Abstract
Purpose
People chronically infected with hepatitis C virus (HCV) have diminished patient-reported outcomes (PROs). This study aimed to compare the impact of elbasvir/grazoprevir (EBR/GZR) treatment versus sofosbuvir with pegylated interferon and ribavirin (SOF/PR) on changes in PROs: 1) during the treatment period and 2) at posttreatment follow-up.
Patients and methods
PRO data collected during the Phase III C-EDGE Head-2-Head (H2H) open-label study was analyzed. In this trial, patients infected with HCV were randomized 1:1 to receive either EBR/GZR or SOF/PR for 12 weeks. Patients self-administered the Short Form-36 version 2 (SF-36v2®) Health Survey Acute (1-week recall) Form and the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) Scale at baseline, during treatment, and posttreatment. Between-group differences in mean change of PRO scores from baseline were estimated during the treatment period and also at the posttreatment follow-up. Effect sizes were calculated to evaluate if the detected change in mean PRO scores is clinically meaningful between groups.
Results
There were 255 patients (99.2% White, 54.1% female, 74.9% treatment naïve) included in the analysis. During the treatment period, significant declines in SF-36v2 scores were observed across all domains for the SOF/PR group. Compared to the SOF/PR group, the EBR/GZR group reported more improvement in scores across all SF-36v2 domain scores at the end of the treatment period. At treatment week 12, the between-group differences for 6 out of the 8 domain scores for these patients reflected at least moderate effects (effect sizes >0.5). No significant between-group differences in change in SF-36v2 scores from baseline were detected posttreatment. The decline in SF-36v2 scores observed during the treatment period for the SOF/PR group returned to near baseline scores or above posttreatment. Treatment with EBR/GZR did not impact fatigue scores, but treatment with SOF/PR led to increased fatigue scores during treatment which resolved by posttreatment follow-up week 12.
Conclusion
This study demonstrated that HCV treatment with EBR/GZR resulted in a significantly better PRO profile as compared to SOF/PR. PROs are an important consideration as worsening PROs experienced during treatment may negatively influence adherence and ultimately contribute to an unfavorable clinical outcome.
Clinicaltrials.gov Identifier
NCT02358044
Introduction
Approximately 2%–3% of the world’s population are chronically infected with hepatitis C virus (HCV),Citation1 which can lead to cirrhosis, end-stage liver disease, hepatocellular carcinoma, and subsequently death.Citation2,Citation3 Even in the absence of liver disease, chronic HCV can lead to worsening patient reported outcomes (PROs), such as health-related quality of life (HRQoL) and fatigue.Citation4–Citation6 HCV treatment can potentially improve PROs; however, the effect of treatment on PROs may vary based on the tolerability profile of the treatment regimen used. For example, treatment regimens that contain interferon or ribavirin have been found to be associated with significantly lower HRQoL while patients are on treatment.Citation6–Citation8 The health domains observed to be negatively affected include role limitations-physical, vitality, social functioning, and role limitations-emotional.Citation7,Citation8 In addition, interferon-based regimens have been associated with increased fatigue, which is a predictor of diminished PROs.Citation6,Citation8–Citation10
The emergence of direct-acting antivirals (DAAs) has revolutionized the treatment of chronic HCV. Patients treated with DAAs have been shown to achieve high sustained viro-logic response (SVR) rates and improved PROs as DAAs have substantially less disabling side effects compared to the conventional pegylated interferon and ribavirin treatment.Citation6,Citation11 Inclusion of PROs in clinical trials of HCV treatment has become increasingly important in recent years given that they provide a more complete assessment of the impact of treatment on the patient’s experience.Citation6,Citation11,Citation12 Further, improved PROs are linked to better treatment adherence, which is integral to the effectiveness of DAA treatment.Citation6,Citation13–Citation16 The potential variation in PROs between regimens can be assessed using data from randomized clinical trials; however, the often-reported change scores in PROs between regimens may be statistically significant, but not clinically meaningful. To ascertain if a change in PRO score is meaningful clinically, the minimal clinically important difference (MCID) is often used as the threshold value.Citation17 MCID is originally defined as “the smallest difference which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient’s management”.Citation18
In the C-EDGE Head-2-Head (H2H) open-label multinational trial, 255 patients were randomized to receive either elbasvir/grazoprevir (EBR/GZR) or sofosbuvir with pegylated interferon and ribavirin (SOF/PR) for the treatment of HCV.Citation19 EBR/GZR was observed to have superior efficacy and safety in patients with HCV genotype 1 or 4 compared with SOF/PR. The aim of this study is to compare the impact of EBR/GZR vs SOF/PR on changes in PROs both during treatment and posttreatment and to assess whether any observed differences in PROs between the two treatment groups are clinically meaningful.
Patients and methods
Study design
This study analyzed the PRO data collected as part of the Phase III C-EDGE H2H study trial conducted in Europe and Turkey. In the trial, patients with HCV genotype 1, 4, and 6 who were either treatment-naïve or had prior treatment failures with pegylated interferon (PR) were randomized 1:1 to receive either EBR (50 mg)/GZR (100 mg) once daily for 12 weeks or SOF 400 mg once daily, pegylated interferon 1.5 µg/ kg once weekly, and weight-based ribavirin for 12 weeks. Details of the study design and results of the C-EDGE H2H trial have been previously reported (ClinicalTrials.gov Identifier: NCT02358044).Citation19 The protocol was approved by the independent ethics committee at each participating site, and all patients provided written informed consent. A list of investigators and independent ethics committee is included in Table S1 of the online supplementary material.
PROs
PROs were collected as exploratory endpoints of the trial. This study analyzed the PRO data collected using the acute Short Form-36 version 2 (SF-36v2) and the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) Scale at the baseline visit, during treatment at week 4 (TW4) and 12 (TW12), and posttreatment follow-up week 12 (FW12). Both PRO instruments were self-administered by patients using an electronic data capture tool.
SF-36v2® Acute (1-week recall)
Form The SF-36v2 Form is a generic questionnaire comprising 36 items assessing one reported health transition item and eight health domains: Physical Functioning (10 items), Role Limitations-Physical (four items), Bodily Pain (two items), General Health (five items), Vitality (four items), Social Functioning (two items), Role Limitations-Emotional (three items), and Mental Health (five items).Citation20 The SF-36v2 has good internal consistency and reliability with Cronbach’s alpha coefficient ranging from 0.78 to 0.93 for all domains.Citation21 The domain scores can be summarized into the physical component summary (PCS) and mental component summary (MCS) scores, which measure overall physical and mental well-being, respectively. The domain scale scores range from 0 to 100, with 100 representing the best health status. The two summary scores were linearly transformed using the population norms to the mean of 50 and a standard deviation (SD) of 10. The SF-36v2 has been used extensively in several studies with HCV populations.Citation8,Citation11,Citation22–Citation28
FACIT-Fatigue Scale
The FACIT-Fatigue Scale version 4 comprises 13 items that assess tiredness, weakness, listlessness, lack of energy, and the impact of these feelings on daily activities and functioning.Citation29 The FACIT-Fatigue has a recall period of 7 days, and items are rated on a 5-point Likert-type response scale. A summary scale score is computed ranging between 0 and 52, where a higher score indicates less fatigue. This instrument has been demonstrated to be reliable and valid in patients with fatigueCitation29 and has been used in HCV-infected populations.Citation11,Citation25,Citation27,Citation30
Statistical analysis
Patients who had at least one dose of study medication and had completed at least one baseline or post-baseline PRO assessment were included in the base analytical cohort. To evaluate the treatment effect on PRO scores, mean change from baseline in PRO scores was calculated at each assessment time point during treatment (TW4 and TW12) and posttreatment (FW12), with corresponding 95% CI. To examine the difference between treatment groups, between-group differences in mean change from baseline in PRO scores were similarly estimated at each PRO assessment time point during treatment (TW4 and TW12) and posttreatment (FW12), with corresponding 95% CI. Missing data were not imputed, and the estimation of mean scores was based on all available data at each time point.
Based on a previous study employing a modified Delphi technique, an expert panel reviewed existing published literature relating to HRQoL in HCV identified using a systematic review and came to the consensus that the Vitality domain of the SF-36 is the most relevant to HCV patients and that a difference of 4.2 points can be used to estimate the MCID in this population.Citation22 However, no MCIDs are available for the remaining domains or component scores of the SF-36v2 or the FACIT-Fatigue for this specific patient population. Therefore, this study computed effect sizes by taking the between-group mean differences in PRO scores from baseline at each assessment time point, divided by the SD of the difference in scores. The effect sizes were then compared to the standardized criteria by Cohen, where 0.2, 0.5, and 0.8 indicate a small, moderate, and large effect, respectively.Citation31
Results
A total of 255 out of the 257 randomized patients were included in the analysis. Two patients in the SOF/PR group did not receive any study medication and were excluded from the analytical cohort. There were 129 patients in the EBR/GZR group and 126 patients in the SOF/PR group. Baseline clinical and demographic characteristics are presented in . The mean age of the patients was 48 years across both study groups. The majority of patients were White (99.2%), female (54.1%), and non-cirrhotic (83.1%). Over three quarters (77.6%) of patients had genotype IL28B non-CC, 82.0% had HCV genotype (GT)1b infection, and 74.9% were treatment naïve. These characteristics were comparable across both treatment groups. No GT6-infected patients were enrolled in this study. Baseline PRO scores are reported in and were similar between treatment groups. Across all PRO assessment time points, none of the patients were missing all SF-36v2 or FACIT-Fatigue assessments. The overall completion rates for the SF-36v2 and FACIT-Fatigue were high, with >92% and >90% of the patients completing the SF-36v2 and FACIT-Fatigue, respectively, at all assessment time points.
Table 1 Baseline clinical and demographic characteristics of study sample
Table 2 Baseline PRO scores of the treatment groups
Change in SF-36v2® scores from baseline
During the treatment period
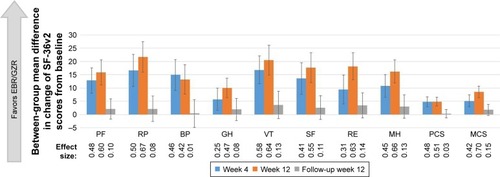
A decrease in SF-36v2 scores was observed from baseline to week 4 of treatment in both treatment groups (), with the SOF/PR treatment group showing a greater decrement in scores. Although this trend of reduction in SF-36v2 scores persisted to week 12 of treatment in the SOF/PR treatment group, improvements in the General Health and Mental Health domains, as well as MCS scores, were observed in the EBR/GZR treatment group at week 12 (). Compared to the SOF/PR treatment group, at TW12, patients in the EBR/GZR treatment group reported a more positive change in SF-36v2 scores across most domains of the SF-36v2 (). Between-group differences in mean change from baseline in PRO scores were at least 4.80 across all domains, with the greatest difference detected for the Role Limitations-Physical (16.55 in TW4; 21.62 in TW12) and Vitality domains (16.81 in TW4; 20.48 in TW12) (). The estimated effect sizes for the between-group change in mean scores ranged from 0.25 to 0.58 at TW4 and 0.42 to 0.70 at TW12. Further, at treatment week 12, except for the Bodily Pain and General Health domains, the effect sizes of the between-group differences of all other domains and summary scores were shown to be at least moderate (>0.50).
Figure 1 Mean change in SF-36v2 scores from baseline.
Abbreviations: BP, bodily pain; EBR/GZR, elbasvir/grazoprevir; GH, general health; MCS, mental component summary; MH, mental health; PCS, physical component summary; PF, physical functioning; RE, role limitations-emotional; RP, role limitations-physical; SF, social functioning; SOF/PR, sofosbuvir with pegylated interferon and ribavirin; VT, vitality.
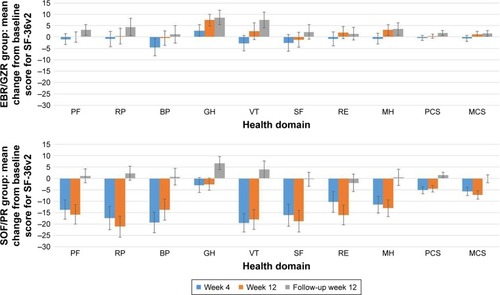
Figure 2 Summary of between-group differences and effect sizes for change in SF-36v2 scores from baseline.
Abbreviations: BP, bodily pain; EBR/GZR, elbasvir/grazoprevir; GH, general health; MCS, mental component summary; MH, mental health; PCS, physical component summary; PF, physical functioning; RE, role limitations-emotional; RP, role limitations-physical; SF, social functioning; VT, vitality.
During the posttreatment follow-up period
After completion of treatment at FW12, mean improvements from baseline scores in five of the eight health domains and both summary scores were observed with the EBR/GZR treatment group (). The declines in SF-36v2 scores observed during the treatment period for the SOF/PR group returned to near baseline scores or above. Although the EBR/GZR group demonstrated more improvement in scores across all health domains and summary scores, there were no statistical differences noted between the treatment groups (95% CI did not exclude 0). There was an overall improvement in SF-36v2 scores from baseline for both treatment groups at FW12, the effect sizes of between-group mean change in scores were small (all <0.20) ().
Change in FACIT-Fatigue Scale scores from baseline
During the treatment period
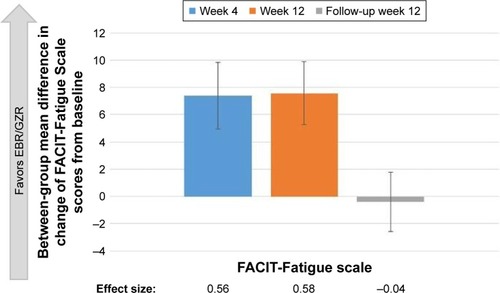
Treatment with EBR/GZR had no significant impact on FACIT-Fatigue Scale scores throughout the treatment period (95% CI did not exclude 0) (). In contrast, treatment with SOF/PR resulted in increased fatigue during the treatment period as indicated by the mean reduction of FACIT-Fatigue Scale scores from baseline (). Significant between- group differences were detected in the mean change of FACIT-Fatigue Scale scores from baseline, at treatment week 4, and at treatment week 12 (7.41 in TW4 and 7.56 in TW12; 95% CI excludes 0) (). Further, the estimated effect sizes for these differences were moderate (both >0.50).
Figure 3 Mean change in FACIT-Fatigue Scale scores from baseline.
Abbreviations: EBR/GZR, elbasvir/grazoprevir; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; SOF/PR, sofosbuvir with pegylated interferon and ribavirin.
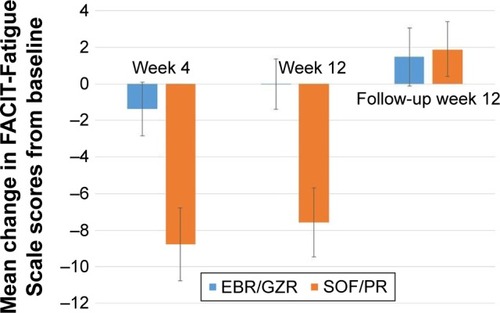
Figure 4 Summary of between-group differences and effect sizes for change in FACIT-Fatigue scale scores from baseline.
Abbreviations: BP, bodily pain; EBR/GZR, elbasvir/grazoprevir; FACIT-Fatigue Scale, Functional Assessment of Chronic Illness Therapy-Fatigue Scale.
During the posttreatment follow-up period
Upon completion of treatment and at FW12, there was no observed impact of EBR/GZR treatment on the FACIT-Fatigue Scale scores. The increase in FACIT-Fatigue Scale scores observed during the treatment period for the SOF/ PR group resolved, and a slight improvement in FACIT-Fatigue Scale scores from baseline was detected at FW12 (mean change =1.89, 95% CI excludes 0). No between-group difference in mean change of FACIT-Fatigue Scale scores from baseline was observed, and the estimated effect size was negligible ().
Discussion
This study examined the impact of EBR/GZR vs SOF/PR on changes in PROs both during and after treatment and assessed whether any observed differences in PROs between the two treatment groups were clinically meaningful. The findings from this study demonstrate that treatment with EBR/GZR resulted in a more favorable PRO profile as compared to treatment with SOF/PR during the treatment period. Specifically, improvements in SF-36v2 scores during treatment as well as posttreatment follow-up period were observed among patients treated with EBR/GZR. In contrast, treatment with SOF/PR resulted in worsening SF-36v2 and FACIT-Fatigue Scale scores during the treatment period. Of note, the between-group differences of the Vitality domain score of the SF-36v2 exceeded the previously established MCID of 4.2 throughout the treatment period. By the end of the treatment at TW12, the between-group differences in mean change scores from baseline for the FACIT-Fatigue Scale, the SF-36v2 summary scales, and most domains of the SF-36v2 had effect sizes that were at least moderate.
The study findings are corroborated by other studies that have also found a negative impact on PROs associated with regimens containing PR during the treatment period, especially when compared to all-oral DAA regimens that do not include PR.Citation6,Citation11,Citation24–Citation26 For example, Younossi et alCitation25 found that patients treated with sofusbuvir/velpatasvir reported improvements in scores of most of the SF-36v2 domains (except Role Limitations-Emotional) and FACIT-Fatigue Scale scores, whereas patients treated with sofosbuvir/ ribavirin reported decrements in these scores during the treatment period. Similar to our study, the impairment in PRO scores observed with the ribavirin-containing treatment group resolved after the treatment was completed.Citation25 This suggests that the deterioration in PROs during treatment with SOF/PR is likely due to treatment-emergent adverse events. In the C-EDGE H2H trial, patients receiving SOF/PR were more likely to report flu-like syndrome, rash/pruritus, and anemia-related adverse events compared to patients receiving EBR/GZR.Citation19
Although we did not detect any clinically meaningful differences between treatment groups by the 12-week post-treatment follow-up period, in the EBR/GZR treatment group there still were significantly improved SF-36v2 scores from baseline in the domains of Physical Functioning, Role Limitations-Physical, General Health, Vitality, Mental Health, and both the PCS and MCS scales. Improvements in SF-36v2 scores started at week 4 of treatment and persisted till posttreatment FW12. These improvements observed at FW12 were also much larger in magnitude as compared to improvements achieved during week 4 and 12 of treatment, which may suggest that the observed PRO improvement in EBR/GZR is likely to persist posttreatment.
Our study results add to the evidence base that patients treated with newer generation DAA regimens without the use of PR result in more favorable PROs during the treatment period, which is likely due to better tolerability when compared to other treatment regimens containing PR. This finding has important implications as nonadherence due to treatment-related side effects or impaired HRQoL can contribute to early treatment discontinuation and, in turn, reduced effectiveness.Citation13,Citation32 Bernstein et al observed that among patients with HCV, fatigue scores and the physical and mental summary scale scores of the SF-36v2 were significantly associated with early discontinuation of HCV treatment.Citation32 Similarly, another study observed that for patients receiving PR-containing regimens, lower physical-related PRO scores, particularly fatigue, were a major driver of treatment nonadherence.Citation13 Taken together, these highlight the importance of minimizing PRO impairment during the treatment period as treatment adherence is associated with higher SVR rates.Citation13,Citation15 In regions such as Eastern Europe and Central Asia where PR is still a standard of practice and access to DAAs varies across countries,Citation33,Citation34 this likely has clinical implications. Based on our study results, patients treated with the 12-week pegylated interferon-containing regimen (ie, SOF/PR), which was the shortest interferon-based regimen available at the time of the study, reported significantly lower PRO scores throughout the treatment period compared to their baseline. This suggests that even a short course of interferon-containing therapy may not be well tolerated. Therefore, to avoid issues of treatment nonadherence or discontinuation associated with impaired on-treatment PROs, it is important to raise awareness and improve treatment access to PR-free DAA regimens in this region.
The study findings should be interpreted in light of several limitations. First, this study analyzed the data collected as part of a clinical trial, which necessitates close monitoring of patients during and posttreatment intervention. Therefore, real-world observational studies are needed to confirm the study results in the clinical setting. In addition, given the open-label design of the C-EDGE H2H trial, it is unknown how much of an impact knowing the treatment assignment might have affected patients’ perceptions of their health status. Due to the small number of nonresponders in this study, the impact of achieving SVR on PROs was not evaluated. This study computed effect sizes of the between-group change in PRO scores and compared them to the standardized interpretation of Cohen to evaluate if the detected differences were clinically meaningful. Although this is a common distribution-based approach of determining MCID, it is known that different approaches tend to yield different values, and the distribution approach is sample-specific where the values calculated depend on the variability of the scores within this study.Citation17
Conclusion
This study demonstrated that HCV treatment with EBR/GZR resulted in a significantly better PRO profile during the treatment period as compared to SOF/PR. Although the improvements in PROs eventually returned to pretreatment levels at the end of therapy, the deterioration of PROs experienced during the treatment periods may negatively influence adherence and ultimately contribute to an unfavorable clinical outcome. Therefore, when deciding on the optimal therapy for HCV treatment, it is important to consider the impact that treatment is likely to have on PROs.
Abbreviation list
| HCV | = | hepatitis C virus |
| HRQoL | = | health-related quality of life |
| EBR/GZR | = | elbasvir/grazoprevir |
| SOF/PR | = | sofosbuvir with pegylated interferon and ribavirin |
| PROs | = | patient-reported outcomes |
| FACIT-Fatigue | = | Functional Assessment of Chronic Illness Therapy-Fatigue |
| DAA | = | direct-acting antiviral |
| MCID | = | minimal clinically important difference |
| C-EDGE H2H | = | C-Edge Head-2-Head |
| PCS | = | physical component summary |
| MCS | = | mental component summary |
| SVR | = | sustained viral response |
| PR | = | pegylated interferon |
Acknowledgments
Initial data analyses were undertaken by Bellinda King-Kallimanis while she was an employee at Pharmerit International, LP. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure
XN, SC, JML, and KEL are employees of Pharmerit International, LP. XN, SC, JML, and KEL are paid consultants to Merck for the purpose of this submitted work. CN, JMA, SP, and HLP are employees/shareholders of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. JQ was involved in this manuscript while she was an employee of Merck & Co., Inc., Kenilworth, NJ, USA. JS received research grants and personal fees from AbbVie, Merck, Gilead, Bristol-Myers Squibb, and Herbacos Recordati, outside of this submitted work. The authors report no other conflicts of interest in this work.
References
- HajarizadehBGrebelyJDoreGJEpidemiology and natural history of HCV infectionNat Rev Gastroenterol Hepatol201310955356223817321
- MaasoumyBWedemeyerHNatural history of acute and chronic hepatitis CBest Pract Res Clin Gastroenterol201226440141223199500
- Hepatitis C Fact Sheet [homepage on the internet]World Health Organization Available from: http://www.who.int/mediacentre/factsheets/fs164/en/AccessedJune 1, 2017
- McdonaldSAHutchinsonSJPalmateerNEDecrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in ScotlandJ Hepatol201358346046623149064
- HsuPCFedericoCAKrajdenMHealth utilities and psychometric quality of life in patients with early- and late-stage hepatitis C virus infectionJ Gastroenterol Hepatol201227114915721679248
- YounossiZHenryLSystematic review: patient-reported outcomes in chronic hepatitis C – the impact of liver disease and new treatment regimensAliment Pharmacol Ther201541649752025616122
- McHutchisonJGWareJEHepatitis Interventional Therapy GroupThe effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivityJ Hepatol200134114014711211891
- HassaneinTCooksleyGSulkowskiMThe impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis CJ Hepatol200440467568115030985
- YounossiZMStepanovaMZeuzemSPatient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE studyJ Hepatol201461222823424713186
- YounossiZMStepanovaMHenryLMinimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C)J Hepatol201460474174724333184
- MarcellinFRouxPProtopopescuCDuracinskyMSpireBCarrieriMPPatient-reported outcomes with direct-acting antivirals for the treatment of chronic hepatitis C: current knowledge and outstanding issuesExpert Rev Gastroenterol Hepatol2017113110
- EvonDMIncorporating patient-reported outcomes into hepatitis C virus treatment studiesClin Gastroenterol Hepatol20141281360136224534544
- YounossiZMStepanovaMHenryLNaderFYounossiYHuntSAdherence to treatment of chronic hepatitis C: from interferon containing regimens to interferon and ribavirin free regimensMedicine20169528e415127428205
- PatelKMchutchisonJGInitial treatment for chronic hepatitis C: current therapies and their optimal dosing and durationCleve Clin J Med200471Suppl 3S8S1215468611
- ShehabTMFontanaRJOberhelmanKMarreroJASuGLLokASEffectiveness of interferon alpha-2b and ribavirin combination therapy in the treatment of naive chronic hepatitis C patients in clinical practiceClin Gastroenterol Hepatol20042542543115118982
- MravčíkVStradaLStolfaJFactors associated with uptake, adherence, and efficacy of hepatitis C treatment in people who inject drugs: a literature reviewPatient Prefer Adherence201371067107524204126
- CopayAGSubachBRGlassmanSDPollyDWSchulerTCUnderstanding the minimum clinically important difference: a review of concepts and methodsSpine J20077554154617448732
- JaeschkeRSingerJGuyattGHMeasurement of health status. Ascertaining the minimal clinically important differenceControl Clin Trials19891044074152691207
- SperlJHorvathGHalotaWEfficacy and safety of elbasvir/ grazoprevir and sofosbuvir/pegylated interferon/ribavirin: A phase III randomized controlled trialJ Hepatol20166561112111927542322
- WareJESF-36 health survey updateSpine200025243130313911124729
- MchorneyCAWareJELuJFSherbourneCDThe MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groupsMed Care199432140668277801
- SpiegelBMYounossiZMHaysRDRevickiDRobbinsSKanwalFImpact of hepatitis C on health related quality of life: a systematic review and quantitative assessmentHepatology200541479080015791608
- FosterGRQuality of life considerations for patients with chronic hepatitis CJ Viral Hepat200916960561119674284
- YounossiZMStepanovaMHenryLNaderFHuntSAn in-depth analysis of patient-reported outcomes in patients with chronic hepatitis c treated with different anti-viral regimensAm J Gastroenterol2016111680881627021197
- YounossiZMStepanovaMSulkowskiMRibavirin-Free Regimen With Sofosbuvir and Velpatasvir Is Associated With High Efficacy and Improvement of Patient-Reported Outcomes in Patients With Genotypes 2 and 3 Chronic Hepatitis C: Results From Astral-2 and -3 Clinical TrialsClin Infect Dis20166381042104827444413
- YounossiZMStepanovaMEstebanRSuperiority of Interferon-Free Regimens for Chronic Hepatitis C: The Effect on Health-Related Quality of Life and Work ProductivityMedicine2017967e591428207507
- YounossiZMStepanovaMFeldJSofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: results from ASTRAL-1 placebo-controlled trialJ Hepatol2016651333926956698
- YounossiZMStepanovaMAfdhalNImprovement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvirJ Hepatol201563233734525795586
- YellenSBCellaDFWebsterKBlendowskiCKaplanEMeasuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement systemJ Pain Symptom Manage199713263749095563
- YounossiZMStepanovaMSulkowskiMWylesDKottililSHuntSPatient-reported outcomes in patients co-infected with hepatitis C virus and human immunodeficiency virus treated with sofosbuvir and velpatasvir: The ASTRAL-5 studyLiver Int201737121796180428470938
- CohenJStatistical Power Analysis for the Behavioral Sciences2nd edHillsdale, NJLawrence Earlbaum Associates1988
- BernsteinDKleinmanLBarkerCMRevickiDAGreenJRelationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patientsHepatology200235370470811870387
- MaistatLGolovinSKravchenkoNKahnTBabikhinaKHepatitis C in Eastern Europe and Central Asia. Civil Society Response to the Epidemic2016 Available from: http://aph.org.ua/wp-content/uploads/2016/07/EECA_HCV_2016_EN.pdfAccessed September 21, 2017
- MaistatLKravchenkoNReddyAHepatitis C in Eastern Europe and Central Asia: a survey of epidemiology, treatment access and civil society activity in eleven countriesHepatol Med Policy2016219
