Abstract
Background
Patient-centered care is respectful to a patient’s preference. All prior clinical trials on patient self-titration algorithms for basal insulin were decided by physicians. We hypothesized that patients and physicians have different preferences.
Patients and methods
Physicians and diabetes patients were asked to choose their preferred insulin glargine self-titration algorithm among 5 algorithms. Algorithm 1, 1 U increase once daily; algorithm 2, 2 U increase every 3 days; algorithm 3, 3 U increase every 3 days; algorithm 4, titration every 3 days according to fasting blood glucose, and algorithm 5, weekly titration 2–8 U based on 3-day mean fasting blood glucose levels.
Results
Eleven (5.2%) out of 210 physicians and 180 (90.9%) out of 198 patients preferred algorithm 1 (χ2=300.4, p=0.000). In contrast, 195 (92.9%) physicians and 18 (9.1%) patients preferred algorithm 2 (χ2=286.6, p=0.000). In addition, 4 (1.9%) physicians but no patients preferred algorithm 3 (χ2=2.099, p=0.124). Neither physicians nor patients chose algorithms 4 or 5. Most physicians preferred algorithm 2 since it is recommended by guidelines, but most patients preferred algorithm 1 for its simplicity.
Conclusion
Patients had different preferences compared with physicians. Attention should be given to patients’ preferences to increase adherence and improve glycemic control.
Introduction
The initiation of basal insulin, as add-on therapy in patients with type 2 diabetes who fail to reach their glycated hemoglobin (HbA1c) target using oral antidiabetic drugs (OADs), is recommended by current guidelines.Citation1,Citation2 Adequate titration of insulin doses is required in order to achieve optimal glycemic control.Citation3 However, many physicians and patients hesitate to titrate insulin dose in the real world. The First Basal Insulin Evaluation Asia study showed that the prescribed mean daily insulin dose increased marginally from 0.20 U/kg/d at initiation to 0.22–0.24 U/kg/d at 6 months.Citation4 The recently published ORBIT study investigating real-life use of basal insulin in patients with uncontrolled type 2 diabetes with OADs showed that the initial dose was 0.18 U/kg and was followed by a mean increase of daily dose of only 0.03 U/kg after 6 months.Citation5 Additionally, only 56.6% of insulin users reported insulin titration at 6 months.Citation5
To ensure adequate titration of insulin doses, Yki-Järvinen et alCitation6 recommended self-adjustment of insulin dose based on home glucose monitoring in 1997. Thereafter, numerous randomized controlled trials using a basal insulin titration algorithm showed that patients with type 2 diabetes can be effectively and safely involved in insulin titration.Citation7–Citation11
However, prior patient self-titration algorithms for basal insulin used in clinical trials were decided by physicians,Citation7–Citation11 which may lead to poor adherence due to neglecting patient preferences. According to the position of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) on patient-centered approach, physicians and patients act as partners, mutually exchanging information and deliberating on options in order to reach a consensus on the therapeutic course of action.Citation12 Importantly, engaging patients in medical decisions may enhance adherence to therapy.Citation12
Numerous basal insulin titration algorithms have been demonstrated to be effective and safe in randomized clinical trials, which can be chosen by patients and physicians.Citation1,Citation2,Citation7–Citation10 We hypothesized that patients have different preferences than physicians for basal insulin titration algorithms. Thus, the aim of this study was to investigate the differences between physicians’ and patients’ preferences for basal insulin titration algorithms and to assess adherence, efficacy, and safety of patient-preferred algorithms.
Patients and methods
Research design and participants
This study comprised of 2 parts – a physician survey and an observational prospective study involving patients.
The physician survey was conducted between August 2016 and December 2016, and involved face-to-face interviews. All participating physicians were from 10 secondary and 10 tertiary hospitals, and had >2 years of experience in prescribing basal insulin. All participating physicians were asked to choose among 5 predefined insulin self-titration algorithms and list reasons spontaneously for their choices. The 5 candidate algorithms were: 1) a daily increase of 1 U (INSIGHT study algorithm);Citation9 2) titration with 2 U every 3 days (AT.LANTUS study algorithm, ADA, EASD, American Association of Clinical Endocrinologists [AACE], and American College of Endocrinology [ACE] recommendations);Citation1,Citation2,Citation8 3) titration with 3 U every 3 days (PREDICTIVE 303 study algorithm);Citation10,Citation13 4) increase insulin dose every 3 days based on fasting blood glucose (FBG) as follows: if FBG value is 7.0–7.9 mmol/L, increase insulin dose by 1 U; if FBG value is 8.0–9.9 mmol/L, increase insulin dose by 10% of total daily dose; if FBG values ≥10 mmol/L, increase insulin dose by 20% of total daily dose (AACE and ACE recommendation);Citation2 and 5) a weekly increase of 2–8 U based on the mean of 3 FBG values ().Citation7,Citation8,Citation14–Citation17
Table 1 Summary of the 5 titration algorithms for insulin glargine
The clinical study involving patients was conducted between January 2016 and February 2017. Type 2 diabetes patients, aged 18–75 years, who failed to reach their HbA1c and FBG targets (<7.0% and 7.0 mmol/L, respectively) using OADs and were recommended to initiate insulin therapy with glargine as add-on to existing OADs were invited to participate and followed up until they reached their FBG target for 3 consecutive days. All patients were asked to visit their treating physicians in the month when their FBG reached the target (second and final study visit). Adherence, efficacy, and safety were recorded during this visit. Exclusion criteria were history of ketoacidosis, noncompliance with daily measurement of FBG, any intention to add additional OADs during study participation, changes in OAD dose at study enrollment, alanine aminotransferase and aspartate aminotransferase >3 times the upper normal limit, serum creatinine ≥120 μmol/L, pregnancy, or use of drugs likely to interfere with glucose control.
The education on insulin administration and FBG monitoring was given according to the local practice of each institution. Patients were provided the insulin titration algorithms presented to physicians, and after detailed explanation of each algorithm, patients were asked to choose a “preferred” insulin titration algorithm to be used during the study and also list reasons spontaneously for their choices. All patients were instructed to start insulin glargine (Lantus®; Aventis Pharma, Strasbourg, France) at an initial dose of 0.2 U/kg and to inject it at the same time each evening (between 21.00 and 22.00 h). Doses were to be self-titrated according to patients’ preferred algorithm and reduced if biochemical or clinical hypoglycemia occurred. During the study, the patients were not allowed to switch from one algorithm to another.
The study was conducted in accordance with the Declaration of Helsinki. Approval was obtained from the institutional ethics committees of the Third Affiliated Hospital of Southern Medical University, the academic ethics review boards of the Second Affiliated Hospital of Guangzhou Medical University, the ethics committees of the Third People’s Hospital of Dongguan, the institutional ethics committees of Foshan Hospital of Southern Medical University, the ethics committees of Guangdong Second Provincial General Hospital, and the ethics committees of Guangzhou First People’s Hospital. All patients provided written informed consent before participation
Preferences, adherence, efficacy, and safety measures
The primary objective of this study was to assess physicians’ and patients’ preferences for the 5 proposed insulin self-titration algorithms.
The secondary objectives included an assessment of adherence, efficacy, and safety of patients’ preferred insulin titration algorithms in reaching FBG levels <7.0 mmol/L. A patient was considered to reach the efficacy target if FBG level was <7 mmol/L on 3 consecutive days, and the first day of the 3 consecutive days was recorded as the date when glucose target was met and the study ended for the patient.
Evaluation of safety included assessment of the proportion of patients who experienced hypoglycemic episodes during the study period. Hypoglycemic episodes were defined as symptoms suggestive of hypoglycemia plus a documented blood glucose level <3.9 mmol/L. Severe hypoglycemia was defined as an episode with symptoms consistent with hypoglycemia during which the patient required the assistance of another person.
Statistical analyses
Significance of differences between patients and physicians was analyzed using the Pearson’s χ2 and Fisher’s exact tests or Student’s t-test. Results are expressed as mean ± SD. Statistical analysis was performed using SPSS Statistical Software 19.0 (IBM Corporation, Armonk, NY, USA), and statistical testing was set at a significance level of α=0.05.
Results
Difference between physicians’ and patients’ preferred algorithms
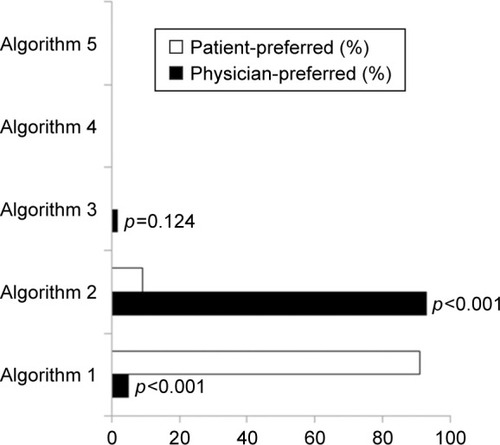
A total of 210 out of 215 screened physicians were willing to receive our survey and 198 out of 230 screened patients were willing to self-titrate their insulin and were included in the analysis of preference of insulin titration algorithms. Eleven (5.2%) out of 210 physicians and 180 (90.9%) out of 198 patients preferred algorithm 1 (χ2=300.4, p=0.000). In contrast, 195 (92.9%) physicians and 18 (9.1%) patients preferred algorithm 2 (χ2=286.6, p=0.000). In addition, 4 (1.9%) physicians but no patients preferred algorithm 3 (χ2=2.099, p=0.124). Neither physicians nor patients chose algorithms 4 or 5 ().
Figure 1 Physician- and patient-preferred algorithms.
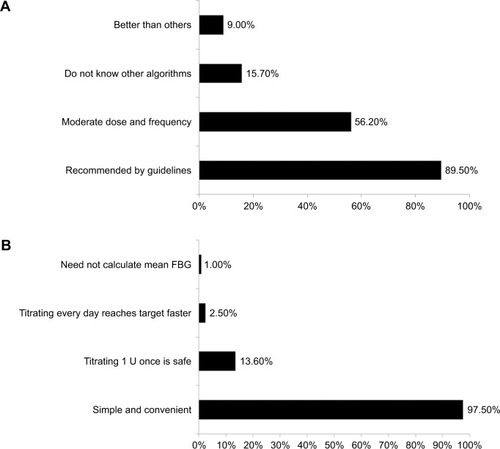
The reasons reported by physicians for choosing algorithm 2 included recommendation by several guidelines, moderation in the titration of insulin dose and frequency of insulin titration, lack of knowledge of other algorithms, or higher efficacy of the algorithm in decreasing glucose values (better than others). For patients, reasons for choosing algorithm 1 included its simplicity, the perception that titrating insulin by 1 U is safe, titrating every day may enable them to reach the target faster, and no requirement to calculate mean FBG ().
Adherence to the algorithms preferred by patients
Ten out of 180 patients using algorithm 1 and 2 out of 18 patients using algorithm 2 were lost to follow-up, and thus not included in the adherence, efficacy, and safety analyses. The baseline characteristics of the patients in the 2 study groups (algorithms 1 and 2) are listed in . A total of 165 (97.1%) patients who preferred algorithm 1 and 13 (81.2%) patients who preferred algorithm 2 titrated their insulin glargine dose according to the algorithm (χ2=5.454, p=0.023).
Table 2 Baseline characteristics of patients who preferred algorithms 1 and 2
Efficacy and safety analyses of the algorithms preferred by patients
FBG reduced from 9.8±1.2 to 6.2±0.5 mmol/L (t=30.793, p=0.000) for algorithm 1 and from 10.1±1.5 to 6.1±0.8 mmol/L for algorithm 2 (t=11.346, p=0.000), respectively. Hypoglycemia was reported in only 1 patient who used algorithm 2, and this patient reported titrating insulin dose with 2 U daily instead of every 3 days.
FBG target was reached in 8.0±4.3 days in patients using algorithm 1 and in 10.8±5.5 days in those using algorithm 2. Mean insulin glargine dose when FBG target was reached was 20.4±4.4 U (0.35±0.09 U/kg) in those using algorithm 1 and 21.7±4.2 U (0.33±0.06 U/kg) in those using algorithm 2.
Discussion
The most frequently used self-titration algorithm for insulin glargine in clinical trials was increasing the does by 2 U every 3 days, until FBG reached the desired target. This was used for the first time in the AT.LANTUS study, and was incorporated in ADA, EASD, and AACE guidelines.Citation1,Citation7,Citation18 In line with these guidelines, the physicians in this study also preferred the AT.LANTUS algorithm (algorithm 2). However, patients preferred the self-titration algorithm for insulin glargine employing 1 U daily increase (algorithm 1), and which was used for the first time in the INSIGHT study.Citation8 Algorithms 4 and 5, which were recommended by AACE and ACE,Citation2 and used in many clinical trials,Citation7,Citation8,Citation14–Citation17 were neither preferred by our physicians nor patients.
The most important reason for patients’ preference was the simplicity of the INSIGHT titration algorithm. This confirms previous observations that complexity of titration algorithms is one of the main barriers in patients’ acceptance of insulin titration.Citation19,Citation20 A simple titration algorithm can offer several benefits, including fewer clinic visits for insulin dose adjustment, permitting timely increase in insulin dose and patients becoming comfortable in managing their insulin dose.Citation21 This facilitates empowerment of patients in the management of diabetes and a higher flexibility of the therapy.Citation21 All these advantages may provide adherence benefits as compared to more complex algorithms. Indeed, in our study, the adherence to self-titration algorithms was as high as 97.1% in the algorithm 1 group and 81.2% in the algorithm 2 group, and was achieved without increasing the consumption of resources commonly used when initiating insulin in patients with type 2 diabetes. Only standard education on insulin administration and FBG and a short explanation and instructions on the preferred self-titration algorithm were provided.
Not only patients but also physicians preferred the relatively simple algorithm. One study showed that if 40 physicians increased fixed dose of 2 U every 3 days, only 1 physician selected a glucose-based titration algorithm of increasing 4 U every 3 days if glucose value was >10 mmol/L and 2 U if glucose value was ≤10 mmol/L.Citation22
In this study, the patients achieved FBG target and had significant reduction within 8–10 days, according to the algorithm used. The time to achieve FBG targets in our study was similar to Pfutzner et al’s study,Citation22 which used the AT.LANTUS algorithm. In their study, 70% of patients achieved a stable insulin glargine dose and FBG within a preset target after a mean of 5±6 days following study enrollment.Citation22 However, they also had daily physician contact via phone and the dose was based on physician’s decision on insulin dose titration.Citation22 In contrast, the time to achieve similar FBG endpoints in many trials was 12–24 weeks.Citation7–Citation9,Citation11,Citation24 This indicated the adherence of our patients to self-preferred algorithms. Another reason is that most of our patients used sulfonylureas, which shortens the FBG target achievement time.Citation23
Hypoglycemia events rarely occurred in both groups in this study perhaps because our FBG target was 7.0 as compared to 5.1–6.1 mmol/L in previous trials.Citation7–Citation10,Citation14–Citation17,Citation25–Citation28
We chose FBG <7.0 mmol/L as efficacy measurement and treatment target and not HbA1c due to several reasons. First, basal insulin is titrated based on FBG levels.Citation1,Citation2 Additionally, patients understand FBG better than HbA1c. Moreover, the majority of randomized clinical trials with basal insulin did not reach their FBG targets of ≤5,Citation13 ≤5.5,Citation5–Citation7 or ≤6.1 mmol/L,Citation10,Citation11,Citation13 and an increased incidence of severe hypoglycemic events was observed and confirmed at FBG values <5.5 mmol/L.Citation29 One study also showed that aggressive titration did not result in better HbA1c values.Citation30 The ADA/EASD position statement states that a FBG level <7.2 mmol/L is sufficient to reach the recommended HbA1c target of <7%.Citation12
We tested whether a higher target could be achieved in real-life conditions. Observational studies of patients in real-life settings, outside of the highly controlled randomized clinical trials, showed that at 3 months after insulin initiation, mean FBG of 7.3–7.4 mmol/L was reachedCitation31 and physicians usually preferred glycemic target of 7.2 rather than 5.5 mmol/LCitation22 in daily practice, which was in line with ADA and EASD position statement (<7.2 mmol/L).Citation1
Interestingly, previous trials with an FBG target of 5.1–6.1 mmol/LCitation7–Citation10,Citation14–Citation17,Citation25–Citation28 attained an endpoint FBG of 6.3–7.6 mmol/L, while we set a higher FBG target of 7.0 mmol/L but attained a lower endpoint FBG of 6.1–6.2 mmol/L. This is in agreement with a global survey which found that physicians would be more aggressive in treating diabetes if there was no concern about hypoglycemia.Citation6
In the present study, a glargine dose of 0.33–0.35 U/kg was required to achieve the FBG target. This dose was lower as compared to that in western populations,Citation7,Citation9,Citation13,Citation24 perhaps due to 2 reasons. First, >80% of our patients also took combined sulfonylureas, which could save insulin glargine dose for enhancing endogenous insulin secretion.Citation32 In the INSIGHT trial, 35.4% of patients used sulfonylureas, combined with 0.41 U/kg and attained an FBG target 6.7 mmol/L. Second, Asian patients with type 2 diabetes may have lower insulin resistanceCitation33 and lower insulin needs as compared to non-Asian populations. In contrast, in the Treat-to-Target study of North American patients with type 2 diabetes, the mean FBG was 6.5 mmol/L over 6 months with insulin glargine doses of 0.48 U/kg.Citation7
Limitations
There are several limitations to this study. First, because patients preferring algorithm 1 were more in number compared to those preferring algorithm 2, the power in comparing the efficacy and safety of the 2 patient-preferred algorithms is limited. Second, whether the patient-preferred algorithm is superior to physician-preferred algorithm in clinical practice needs to be further clarified using a randomized trial. Last, our research was a short-term observation based on FBG, so the question of whether patient-preferred algorithm has a long-term advantage based on HbA1c needs further study.
Conclusion
This study showed that even in real-life settings, patients with type 2 diabetes inadequately controlled with OADs, who were recommended insulin initiation, preferred different algorithms as compared to the physicians. Most patients preferred 1 U insulin glargine increase once daily for its simplicity. A simple algorithm had higher adherence, resulted in adequate titration of insulin doses, and was efficient and safe in decreasing FBG. Hence, attention should be given to patients’ preferences in self-titration algorithms.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Wangen Li contributed to the design of the study, data analysis, and manuscript preparation and overviewing. Other coauthors participated in the patient recruitment, implementation of the study, safety monitoring, and manuscript overviewing. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the Health and Family Planning Commission of Guangzhou Municipality (grant number: 20171A011301) and Science and Technology project of Guangzhou (grant numbers: 201707010365 and 201707010045).
Disclosure
The authors report no conflicts of interest in this work.
References
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of DiabetesDiabetes Care201538114014925538310
- GarberAJAbrahamsonMJBarzilayJIConsensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summaryEndocr Pract20162218411326731084
- GarberAJThe importance of titrating starting insulin regimens in patients with type 2 diabetesDiabetes Obes Metab200911Suppl 51013
- TsaiSTPathanFJiLFirst insulinization with basal insulin in patients with type 2 diabetes in a real-world setting in AsiaJ Diabetes20113320821621631903
- JiLZhangPZhuDObservational Registry of Basal Insulin Treatment (ORBIT) in patients with type 2 diabetes uncontrolled with oral antihyperglycaemic drugs: real-life use of basal insulin in ChinaDiabetes Obes Metab201719682283028105735
- Yki-JärvinenHRyysyLKauppilaMEffect of obesity on the response to insulin therapy in noninsulin-dependent diabetes mellitusJ Clin Endocrinol Metab19978212403740439398709
- RiddleMCRosenstockJGerichJInsulin Glargine 4002 Study InvestigatorsThe treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patientsDiabetes Care200326113080308614578243
- DaviesMStormsFShutlerSBianchi-BiscayMGomisRATLANTUS Study GroupImprovement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargineDiabetes Care20052861282128815920040
- GersteinHCYaleJFHarrisSBIssaMStewartJADempseyEA randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) studyDiabet Med200623773674216842477
- MeneghiniLKoenenCWengWSelamJLThe usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes – results of the randomized, controlled PREDICTIVE 303 studyDiabetes Obes Metab20079690291317924873
- GargSKAdmaneKFreemantleNPatient-led versus physician-led titration of insulin glargine in patients with uncontrolled type 2 diabetes: a randomized multinational ATLAS studyEndocr Pract201521214315725297660
- InzucchiSEBergenstalRMBuseJBAmerican Diabetes Association (ADA)European Association for the Study of Diabetes (EASD)Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Diabetes Care20123561364137922517736
- BlondeLMerilainenMKarweVRaskinPTITRATE Study GroupPatient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets – the TITRATE studyDiabetes Obes Metab200911662363119515182
- RosenstockJDaviesMHomePDLarsenJKoenenCSchernthanerGA randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetesDiabetologia200851340841618204830
- KennedyLHermanWHStrangePHarrisAGOAL AIC TeamImpact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trialDiabetes Care20062911816373887
- FritscheASchweitzerMAHaringHU4001 Study GroupGlimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trialAnn Intern Med20031381295295912809451
- RaskinPAllenEHollanderPINITIATE Study GroupInitiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogsDiabetes Care200528226026515677776
- VaagALundSSInsulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analoguesEur J Endocrinol2012166215917021930715
- PeyrotMBarnettAHMeneghiniLFSchumm-DraegerPMInsulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy studyDiabet Med201229568268922313123
- DaileyGAurandLStewartJAmeerBZhouRComparison of three algorithms for initiation and titration of insulin glargine in insulin-naive patients with type 2 diabetes mellitusJ Diabetes20146217618323931125
- KhuntiKDaviesMJKalraSSelf-titration of insulin in the management of people with type 2 diabetes: a practical solution to improve management in primary careDiabetes Obes Metab201315869070023253563
- PfutznerAStratmannBFunkeKReal-world data collection regarding titration algorithms for insulin glargine in patients with type 2 diabetes mellitusJ Diabetes Sci Technol20161051122112927325389
- Yki-JarvinenHCombination therapies with insulin in type 2 diabetesDiabetes Care200124475876711315844
- Yki-JarvinenHKauppinen-MakelinRTiikkainenMInsulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET studyDiabetologia200649344245116456680
- Yki-JarvinenHJuurinenLAlvarssonMInitiate Insulin by Aggressive Titration and Education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groupsDiabetes Care20073061364136917384341
- HeineRJVan GaalLFJohnsDMihmMJWidelMHBrodowsRGExenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trialAnn Intern Med2005143855956916230722
- JankaHUPleweGRiddleMCKliebe-FrischCSchweitzerMAYki-JarvinenHComparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetesDiabetes Care200528225425915677775
- EliaschewitzFGCalvoCValbuenaHHOE 901/4013 LA Study GroupTherapy in type 2 diabetes: insulin glargine vs NPH insulin both in combination with glimepirideArch Med Res200637449550116715577
- StrangePTreat-to-target insulin titration algorithms when initiating long or intermediate acting insulin in type 2 diabetesJ Diabetes Sci Technol20071454054819885117
- ZinmanBFulcherGRaoPVInsulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomised, open-label, phase 2 trialLancet2011377976992493121396703
- SchreiberSAHaakTInsulin glargine benefits patients with type 2 diabetes inadequately controlled on oral antidiabetic treatment: an observational study of everyday practice in 12,216 patientsDiabetes Obes Metab200791313817199716
- Yki-JarvinenHRyysyLNikkilaKTulokasTVanamoRHeikkilaMComparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trialAnn Intern Med1999130538939610068412
- KodamaKTojjarDYamadaSTodaKPatelCJButteAJEthnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysisDiabetes Care20133661789179623704681