common errors for both DPI and MDI were expiration before the dose, apnea after the dose, waiting between two consecutive doses, and finally gargle after a dose of corticosteroids.Citation57,Citation58
However, the ideal device should have a low internal resistance, allow a good reproducibility of the dose, generate a high fraction of fine particles, and, therefore, determine a high pulmonary deposition. At the same time, a good device should be easy to use, have a system for checking the dose inhalation and a simple system for the counting of doses, and should be resistant to the action of any external agents, such as humidity or trauma.
The ELLIPTA device, which is the tool to administrate FF/UMEC/VI, is a device with a dose counting system and a medium-low resistance system, which does not dispense preestablished suspensions, but determines the mixing of the doses of the different drugs at the time of the activation of the flow from the patient. It does not have any humidity control systems, so there is a deadline after 6 weeks.Citation59,Citation60 Several studies have demonstrated the efficacy of this device (). Svedsater et al have reported a positive opinion with the use of ELLIPTA in patients with asthma and COPD already in treatment with other devices. This was due to the lowest number of steps to be taken in order to inhale the drug, the compactness of the device and the size, the ease in remembering the operation, the size of the mouthpiece, and other features.Citation61 This was confirmed by van der Palen et alCitation62 who showed fewer errors in patients using ELLIPTA compared with naive patients who used other devices.
Table 1 Studies comparing ELLIPTA with other devices
ELLIPTA was compared with both DiskusCitation63 and Handihaler devices.Citation64 In both the studies, the patients expressed preference for ELLIPTA regarding the ease of use and therefore the characteristics of the device itself. Accordingly, Komase et al reported similar results in a Japanese population, demonstrating a preference of patients for ELLIPTA in particular, for ease of use, number of steps needed, and time taken to operate the inhaler.Citation65
Several recent randomized controlled trials evaluated the efficacy and safety of triple ICS/LABA/LAMA therapy using a single fixed-dose combined inhaler for patients with COPD.Citation19,Citation20
Bremner showed that FF/UMEC/VI administered with only one inhaler was not inferior to FF/VI plus UMEC administered at the same dosages but in two separate devices, in terms of improvement of FEV1. His findings confirm that triple therapy with a single inhaler with FF/UMEC/VI offers similar efficacy, health, and quality of life, such as the triple therapy administered with two separate inhalers.Citation66
At the final study visit, patients were also asked to express a preference regarding the device: among patients who expressed a preference, there was a greater preference for the ELLIPTA inhaler than the Turbuhaler.Citation45
Safety
Pharmacokinetic studies do not suggest clinically relevant pharmacokinetic differences between FF, UMEC, or VI intake when given as a triple therapy compared to separate FF/VI and UMEC/VI. It can, therefore, be assumed that the lung dose and safety of all three agents, delivered by a single inhaler, are similar to those of the approved FF/VI and UMEC/VI therapies.Citation67
Two randomized trials were conducted to study the safety and efficacy of UMEC added to FF/VI.Citation37,Citation67 When the use of FF/VI or UMEC was compared with the triple combination of UMEC/FF/VI, no major adverse or side effects were observed, and the therapy was generally well tolerated. In fact, few adverse events were observed, and the experimenters did not consider that they were to be attributed to the therapy in progress. The most common side effects reported include nasopharyngitis, headache, and back pain.Citation38 In both studies on this combination, totally, six deaths were reported but were not considered to be related to study treatment. Only one serious adverse event of diabetes mellitus was reported, which was shown to be a previously undiagnosed case of type 2 diabetes mellitus.Citation37,Citation67
Conclusion
The inhaler triple therapy with ICS/LAMA/LABA has demonstrated efficacy and safety in clinical studies, obviously with a variability based on the endpoint considered. The triple therapy has in fact shown efficacy in terms of respiratory function and quality of life.
An important point among the considerations regarding the triple therapy is the identification of the subgroup of COPD patients deserving to be treated with this protocol. Therefore, a correct diagnosis and stratification of the disease’s severity are needed. In other terms, it is necessary and fundamental to identify the group of patients that can best respond to the combined treatment with ICS/LAMA/LABA.
Currently, several inhaler combinations in triple therapy are being produced. In particular, the FF/UMEC/VI combination has demonstrated efficacy and safety, with a single administration a day, through a DPI device, especially after the recent publication of IMPACT study which has added new data.
The inhaler combination FF/UMEC/VI has shown a good patient adherence and preference from patients, probably linked to a greater easy handling and ease of use of simultaneous administration of three drugs, offering the potential for better compliance and results in patients with advanced COPD. Obviously, we think that an adequate choice of treatment for the patient is fundamental even for this combination.
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- MiravitllesMSorianoJBGarcia-RioFPrevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activitiesThorax2009641086386819553233
- Lopez-CamposJLTanWSorianoJBGlobal burden of COPDRespirology2016211142326494423
- SanduzziABalboPCandoliPCOPD: adherence to therapyMultidiscip Respir Med2014916025485108
- ChrystynHSmallMMilliganGHigginsVGilEGEstruchJImpact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPDRespir Med2014108235836524209768
- FromerLGoodwinEWalshJCustomizing inhaled therapy to meet the needs of COPD patientsPostgrad Med20101222839320203459
- BuelsKSFryerADMuscarinic receptor antagonists: effects on pulmonary functionHandb Exp Pharmacol2012208317341
- CorsicoAFulgoniPBeccariaMEffects of exercise and beta 2-agonists on lung function in chronic obstructive pulmonary diseaseJ Appl Physiol (1985)20029362053205812391117
- BarnesPJInhaled corticosteroidsPharmaceuticals (Basel)20103351454027713266
- CalzettaLMateraMGCazzolaMPharmacological interaction between LABAs and LAMAs in the airways: optimizing synergyEur J Pharmacol201576116817325981302
- CurrieGPLipworthBJPharmacological management-inhaled treatmentBMJ200633275551439144116777889
- BarnesPJInhaled corticosteroids in COPD: a controversyRespiration2010802899520501985
- HarriesTHSeedPTJonesSSchofieldPWhitePChronic obstructive pulmonary disease hospital admissions and drugs-unexpected positive associations: a retrospective general practice cohort studyNPJ Prim Care Respir Med2014241400624842126
- LeeSDXieCMYunusFEfficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East AsiaRespirology201621111912726394882
- SinghDBrooksJHaganGCahnAO’ConnorBJSuperiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPDThorax200863759259818245142
- JungKSParkHYParkSYKorea Chronic Obstructive Pulmonary Disease study groupComparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled studyRespir Med2012106338238921975275
- LiuYShiHSunXBenefits of adding fluticasone propionate/salmeterol to tiotropium in COPD: a meta-analysisEur J Intern Med201425549149524816076
- MalerbaMNardinMSantiniGMoresNRadaeliAMontuschiPSingle-inhaler triple therapy utilizing the once-daily combination of fluticasone furoate, umeclidinium and vilanterol in the management of COPD: the current evidence base and future prospectsTher Adv Respir Dis201812175346661876077929537340
- VestboJPapiACorradiMSingle inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trialLancet2017389100821919192928385353
- SinghDPapiACorradiMSingle inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trialLancet20163881004896397327598678
- AaronSDVandemheenKLFergussonDCanadian Thoracic Society/Canadian Respiratory Clinical Research ConsortiumTiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2007146854555517310045
- ManoharanAShortPMAndersonWJLipworthBJImpact of long-acting bronchodilators and exposure to inhaled corticosteroids on mortality in COPD: a real-life retrospective cohort studyLung2014192564965224952426
- KraemerMEllisABaldwinMJansenJPCapkun-NiggliGCopeSPRS5 dual bronchodilation with indacaterol and tiotropium in combination versus triple therapy, fixed-dose combinations, and monotherapy in COPD. A network meta-analysis of FEV1Value Health2012157A560
- MagnussenHDisseBRodriguez-RoisinRWISDOM InvestigatorsWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117
- BrusselleGPriceDGruffydd-JonesKThe inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UKInt J Chron Obstruct Pulmon Dis2015102207221726527869
- Di MarcoFSantusPTerraneoSCharacteristics of newly diagnosed COPD patients treated with triple inhaled therapy by general practitioners: a real world Italian studyNPJ Prim Care Respir Med20172715128883469
- KewKMSeniukovichAInhaled steroids and risk of pneumonia for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20143CD010115
- YangIAClarkeMSSimEHFongKMInhaled corticosteroids for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev20127CD002991
- ChoKHKimYSLintonJANamCMChoiYParkECEffects of inhaled corticosteroids/long-acting agonists in a single inhaler versus inhaled corticosteroids alone on all-cause mortality, pneumonia, and fracture in chronic obstructive pulmonary disease: a nationwide cohort study 2002–2013Respir Med2017130758429206637
- BabuKSMorjariaJBUmeclidinium in chronic obstructive pulmonary disease: latest evidence and place in therapyTher Adv Chronic Dis201784–5819128491268
- MalerbaMMorjariaJBRadaeliADifferential pharmacology and clinical utility of emerging combination treatments in the management of COPD-role of umeclidinium/vilanterolInt J Chron Obstruct Pulmon Dis2014968769525061288
- HananiaNAFeldmanGZachgoWThe efficacy and safety of the novel long-acting beta2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trialChest2012142111912722241764
- ZhengJZhongNNewlandsAChurchAGohAHEfficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo-controlled studyInt J Chron Obstruct Pulmon Dis2015101753176726366068
- CazzolaMSegretiAMateraMGNew developments in the combination treatment of COPD: focus on umeclidinium/vilanterolDrug Des Devel Ther2013712011208
- KerwinEMKalbergCJGalkinDVUmeclidinium/vilanterol as step-up therapy from tiotropium in patients with moderate COPD: a randomized, parallel-group, 12-week studyInt J Chron Obstruct Pulmon Dis20171274575528280319
- SilerTMDonaldACO’DellDChurchAFahyWAA randomized, parallel-group study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25 mug on health-related quality of life in patients with COPDInt J Chron Obstruct Pulmon Dis20161197197927274218
- SilerTMKerwinESousaARDonaldAAliRChurchAEfficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studiesRespir Med201510991155116326117292
- LipsonDABarnacleHBirkRFULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2017196443844628375647
- BremnerPRBirkRBrealeyNIsmailaASZhuCQLipsonDASingle-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: a randomized non-inferiority studyRespir Res20181911929370819
- LipsonDABarnhartFBrealeyNIMPACT InvestigatorsOnce-daily single-inhaler triple versus dual therapy in patients with COPDN Engl J Med2018378181671168029668352
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaireAm Rev Respir Dis19921456132113271595997
- KatsuraHYamadaKKidaKUsefulness of a linear analog scale questionnaire to measure health-related quality of life in elderly patients with chronic obstructive pulmonary diseaseJ Am Geriatr Soc20035181131113512890078
- BarrJTSchumacherGEFreemanSLeMoineMBakstAWJonesPWAmerican translation, modification, and validation of the St. George’s respiratory questionnaireClin Ther20002291121114511048909
- GuptaNPintoLMMoroganABourbeauJThe COPD assessment test: a systematic reviewEur Respir J201444487388424993906
- TabbererMLomasDABirkROnce-daily triple therapy in patients with COPD: patient-reported symptoms and quality of lifeAdv Ther2018351567129313286
- IsmailaASBirkRShahDOnce-daily triple therapy in patients with advanced COPD: healthcare resource utilization data and associated costs from the FULFIL trialAdv Ther20173492163217228875459
- AlbertsonTEHarperRMurinSSandrockCPatient considerations in the treatment of COPD: focus on the new combination inhaler umeclidinium/vilanterolPatient Prefer Adherence2015923524225673975
- LibbyAMFishDNHosokawaPWPatient-level medication regimen complexity across populations with chronic diseaseClin Ther2013354385e1398.e123541707
- Carr-LopezSMShekALastimosaJMedication adherence behaviors of medicare beneficiariesPatient Prefer Adherence201481277128425258521
- SainiSDSchoenfeldPKaulbackKDubinskyMCEffect of medication dosing frequency on adherence in chronic diseasesAm J Manag Care2009156e22e3319514806
- ColemanCILimoneBSobierajDMDosing frequency and medication adherence in chronic diseaseJ Manag Care Pharm201218752753922971206
- ToyELBeaulieuNUMcHaleJMTreatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costsRespir Med2011105343544120880687
- YuAPGuerinAde LeonDPClinical and economic outcomes of multiple versus single long-acting inhalers in COPDRespir Med2011105121861187121807487
- LavoriniFInhaled drug delivery in the hands of the patientJ Aerosol Med Pulm Drug Deliv201427641441825238005
- MelaniASInhalatory therapy training: a priority challenge for the physicianActa Biomed200778323324518330086
- DarbaJRamirezGSicrasAFrancoliPTorvinenSSanchez-de la RosaRThe importance of inhaler devices: the choice of inhaler device may lead to suboptimal adherence in COPD patientsInt J Chron Obstruct Pulmon Dis2015102335234526604733
- RamadanWHSarkisATPatterns of use of dry powder inhalers versus pressurized metered-dose inhalers devices in adult patients with chronic obstructive pulmonary disease or asthma: an observational comparative studyChron Respir Dis201714330932028774201
- AroraPKumarLVohraVEvaluating the technique of using inhalation device in COPD and bronchial asthma patientsRespir Med2014108799299824873874
- JonesTLNevilleDMChauhanAJThe Ellipta® in asthma and chronic obstructive pulmonary disease: device characteristics and patient acceptabilityTher Deliv20189316917629424288
- GrantACWalkerRHamiltonMGarrillKThe ELLIPTA® dry powder inhaler: design, functionality, in vitro dosing performance and critical task compliance by patients and caregiversJ Aerosol Med Pulm Drug Deliv201528647448526372466
- SvedsaterHDalePGarrillKWalkerRWoepseMWQualitative assessment of attributes and ease of use of the ELLIPTA dry powder inhaler for delivery of maintenance therapy for asthma and COPDBMC Pulm Med2013137224314123
- van der PalenJThomasMChrystynHA randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devicesNPJ Prim Care Respir Med2016261607927883002
- Yun KirbySZhuCQKerwinEMStanfordRHGeorgesGA preference study of two placebo dry powder inhalers in adults with COPD: ELLIPTA® Dry Powder Inhaler (DPI) versus DISKUS® DPICOPD201613216717526516724
- CollisonKAPatelPPreeceAFStanfordRHSharmaRKFeldmanGA randomized clinical trial comparing the ELLIPTA and HandiHaler dry powder inhalers in patients with COPD: inhaler-specific attributes and overall patient preferenceCOPD2018151465029227727
- KomaseYAsakoAKobayashiASharmaREase-of-use preference for the ELLIPTA® dry powder inhaler over a commonly used single-dose capsule dry powder inhaler by inhalation device-naive Japanese volunteers aged 40 years or olderInt J Chron Obstruct Pulmon Dis201491365137525525354
- BremnerPRBirkRBrealeyNIsmailaASZhuCQLipsonDASingle-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: a randomized non-inferiority studyRespir Res20181911929370819
- BrealeyNGuptaARenauxJMehtaRAllenAHendersonAPharmacokinetics of fluticasone furoate, umeclidinium, and vilanterol as a triple therapy in healthy volunteersInt J Clin Pharmacol Ther201553975376426227101
