Abstract
Purpose
Patients treated with warfarin must adhere to frequent monitoring, dietary restrictions, and complicated dose adjustments. Apixaban, a direct factor Xa inhibitor, is an alternative to warfarin that may reduce patient burdens associated with warfarin therapy. However, there is limited evidence pertaining to patient satisfaction with anticoagulant therapies in Japanese patients. The purpose of this observational study was to investigate changes in patient satisfaction after switching from warfarin to apixaban.
Patients and methods
Nonvalvular atrial fibrillation (NVAF) patients who were scheduled to switch anticoagulants from warfarin to apixaban were enrolled and treated with apixaban for 12 weeks. Patient satisfaction was assessed before the change in medication and after 12 weeks of treatment with apixaban using the Anti-Clot Treatment Scale (ACTS), a patient-reported instrument for measuring satisfaction with anticoagulation treatment. The ACTS includes a 12-item burden scale (maximum 60 points) and a 3-item benefit scale (maximum 15 points).
Results
Among 732 NVAF patients enrolled, the full analysis set consisted of 697 patients who completed two ACTS assessments (one before the medication change and one 12 weeks after the change). Mean (±standard deviation) patient age was 76.2±9.1 years and mean CHADS2 score was 2.5±1.3. There were no significant changes in ACTS benefit scores. However, ACTS burden scores showed significant improvements at Week 12 compared to baseline (55.6±5.3 at Week 12 and 49.7±8.7 at baseline; P<0.0001). Factors associated with changes in ACTS burden scores from the multiple logistic regression analysis were age ≥70 years (odds ratio [OR]: 1.86; 95% confidence interval [CI]: 1.12–3.10; P=0.0169), baseline ACTS burden score (OR: 0.79; 95% CI: 0.75–0.82; P<0.0001), and use of non-steroidal anti-inflammatory drugs/antiplatelet drugs (OR: 0.60; 95% CI: 0.36–1.00; P=0.0499).
Conclusion
Switching from warfarin to apixaban improved patient satisfaction with anticoagulant therapy in Japanese patients with NVAF by reducing burden of treatment.
Plain language summary
For many years, warfarin has been the standard of care in oral anticoagulation for the prevention of ischemic stroke in patients with nonvalvular atrial fibrillation (NVAF). However, patients treated with warfarin must adhere to frequent monitoring, dietary restrictions, and complicated dose adjustments. We investigated changes in patient satisfaction after switching from warfarin to apixaban. The results showed that switching from warfarin to apixaban improved patient satisfaction with anticoagulant therapy in Japanese patients with NVAF by reducing burden of treatment and that apixaban may be a suitable alternative to warfarin to reduce patient burdens associated with warfarin therapy.
Introduction
It is well known that atrial fibrillation (AF) is strongly associated with ischemic stroke. Furthermore, major cardiovascular risk factors including age, male gender, hypertension, elevated blood pressure, heart failure, and obesity are known to increase the risk of AF.Citation1–Citation3 Studies show that the prevalence of AF is increasing in the aging Japanese population.Citation4–Citation6 Warfarin has been widely used to prevent the occurrence of stroke as a complication of AF. However, warfarin can be difficult for patients to manage, as it requires regular monitoring of prothrombin time/international normalized ratio (PT-INR) and also as it interacts with many foods and medications.Citation7 It has been also suggested that warfarin therapy may be associated with an increase in arterial stiffness.Citation8–Citation10 Therefore, development of direct oral anticoagulant (DOAC) drugs offers potential benefits for patients requiring anticoagulant therapy.
Apixaban is an oral direct factor Xa inhibitor with potent antithrombotic effects. The ARISTOTLE trial showed non-inferiority and superiority of apixaban over warfarin in the primary efficacy endpoint (stroke or systemic embolism) and superiority in the primary safety endpoint (major bleeding) in patients with nonvalvular AF (NVAF).Citation11 The 2013 Guidelines for Pharmacotherapy of Atrial Fibrillation issued by the Japanese Circulation Society recommend that “when both warfarin and DOACs are indicated, the use of DOACs is desirable”.Citation12 However, the 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society state that “if patients are stable, easily controlled, and satisfied with warfarin therapy, it is not necessary to change to one of the newer agents”. The guidelines do, however, recommend discussing DOACs as an option for patients who are appropriate candidates.Citation12 In this context, patient satisfaction becomes an important factor in selecting or changing anticoagulant therapy in patients with AF. Additionally, patient preference may have a large impact on long-term adherence to prescribed medicationsCitation13,Citation14 and, consequently, on clinical prognosis.
A previous study reported that 58% of patients, particularly those >70 years of age or male, hoped to switch from warfarin to a DOAC.Citation15 Furthermore, Elewa et alCitation16 reported that although patient satisfaction with warfarin therapy was adequate, many patients still hoped to switch to DOACs to reduce hospital visits and to avoid interactions with medications and diet. In addition, it was shown that the price of DOACs is a major barrier to switching to anticoagulants.Citation7 Unfortunately, in Japan, there are scant data regarding patient satisfaction with prescribed anticoagulants among patients with NVAF in real-world clinical settings. Such data could help inform clinical decisions surrounding anticoagulant choice – specifically, whether switching from warfarin to a DOAC would be beneficial for patients.Citation17 In this study, we investigated the levels of patient satisfaction according to the Anti-Clot Treatment Scale (ACTS) when warfarin was switched to apixaban in Japanese patients with NVAF.
Patients and methods
Study design
This was a prospective, short-term, multicenter, single-arm, observational study designed to evaluate changes in patient satisfaction associated with switching from warfarin to apixaban. This study was conducted in compliance with all international and local rules including the Ethical Guidelines for Medical and Health Research Involving Human Subjects, the Declaration of Helsinki, and Japan’s Act on the Protection of Personal Information. The study was approved by institutional ethics committees or the central ethical committee. Written informed consent was obtained from every patient. The study is registered with UMIN-CTR, a clinical trial registry in Japan (study ID: UMIN000018970).
Study sites
This study was conducted at a total of 149 institutions in Japan. Hospitals accounted for 58 of all the study sites and institutions specializing in cardiology accounted for 125 sites.
Study population
This was an observational study without interventions or force to change medications by physicians and, therefore, the study protocol did not determine the treatment prescribed by physicians. Only NVAF patients whose anticoagulant was being switched from warfarin to apixaban for reasons unrelated to this study, such as medically important reasons (strict adherence to treatment guidelines, labile PT-INR control, potential drug–drug interactions, etc) and patient request, were enrolled in this study. Patients were excluded for contraindications or off-label use of apixaban. The enrollment period was between September 2015 and October 2016.
Outcomes
The primary objective of this study was to evaluate the change in patient satisfaction after switching from warfarin to apixaban in real-world settings in Japan. The primary endpoint was the change in patient satisfaction from baseline to Week 12 after switching to apixaban to evaluate the short-term impact on patient satisfaction; this was assessed using the ACTS burden and benefit scales. ACTS is an instrument that evaluates patient-reported satisfaction with anticoagulation therapy. It includes the 12-item ACTS burden scale (maximum of 60 points) and the 3-item ACTS benefit scale (maximum of 15 points).Citation18 The former includes questions regarding limitations of daily activities due to bleeding concerns and inconvenience associated with anticoagulant therapy. The latter includes questions regarding satisfaction or reassurance of anticoagulant therapy (Table S1 for details). The secondary objective of this study was to identify the key factors for improvement in patient satisfaction when switching from warfarin to apixaban.
Data collection
At each site, data were collected from medical records by investigators and their clinical research coordinators and entered into Viedoc (Uppsala, Sweden), an electronic data capture (EDC) system developed by DOT WORLD Co., Ltd. (Tokyo, Japan). Patients answered the ACTS and study-specific questionnaire by themselves for baseline characteristics, which were then faxed to Mebix, Inc. (Tokyo, Japan), a contract research organization. Mebix then entered questionnaire data into the EDC system and ensured the accuracy and validity of the data. Source data verification was conducted randomly at 10% of the study sites to confirm consistency between the original medical records and data entered into the EDC. Based on patient background data, CHADS2 and CHA2DS2-VASc scores (scores to evaluate the risk of stroke) and HAS-BLED scores (score to evaluate the risk of bleeding) were calculated.
Statistical methods
We separately compared ACTS burden and benefit scores at baseline to scores reported 12 weeks after patients switched to apixaban using the paired t-test with a two-sided significance level of 5%. In addition, the Wilcoxon rank sum test was conducted with a significance level of 0.05 (two-sided) to assess the robustness of the paired t-test. Descriptive statistics were used to describe demographics, clinical characteristics, and patient satisfaction from the ACTS.
To identify independent factors affecting patient satisfaction after switching to apixaban, we performed a multiple logistic regression analysis using the changes in each ACTS score (burden and benefit) as dichotomous variables: “improvers” (patients whose ACTS scores improved at Week 12) and “non-improvers” (those whose ACTS scores decreased or did not change at Week 12). Univariate logistic regression models were fitted for each variable and variables with P-values <0.2 were candidates for a subsequent stepwise selection procedure. Variables that were considered likely to show an association with each other were paired, and the relationship between the variables in each pair was investigated and reported as the Pearson product-moment correlation coefficient. If the correlation coefficient was >0.9, one of the variables was removed from the analysis to avoid multicollinearity. Gender, age (categorized into three age ranges: <70, 70–79, and ≥80 years), ACTS scores at baseline, history of bleeding, and history of stroke, as well as presence of concomitant diseases such as hypertension, diabetes, heart failure, and myocardial infarction, and use of concomitant medications (eg, non-steroidal anti-inflammatory drugs [NSAIDs] and/or antiplatelet drugs) were examined as covariates. The constructed model (“base model”) included gender and factors with P-values <0.2: age (≥70 years), ACTS scores at baseline, history of stroke, and use of NSAIDs, and/or antiplatelet drugs. In Model 1 and Model 2, other variables to be examined were added to the base model. Factors with a P-value <0.05 were considered to be significantly associated with the outcomes.
Results
From September 2015 to October 2016, 732 patients with NVAF were enrolled in the study; of these, 697 patients met the criteria of having at least two ACTS assessments (one before and one after switching medications) for inclusion in the full analysis set.
Patient characteristics
Demographic and clinical characteristics of the patients are shown in . A majority of patients were ≥70 years of age and were male. Creatinine clearance was 60.8±43.0 (mean ± SD) mL/min, suggesting that many patients had slight renal impairment. Mean CHADS2 and CHA2DS2-VASc scores indicated that patients registered in the study had a relatively higher risk of stroke. INR at baseline was 2.0±0.6, which is within the therapeutic range recommended in the treatment guidelines. A majority of patients did not earn income by working, and in more than half of the patients, the self-pay portion of total medical costs was 10%.
Table 1 Patient characteristics in FAS
Patient satisfaction
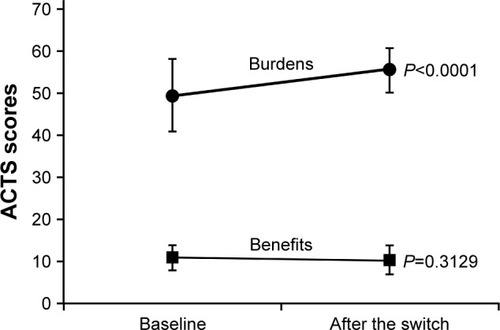
shows the changes in ACTS burden score and ACTS benefit score from baseline to Week 12 after switching to apixaban. The ACTS burden score significantly improved at Week 12 compared to baseline (55.6±5.3 vs 49.7±8.7; P<0.0001). However, there was no significant change in the ACTS benefit score (Week 12, 10.5±2.8 vs baseline, 10.4±3.3; P=0.3129).
Figure 1 Changes in ACTS burden score and ACTS benefit score.
Abbreviations: ACTS, Anti-Clot Treatment Scale; FAS, full analysis set; SD, standard deviation.
Among the components of the ACTS burden score, the question 5 Limit eat/drink showed the largest improvement (mean [range] score at baseline: 3.3 [3.2–3.4]; Week 12: 4.7 [4.7–4.8]). In contrast to the burden score, there was no component of the ACTS benefit score that changed substantially after switching to apixaban (Table S1). shows the differences in patient demographic and clinical characteristics between patients whose ACTS burden score improved (“improvers”) and those whose ACTS burden score did not improve (“non-improvers”). CHA2DS2-VASc and CHADS2 scores were higher in improvers than in non-improvers. The percentage of patients with a history of stroke was significantly (P<0.05) higher in the improver group. More patients in the improver group compared to the non-improver group had received apixaban 2.5 mg twice daily (BID). The use of NSAIDs and/or antiplatelet drugs was also slightly lower in the improver group versus the non-improver group, although the difference was not statistically significant (19.7% vs 23.6%, P=0.2836). In addition, there were significant differences in the reasons for changing to apixaban from patients’ perspectives (such as dietary restrictions and recommendations from persons other than physicians), as well as physicians’ perspectives (including bleeding risk associated with warfarin; P<0.05). Table S2 provides detailed results of the background differences between ACTS benefit score in improvers and non-improvers.
Table 2 Patient characteristics among ACTS burden “improvers” and “non-improvers”
Multiple logistic regression analysis
We conducted a multiple logistic regression analysis to investigate factors associated with changes in ACTS burden and benefit scores. As shown in “Patients and methods” section, we preliminarily built two logistic regression models and ultimately obtained the base model (Model 3) to identify factors that predicted improvement in ACTS burden score. The base model revealed that age, baseline ACTS score, history of stroke, and use of NSAIDs/antiplatelet drugs were significantly associated (P<0.1) with improvement of the ACTS burden score ().
Table 3 Logistic regression analysis to identify factors associated with improvement of ACTS burden scores
Other factors were added to the base model and multiple logistic regression analysis. We found that the following factors were not significant predictors for ACTS burden improvement: frequency in range (FIR), CHADS2, CHA2DS2-VASc, and HAS-BLED scores; patient’s understanding of anticoagulant therapy; patient’s perspective on switching to apixaban; socioeconomic factors (income from employment and self-pay ratio); and physician’s perspective on switching to apixaban (Tables S3–S5).
The multiple logistic regression analysis yielded several independent factors associated with improvement of ACTS benefit score after switching to apixaban: ACTS benefit score at baseline, age ≥70 years, history of bleeding, history of acute myocardial infarction, use of NSAIDs/antiplatelet drugs, and use of apixaban 5 mg daily (Table S6).
Discussion
In this study, we investigated changes in patient satisfaction, as measured by the ACTS score, in Japanese patients with NVAF who switched from warfarin to apixaban. To our knowledge, this is one of the few studies that demonstrates a significant improvement in patient satisfaction with anticoagulant therapy in patients with NVAF. Additionally, we identified factors associated with improvement of ACTS burden and benefit scores using a multiple logistic regression analysis.
Patient satisfaction with pharmacological therapy is very important in clinical practice. Satisfaction with and preference for a given pharmaceutical regimen may have a significant impact on medication adherence,Citation13,Citation14,Citation17 and higher satisfaction is associated with better anticoagulation control.Citation19 Therefore, patient satisfaction with anticoagulant therapy should be considered an important component of maintaining optimal medication adherence.
The results of the current study show that ACTS scores improved after switching from warfarin to apixaban. This finding may stem from patients’ relief from the restrictions and requirements associated with warfarin therapy, which are not needed with DOAC treatment. For patients taking warfarin, anticoagulation status should be routinely monitored with PT-INR measurements; such monitoring is not performed for patients taking DOACs. In addition, patients on warfarin must adhere to dietary restrictions and avoid medications that interact with warfarin. These constraints may impose a substantial burden on patients with NVAF who are taking warfarin. Furthermore, restriction on food and drink (including alcohol) is an area in which patients are most dissatisfied with warfarin therapy. Because DOACs are not subject to the same dietary and drug interactions as warfarin, switching to apixaban largely improved the dissatisfaction associated with food and drink restrictions in our survey. When considering individual questions, scores for two treatment burden-related questions – No 6 and No 8 – both improved after the switch. Taken together, the increased number of daily doses (once daily to BID) could not have had a large impact on patients’ burdens. These results suggest that physicians should give special consideration to patients’ dietary preferences before starting anticoagulant therapy and understand the potential frustrations with warfarin-related restrictions. Bleeding tendency during warfarin treatment has been suggested to have an impact on patient satisfaction; therefore, switching from warfarin to apixaban, an anticoagulant that is associated with relatively fewer bleeding events, could decrease patient burden. Surprisingly, history of bleeding and concerns about future bleeding were not significantly associated with improvement of patient satisfaction in the current study. The reasons for this finding are uncertain; however, the results might be explained, at least in part, by the suboptimal INR control during warfarin treatment in Japan. It is well known that concerns of bleeding often lead physicians, particularly non-cardiologists or non-neurologists responsible for primary prevention of stroke, to frequently use low doses of warfarin to control INR values below or around the lower limit of the recommended therapeutic range. This may account for the lower incidence of bleeding and less concerns about a possibility of bleeding. Further investigations, particularly stratified by experience of bleeding events or by INR control status, are needed to validate these results.
Although ACTS burden scores improved, there were no significant changes in ACTS benefit scores after switching to apixaban. Relatively high baseline score may not have allowed sufficient room for improvement. In the SAFARI study, researchers investigated patient satisfaction with rivaroxaban treatment for stroke prevention in AF patients and found that the ACTS benefit score significantly increased by 0.4±2.9 points (P<0.001), which might have been too marginal to be considered clinically relevant.Citation20
A multiple logistic regression analysis revealed ACTS burden score at baseline as one of the factors significantly associated with improvement of ACTS burden scores following treatment, suggesting that lower level of satisfaction with anticoagulant therapy at baseline was associated with a higher likelihood to be satisfied with a new anticoagulant after switching. Physicians should, therefore, communicate effectively with patients to assess the levels of satisfaction with anticoagulant therapy, and, if inadequate, consider switching to a new medication.
Patients aged >70 years were more likely to show improved ACTS burden scores after switching to apixaban. The reason for this finding is unclear. It is possible that warfarin-related food restrictions are more of a burden for elderly patients who may prefer traditional Japanese foods, such as fermented soybeans and vegetables that contain vitamin K, and are therefore limited in the diets of patients on warfarin. Similarly, a previous study reported that 58% of patients, particularly males and patients aged >70 years, were interested in switching from warfarin to a DOAC.Citation15
In addition to these two factors, we also found that the use of NSAIDs/antiplatelet drugs was a negative independent factor for improved patient satisfaction according to the ACTS burden score. For patients on warfarin, concomitant use of these medications may not only increase the incidence of bleeding but may also heighten patients’ apprehension of a bleeding event. Patients’ daily lives may be more limited when using these drugs together. Therefore, patients on warfarin may worry about bleeding events and may only find relief from regular monitoring of PT-INR. In one study comparing apixaban and warfarin, apixaban reduced bleeding risk regardless of concomitant antiplatelet medications.Citation21 Taken together, these results indicate that apixaban may reduce the burden associated with bleeding.
The high price of DOACs compared to the much lower price of warfarin is one of the major barriers to switching therapies,Citation16 and, moreover, patient satisfaction may worsen in response to increased drug costs. However, in our study, self-pay ratio was unrelated to patient satisfaction, despite the higher cost of apixaban. In Japan, the national health insurance system covers all citizens at a standard level, and the self-pay portion of total medical costs, including drug costs, is only 0%–30%. This may be a major reason why increased drug costs due to the switch did not have a large impact on changes in patient satisfaction.
Our results illustrate the roles that patient satisfaction, opinion, and preference play in the process of selecting or changing anticoagulant therapy in patients with AF. Sustained improvements in patient satisfaction may be a crucial factor for maintaining adherence to anticoagulant medications.
Limitations
There were some limitations to this study. First, as an observational study, only patients who had already been scheduled to switch from warfarin to apixaban were enrolled. Therefore, it is likely that patients who were fully satisfied with warfarin may not have been included, which, in turn, may have significantly impacted our results. Second, changes in patient satisfaction were investigated at a single time point (12 weeks after switching to apixaban), and long-term changes were not captured in this study; longer-term assessment is necessary for a more comprehensive analysis of patient satisfaction with apixaban. Third, major cardiovascular risk factors such as oxidative stress, diabetes mellitus, smoking, hypertension, obesity, and heart failure could be associated with the development and progression of AF, and patients included in the current study may have been receiving treatment for these pathological conditions.Citation22 More importantly, such treatments might also have an influence on patient satisfaction but were not considered in the current analysis.
Conclusion
Switching from warfarin to apixaban reduced patient-reported treatment burden and thereby improved patient satisfaction with anticoagulant therapy in Japanese patients with NVAF.
Author contributions
YK, TI, KK, TH, and MY were involved in designing the study, interpreting the obtained results, and critically reviewing the drafted manuscript. MK was involved in designing the study, managing the project, interpreting the obtained results, and critically reviewing the drafted manuscript. MC and MI were involved in designing the study, preparing a statistical analysis plan, managing the project, interpreting the obtained results, and drafting the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank the doctors who participated in this study (see Table S7 for details). We would like to acknowledge the contribution of Mebix, Inc. to this study including site selection, site monitoring, study management, and data management, and DOT WORLD Co., Ltd. for developing the EDC. We sincerely appreciate MediStatLab Co., Ltd. for its contributions to statistical analysis. We thank Ms. Pearl Gomes from Cactus Communications K.K. for editing the manuscript. This study and editing of the manuscript were supported by Bristol-Myers Squibb K.K and Pfizer Japan Inc.
Disclosure
YK received honoraria and consulting fees (for speaker, writer, and/or adviser) from Bayer Yakuhin Ltd., Bristol-Myers Squibb K.K., Daiichi Sankyo K.K., Nippon Boehringer Ingelheim, and Pfizer Japan Inc., and research grants from Daiichi Sankyo K.K. and Nippon Boehringer Ingelheim. TI received research grants from Bristol-Myers Squibb K.K., Daiichi Sankyo K.K., and Nippon Boehringer Ingelheim, and honoraria and consulting fees from Bayer Yakuhin Ltd., Bristol-Myers Squibb K.K., Daiichi Sankyo K.K., Nippon Boehringer Ingelheim, and Pfizer Japan Inc. KK received research grants from Bayer Yakuhin Ltd., Daiichi Sankyo K.K., and Nippon Boehringer Ingelheim, and honoraria and consulting fees from Bayer Yakuhin Ltd., Daiichi Sankyo K.K., Nippon Boehringer Ingelheim, and Pfizer Japan Inc. TH received honoraria and consulting fees from Bayer Yakuhin Ltd., Bristol-Myers Squibb K.K., Daiichi Sankyo K.K., Nippon Boehringer Ingelheim, and Pfizer Japan Inc. MY received a research grant from Nippon Boehringer Ingelheim and honoraria and consulting fees from Bayer Yakuhin Ltd., Bristol-Myers Squibb K.K., Daiichi Sankyo K.K., Nippon Boehringer Ingelheim, and Pfizer Japan Inc. MK is an employee of Bristol-Myers Squibb K.K. MC and MI are employees of Pfizer Japan Inc. The authors report no other conflicts of interest in this work.
References
- BenjaminEJLevyDVaziriSMD’AgostinoRBBelangerAJWolfPAIndependent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart StudyJAMA1994271118408448114238
- KokuboYWatanabeMHigashiyamaANakaoYMKusanoKMiyamotoYDevelopment of a basic risk score for incident atrial fibrillation in a Japanese general population – the Suita StudyCirc J201781111580158828539563
- WangTJPariseHLevyDObesity and the risk of new-onset atrial fibrillationJAMA2004292202471247715562125
- MorinDPBernardMLMadiasCRogersPAThihalolipavanSEstesNA3rdThe state of the art: atrial fibrillation epidemiology, prevention, and treatmentMayo Clin Proc201691121778181027825618
- ColillaSCrowAPetkunWSingerDESimonTLiuXEstimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult populationAm J Cardiol201311281142114723831166
- GoASHylekEMPhillipsKAPrevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) StudyJAMA2001285182370237511343485
- DantasGCThompsonBVMansonJATracyCSUpshurREPatients’ perspectives on taking warfarin: qualitative study in family practiceBMC Fam Pract200451515268764
- SiltariAVapaataloHVascular calcification, vitamin K and warfarin therapy – possible or plausible connection?Basic Clin Pharmacol Toxicol Epub2017621
- MozosIStoianDLucaCTCrosstalk between vitamins A, B12, D, K, C, and E status and arterial stiffnessDis Markers20172017878497128167849
- YamamotoKKoretsuneYAkasakaTEffects of vitamin K antagonist on aortic valve degeneration in non-valvular atrial fibrillation patients: prospective 4-year observational studyThromb Res2017160697529121522
- GrangerCBAlexanderJHMcMurrayJJApixaban versus warfarin in patients with atrial fibrillationN Engl J Med20113651198199221870978
- JanuaryCTWannLSAlpertJS2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm SocietyCirculation2014130232071210424682348
- BarbosaCDBalpMMKulichKGermainNRofailDA literature review to explore the link between treatment satisfaction and adherence, compliance, and persistencePatient Prefer Adherence20126394822272068
- LabaTLEssueBKimmanMJanSUnderstanding patient preferences in medication nonadherence: a review of stated preference dataPatient20158538539525404203
- AttayaSBornsteinTRonquilloNStudy of warfarin patients investigating attitudes toward therapy change (SWITCH Survey)Am J Therap201219643243522198071
- ElewaHFDeRemerCEKellerKGujralJJoshuaTVPatients satisfaction with warfarin and willingness to switch to dabigatran: a patient surveyJ Thromb Thrombolysis201438111512023918529
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- CanoSJLampingDLBamberLSmithSThe Anti-Clot Treatment Scale (ACTS) in clinical trials: cross-cultural validation in venous thromboembolism patientsHealth Qual Life Outcomes20121012023013426
- WangYKongMCLeeLHNgHJKoYKnowledge, satisfaction, and concerns regarding warfarin therapy and their association with warfarin adherence and anticoagulation controlThromb Res2014133455055424448058
- HanonOChaussadeEGuerangerPGrusonEBonanSGayAPatient-reported treatment satisfaction with rivaroxaban for stroke prevention in atrial fibrillation. A French observational study, the SAFARI StudyPLoS One20161112e016621827935987
- AlexanderJHLopesRDThomasLApixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trialEur Heart J201435422423224144788
- GasparovaIKubatkaPOpatrilovaRPerspectives and challenges of antioxidant therapy for atrial fibrillationNaunyn Schmiedebergs Arch Pharmacol20173901114
