Abstract
Purpose
Adherence to medication can be assessed by various self-report questionnaires. One could hypothesize that survey respondents tend to answer questions in a manner that will be viewed favorably by others. We aimed to answer if anonymous and nonanonymous responses to a questionnaire on medication adherence differ.
Patients and methods
Adherence was assessed with the German Stendal Adherence with Medication Score (SAMS), which includes 18 questions with responses based on a 5-point Likert scale. Anonymous data from 40 subjects were collected during a symposium for patients with Parkinson’s disease (PD), and nonanonymous data were obtained from 40 outpatient-clinic PD patients at the Department of Neurology.
Results
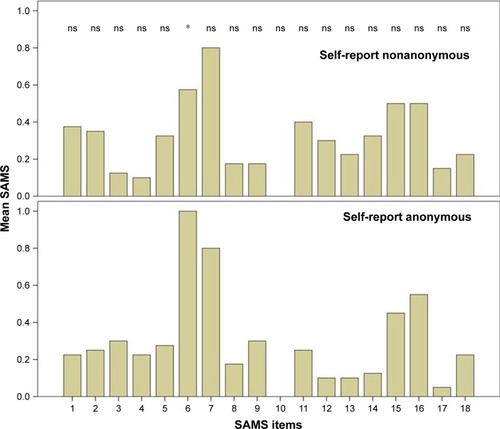
The two groups (anonymous self-reported questionnaire and nonanonymous) did not differ in terms of demographical characteristics and the SAMS sum score. However, anonymously collected data showed significant higher scoring for the item 6 (“Do you forget your medications?”) than the data collected nonanonymously (P=0.017). All other items of the SAMS did not significantly differ between both groups.
Conclusion
Overall assessment of adherence does not depend on whether the patient remains anonymous or not. There seems to be no relevant social desirability bias in nonanonymous responses.
Introduction
Treatment of neurological disorders commonly includes long-term pharmacological therapy. However, for various reasons, many people do not follow the instructions they are given for prescribed treatments (nonadherence).Citation2–Citation6 Nonadherence can be assessed with direct or indirect methods. Direct methods include visually observed therapy or metabolites in the blood and have limited practicality within routine clinical use. Many researchers justify indirect methods including self-report questionnaires.Citation1,Citation7–Citation10 However, does it make a difference when adherence was assessed anonymously or nonanonymously and when the patients know the researcher/physician? To answer this question, we aimed to compare anonymous vs nonanonymous responses to a questionnaire on medication adherence. We chose a cohort of patients with Parkinson’s disease (PD), because it involves a common chronical disorder with patients ingesting several drugs per day.Citation14
Methods
The study was approved by the local ethics committee of the Jena University Hospital. Written informed consent was obtained from all participating patients prior to enrolment in the study, and the study was conducted in accordance with the Declaration of Helsinki. Anonymous data from 40 subjects were collected during a symposium for PD patients in the Jena University organized by the Department of Neurology at the Jena University Hospital. This regular symposium is open for all patients with PD from Thuringia and neighboring regions. In preparation of the symposium, invitations were sent to regional Patient Support Groups as well as neurological outpatient clinics. Nonanonymous data from patients known to the researchers (TP, DS, CP) were obtained from 40 outpatient-clinic PD patients at the Department of Neurology between September and October 2017. Additional demographical data collected are listed in . We used the German Stendal Adherence with Medication Score (SAMS) questionnaire, an extension of the validated German Essen Compliance Score, to assess adherence ().Citation11–Citation13 The SAMS includes 18 questions with responses based on a 5-point Likert scale. In accordance with the cumulative scale 0–72, 0 indicates complete adherence and 72 indicates complete nonadherence (SAMS items are given in ). SPSS (version 23.0; IBM Corporation, Armonk, NY, USA) was used for statistical analyses. There is no established threshold to determine nonadherence. It is generally considered that suboptimal adherence becomes clinically significant when <80% of prescribed medication is taken.Citation15–Citation17 In our study, the highest 25% of all SAMS scores were categorized as nonadherent.Citation15 This leads to a study and sample-specific SAMS cutoff of 7 points for a clinical meaningful nonadherence. The patients were then categorized into 1) fully adherent (SAMS=0), 2) moderate nonadherent (SAMS=1–7), and 3) nonadherent (SAMS >7). Sample size calculation was performed for the SAMS sum score as the main outcome. A sample size of 34 participants per group was found to determine if both groups are equivalent (power=0.8, α=0.05, sampling ratio=1). After checking for outliers and normality, either the t-test or chi-squared test were used for comparison between both groups with Bonferroni correction for multiple comparisons. Kolmogorov–Smirnov test was used to determine similarity between both groups. Statistical significance was set at P<0.05. The datasets generated during the current study are available from the corresponding author on reasonable request for scientific purpose only.
Table 1 Characteristics of both groups (nonanonymous self-report and anonymous self-report) and items of the Stendal Adherence to Medication Score
Table 2 Stendal Adherence to Medication Score (SAMS)
Results
Overall 32 female and 48 male patients with PD with a mean age of 71 years (SD=9, range 44–83) were included in the analysis. The two groups (anonymous self-reported questionnaire and nonanonymous) did not differ in terms of age, gender, marital status, graduation, occupation, the number of drugs used per day, and the mean SAMS sum score (chi-squared test and t-test, respectively; P>0.05) (detailed in ). The mean SAMS sum score of both groups did not differ in the Kolmogorov–Smirnov test (P=0.91). According to the SAMS, 13 (16%) patients were fully adherent (SAMS=0), 46 (56.8%) showed moderate nonadherence, and 21 (25.9%) were found to be clinically meaningful nonadherent. The numbers of fully adherent, moderate nonadherent, and nonadherent patients did not significantly differ between anonymously and nonanonymously collected data (chi-squared test, P>0.05). Anonymously collected data showed significantly higher scoring for the item 6 (“Do you forget your medications?”) than the data collected nonanonymously (P=0.017 with Bonferroni correction). All other items of the SAMS did not significantly differ between both groups ().
Figure 1 Comparison of each item of the Stendal Adherence to Medication Score (SAMS) between both groups.
Discussion
Pharmacotherapy in patients with PD is often suboptimal, and nonadherence is influenced by various factors, such as disease stage, motor complications, complexity of therapeutic schedule, or the presence of depression.Citation18 The prevalence of the observed nonadherence agrees with other epidemiological studies.Citation18,Citation19 Our study indicates that overall assessment of adherence does not depend on whether the patient remains anonymous or not. Solely, for the item “Do you forget your medications?” did patients more frequently report forgetfulness if they were asked anonymously. This also implies that this general question probably does not add significant knowledge about the individual adherence of a patient. It seems more appropriate to convey to the patient that forgetting is a normal human trait and to rephrase the question to, “If you forget your medication, is it more likely to happen in the morning, at noon or in the evening?” Our data suggest that adherence questionnaires can also be used by physicians for regular care to determine reasons of nonadherence.
This study is not free of limitations. Although the results are likely to be transferable to other groups of patients, it should be noted that it was restricted to patients with PD, because they usually need long-term medical treatment with several drugs per day. Due to the study design, we cannot guarantee that other cofounders, such as disease severity or disease stage (which were not assessed in the anonymous cohort), were perfectly matched. However, to the group with the nonanonymous self-report, we only included patients from our outpatient hospital who were mobile and able to walk in order to make them comparable to the anonymous patients who had participated in the symposium.
Conclusion
We found that the overall assessment of adherence, as indicated by the SAMS sum score, does not depend on whether the patient remains anonymous or not. There seems to be no social desirability bias in nonanonymous responses. Therefore, the SAMS questionnaire can be used as valid tool for physicians to detect nonadherence in their patients.
Author contributions
Research idea and study design: TP. Data collection: TP, DS, CP. Data analysis/interpretation: TP, DS, GHF, AK. Preparation of the manuscript: TP. Revision and approval of the manuscript: CP, OW, JG. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Nasim Kroegel for proofreading and Sarah Mendorf, Jenny Wilde, Maria-Theresa Gruber, and Juliane Nößler for acquisition of data.
Disclosure
The authors report no conflicts of interest in this work.
References
- Pérez-EscamillaBFranco-TrigoLMoullinJCMartínez-MartínezFGarcía-CorpasJPIdentification of validated questionnaires to measure adherence to pharmacological antihypertensive treatmentsPatient Prefer Adherence2015956957825926723
- BrownMTBussellJKMedication adherence: WHO cares?Mayo Clin Proc201186430431421389250
- YapAFThirumoorthyTKwanYHSystematic review of the barriers affecting medication adherence in older adultsGeriatr Gerontol Int201616101093110126482548
- ConnVSRupparTMMedication adherence outcomes of 771 intervention trials: systematic review and meta-analysisPrev Med20179926927628315760
- ChecchiKDHuybrechtsKFAvornJKesselheimASElectronic medication packaging devices and medication adherenceJAMA2014312121237124725247520
- SlomskiAPill reminders don’t improve adherenceJAMA2017317242476
- NieuwlaatRWilczynskiNNavarroTInterventions for enhancing medication adherenceCochrane Database Syst Rev201411CD000011
- MoriskyDEAngAKrousel-WoodMWardHJPredictive validity of a medication adherence measure in an outpatient settingJ Clin Hypertens2008105348354
- MoriskyDEGreenLWLevineDMConcurrent and predictive validity of a self-reported measure of medication adherenceMed Care198624167743945130
- ShalanskySJLevyARIgnaszewskiAPSelf-reported Morisky score for identifying nonadherence with cardiovascular medicationsAnn Pharmacother20043891363136815238622
- FrankeGHJaglaMReimerJHaferkampLTürkTWitzkeOErfassung von Medikamenten-compliance bei erfolgreich nierentransplanti-erten mit einer erweiterten version des morisky-scores – dem Essener Compliance Score (ECS) [Detection of adherence to medication with an extended version of the Morisky score (Essener Compliance Score, ECS) in kidney transplanted patients]PPmP – Psychotherapie Psychosomatik Medizinische Psychologie200959027993
- JägerSFGReimerJGallCHaferkampLTürkTWitzkeODer Zusammenhang zwischen Medikamenten-Compliance und gesundheitsbezogener Lebensqualität bei Nierentransplantierten [Relations-ship between adherence to medication and health related quality of life in kidney transplanted patients]AK Klinische Psychologie im BDP: Psychische Störungen in der somatischen Rehabilitation20097993
- TürkTFGJaglaMHaferkampLReimerJKribbenAWitzkeODevelopment of the Essen compliance score – measurement of adherence in kidney transplant patientsAm J Transpl20099Suppl 2A296
- BainbridgeJLRuscinJMChallenges of treatment adherence in older patients with Parkinson’s diseaseDrugs Aging200926214515519220071
- DimatteoMRVariations in patients’ adherence to medical recommendations: a quantitative review of 50 years of researchMed Care200442320020915076819
- MalekNHeathCAGreeneJA review of medication adherence in people with epilepsyActa Neurologica Scandinavica2017135550751527781263
- OffordSLinJWongBMirskiDBakerRAImpact of oral anti-psychotic medication adherence on healthcare resource utilization among schizophrenia patients with medicare coverageCommun Mental Health J2013496625629
- StrakaIMinarMGazovaAValkovicPKyselovicJClinical aspects of adherence to pharmacotherapy in Parkinson disease: a PRISMA-compliant systematic reviewMedicine20189723e1096229879046
- DurandHHayesPMorrisseyECMedication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysisJ Hypertens201735122346235728777133
