Abstract
Background
Antiretroviral therapy (ART), when taken consistently, reduces morbidity and mortality associated with human immunodeficiency virus and viral transmission. Suboptimal treatment adherence is associated with regimen complexity and high tablet burden. Single-tablet regimens (STRs) provide a complete treatment regimen in a single tablet. This study examined the relationship between STRs (vs multiple-tablet regimens [MTRs]), treatment adherence, and viral suppression.
Methods
A systematic review was conducted to identify studies investigating at least one of the following: (1) STR/MTR use and adherence; (2) levels of adherence and viral suppression; and (3) STR/MTR use and viral suppression. Meta-analysis was performed to assess the relationship between STR vs MTR use and adherence in observational settings at ≥95% and ≥90% adherence thresholds.
Results
In total, 29 studies were identified across the three objectives; two studies were relevant for all objectives. STRs were associated with higher treatment adherence than MTRs in 10/11 observational studies: a 63% greater likelihood of achieving ≥95% adherence (95% CI=1.52–1.74; P<0.001) and a 43% increase in the likelihood of achieving ≥90% adherence (95% CI=1.21–1.69; P<0.001). Higher adherence rates were associated with higher levels of viral suppression in 13/18 studies. Results were mixed in five studies investigating the association between STR or MTR use and viral suppression.
Conclusion
Although the direct effect of STRs vs MTRs on viral suppression remains unclear, this study provided a quantitative estimate of the relationship between STRs and ART adherence, demonstrating that STRs are associated with significantly higher ART adherence levels at 95% and 90% thresholds. Findings from the systematic review showed that improved adherence results in an increased likelihood of achieving viral suppression in observational settings. Future research should utilize similar measures for adherence and evaluate viral suppression to improve assessment of the relationship between pill burden, adherence, and viral suppression.
Introduction
The introduction of highly effective antiretroviral therapy (ART) has transformed the treatment of people living with human immunodeficiency virus (PLWH). When patients are optimally adherent to potent combination ART, human immunodeficiency virus (HIV) is transformed from a potentially fatal condition to a manageable chronic disease. Current national guidelines in the USA recommend a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with an integrase strand transfer inhibitor (INSTI) for initial treatment in ART-naïve individuals.Citation1
Combination ART has multiple individual and societal benefits mediated by achieving viral suppression, which reduces HIV-associated morbidity and mortality, increases health-related quality of life (HRQoL), and prevents HIV transmission. However, ART regimens differ in dosing complexity, toxicity, and tolerability – factors that influence adherence to treatment and outcomes.Citation2–Citation6 Suboptimal adherence reduces the likelihood of viral suppression,Citation4,Citation7 which in turn increases the risk of transmissionCitation8 and the development of drug resistance, thereby limiting future treatment options.Citation7 Suboptimal adherence has both clinical and economic consequences, including accelerated disease progression and mortality,Citation9–Citation11 decreased HRQoL,Citation12 and higher healthcare costs.Citation13–Citation15
Single-tablet regimens (STRs), which combine a complete treatment regimen into a single fixed-dose tablet, have the potential to address regimen complexity and high pill burden. STRs have been shown to improve adherence to antihypertensive agents.Citation16 Generally, studies assessing real-world medication-taking behaviors in PLWH show greater adherence to STRs than to multiple-tablet regimens (MTRs).Citation6,Citation17–Citation19 In a study published in 2000, high adherence (taking ≥95% of prescribed doses) was associated with greater viral suppression, and avoidance of drug resistance and HIV-associated complications.Citation20 More recently, in 2015, a meta-analysis looked at the impact of pill burden on viral suppression.Citation21 While the authors found that STRs were associated with greater viral suppression than MTRs, the results were based on only three studies and did not include sensitivity analyses. The development of new treatment options, including several STRs with improved tolerability and efficacy, warrants a stepwise appraisal of the literature on adherence and outcomes with emphasis on the impact of STRs vs MTRs. Specifically, these new regimens may improve the relationship between adherence and viral suppression, potentially reducing the adherence thresholds needed for treatment success. Results of a recent meta-analysis suggest that adherence of 80%–90% may be adequate to achieve viral suppression.Citation22 In the current era of newer, more potent, treatment options, it is important to understand whether the older paradigms associating regimen simplicity (STR vs MTR), adherence, and patient outcomes remain valid. Furthermore, given demographic changes, with more than half of PLWH in the USA being over the age of 50 years,Citation23 the high prevalence of age-related comorbidities, such as cardiovascular disease,Citation24 may complicate HIV management or adherence due to higher pill burden and polypharmacy.Citation25 The results of the current study extend findings from earlier research,Citation20,Citation21 taking into account newer regimens and emerging data on the level of adherence required for successful viral suppression.
Adherence is most likely best assessed in observational studies in which patients’ medication usage may more closely reflect actual clinical practice, because real-world studies often find poor long-term adherence.Citation26 Adherence can be higher in randomized controlled trials (RCTs) due to factors such as careful selection of participants or more intensive follow-up;Citation27 however, RCTs may provide useful information about the effect of adherence on virologic outcomes. We therefore conducted a systematic review to appraise the published literature reporting associations between STR vs MTR use and adherence to ART in observational studies alone, and that reporting the effects of adherence or STR vs MTR use on virologic outcomes in either observational studies or RCTs.
Methods
Search strategy
A systematic literature review was performed to identify studies assessing at least one of the following objectives: 1) the association between STR vs MTR use and adherence in observational settings; 2) the association between adherence (in either observational studies or RCTs) and viral suppression; and 3) the association between STR vs MTR use and viral suppression (in either observational studies or RCTs).
The systematic literature review was performed in two parts. An initial search of MEDLINE In-Process was completed on September 6, 2013, to identify any studies that were relevant to the three objectives that were published between 2006 and 2013. A follow-up search using the same search criteria and database was conducted on September 14, 2016, to identify relevant literature published since the initial search (2013–2016) to account for more contemporary regimens. Search terms and categories were consistent across both elements of the systematic review ().
Supplemental searches were conducted to identify recent relevant studies published in the proceedings of the following conferences (2013–2016): International AIDS Society Conference, International AIDS Conference, Conference on Retroviruses and Opportunistic Infections, American Society for Microbiology Interscience Conference on Antimicrobial Agents and Chemotherapy (2015), European AIDS Clinical Society Conference, and Infectious Disease Week. The 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelinesCitation28 were followed for both initial and current searches.
Eligibility criteria
The titles and abstracts of identified publications were screened manually by one reviewer (OE) against pre-specified eligibility criteria for each objective (). Literature eligible for inclusion included full-text reports of original research published in English, conducted in North America or the European Union (EU), and involving adults diagnosed with HIV. Studies finding that patients received once-daily ART and those with a one-pill regimen arm with no confirmation that treatment was administered once daily were included; studies in which all patients received more-than-once-daily regimens were excluded. For objective 1, publications from observational studies (including prospective or retrospective non-randomized studies, disease registries or databases, electronic medical records, and claims data) reporting the effects of STRs vs MTRs on adherence were included. Observational studies and RCTs investigating the association between adherence and treatment efficacy or effectiveness were included for objective 2. Studies researching the effect of treatment regimen (STR vs MTR) on treatment efficacy or effectiveness in the observational setting were included for objective 3.
Full-text versions of all publications meeting the eligibility criteria at initial screening were reviewed. Once eligibility was confirmed, data were extracted manually into pre-defined summary tables for each objective.
Information extracted from relevant studies included details of the study (enrollment start year, study end year, study location [EU or North America], sample size, and period of follow-up), patient characteristics (age, sex, CD4+ count, baseline HIV RNA level, and previous treatment), and adherence and efficacy results (eg, method of assessing adherence, viral suppression measures, and definitions).
Meta-analysis
A meta-analysis was performed to assess the relationship between STR vs MTR use and adherence at the ≥95% and ≥90% thresholds (objective 1) using random- and fixed-effects models. Heterogeneity between studies was examined using Q and I2 statistics.Citation29,Citation30 A random-effects model was used in each analysis, and an additional fixed-effects model was used when heterogeneity among studies was not significant at the P,0.05 level. Publication bias for the ≥95% adherence outcome was determined by plotting the odds ratio (OR) against the inverse standard error and visually assessing the symmetry of funnel plots and statistical significance confirmed using Egger’s and Begg’s tests.Citation31 Data management and statistical analyses were undertaken using Stata (StataCorp LLC, College Station, TX, USA).
Sensitivity analyses were performed to examine the following potential treatment effect modifiers on the influence of STR vs MTR use on adherence (≥95%): a) studies in which once-daily dosing was confirmed in the publication compared with those studies in which the daily dose could not be confirmed; b) studies in which adherence was measured using the medication possession ratio (MPR) vs the proportion of days covered (PDC); and c) studies conducted in North America compared with those in the EU.
A meta-regression was performed to assess major moderators of between-study heterogeneity (age, sex, race, and study period) in data from studies reporting ≥95% adherence to treatment.
Results
Search results
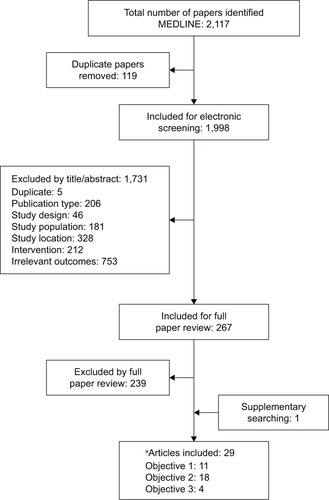
Overall, 2,117 relevant publications were initially identified, of which 119 were duplicates. Of the remaining 1,998 titles and abstracts screened, 1,731 were excluded, leaving 267 that qualified for full-text review (). Ultimately, 29 studies met the inclusion criteria for one or more of the review objectives: 11 for objective 1, 18 for objective 2, and four for objective 3 (two studies were relevant for all three objectives).Citation5,Citation32
Figure 1 PRISMA diagram.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Objective 1 – comparison of STR vs MTR use and adherence
Eleven studies (10 full papersCitation5,Citation32–Citation41 and one conference abstractCitation37) described the association between STR or MTR use and treatment adherence. Of these studies, nine were conducted in the USACitation5,Citation32–Citation36,Citation39–Citation41 and two were conducted in the EU ().Citation37,Citation38 Six studies used the MPR method to assess adherence,Citation32–Citation35,Citation38,Citation39 four used the PDC method,Citation36,Citation37,Citation40,Citation41 and, in one study, patients self-reported their adherence ().Citation5
Table 1 Characteristics of observational studies identified by the systematic literature review that assess the relationship between adherence and use of STR vs MTR (objective 1)
In all studies, the proportion of patients reaching a defined minimum threshold of adherence was determined. Eight studies reported the proportion of patients with ≥95% adherence.Citation5,Citation33,Citation35–Citation37,Citation39–Citation41 Some publications reported the proportion of patients with ≥90%,Citation32,Citation34,Citation35,Citation40 >90%,Citation38 ≥80%,Citation40 and >80%Citation38 adherence throughout the study. Ten of 11 eligible studies found that STRs were associated with higher adherence than MTRs.Citation5,Citation33–Citation41 The differences were statistically significant in nine studies.Citation5,Citation33–Citation37,Citation39–Citation41 Of the remaining two studies, one investigated adherence to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based (STR – consisting of efavirenz, emtricitabine, and tenofovir) and protease inhibitor (PI)-based (MTR) regimens, and found that patients using MTRs had significantly higher rates of adherence than those using STRs.Citation32 In this study, however, multivariate analysis showed that treatment-experienced patients were 52% less likely than treatment-naïve patients to achieve 95% adherence, with the STR arm having a higher proportion of treatment-experienced patients than the MTR arm (75.2% vs 61.5%).Citation32 The other study found no significant difference in >90% adherence between patients receiving STRs and those receiving MTRs.Citation38
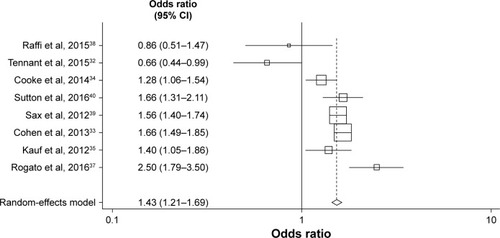
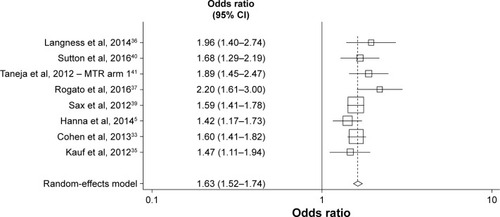
The meta-analysis, involving eight studies describing nine comparisons, compared the associations between STR vs MTR use and adherence at the ≥95% adherence threshold.Citation5,Citation33,Citation35–Citation37,Citation39–Citation41 In the random-effects model, STR use was 1.72-fold more likely to be associated with ≥95% treatment adherence than MTR use (95% CI=1.54–1.93; P<0.001).
A funnel plot using estimates of ≥95% adherence from all studies included in the meta-analysis found that the nevi-rapine (NVP)-based regimen plus at least two NRTIs arm in the 2012 study of Taneja et alCitation41 was an outlier (Egger’s test, P=0.050, Begg’s test, P=0.048). Excluding this arm, an updated funnel plot showed no evidence of publication bias using Egger’s (P=0.185) or Begg’s (P=0.174) tests (). The revised fixed-effects meta-analysis excluding the NVP arm resulted in similar findings, with STRs remaining significantly associated with ≥95% adherence than MTRs (OR=1.63; 95% CI=1.52–1.74; P,0.001).
Figure 2 Objective 1 – meta-analysis comparing the effects of STRs and MTRs on optimal (≥95%) adherence levels. Excluding nevirapine-based regimen plus at least two nucleoside reverse transcriptase inhibitors arm in Taneja et al.Citation41
Sensitivity analyses using meta-regression () confirmed no significant differences in OR for ≥95% adherence in studies in which once-daily dosing was confirmed in the publications compared with those in which once-daily dosing could not be confirmed (P=0.368), in studies in which adherence was measured by MPR or PDC (P=0.056), and in those conducted in the USA vs the EU (P=0.098).
Table 2 Sensitivity analysis of moderators for the association between STR vs MTR use and adherence (objective 1)
Meta-regression analyses did not find a significant effect of sex (P=0.914), age (P=0.898), or race (P=0.412) on the association between STR or MTR use and treatment adherence. The effect of the study period had to be considered at the study-level because this variable did not differ between STR and MTR groups in any one study. Meta-regression did not demonstrate any significant effect of study start or end date on the results of the meta-analysis (P=0.419).
A meta-analysis also compared the effects of STRs and MTRs on lower adherence thresholds (≥90%), which were reported in eight studies ().Citation32–Citation35,Citation37–Citation40 Findings were similar to those for ≥95% adherence, with an OR in favor of STR of 1.43 (random-effects model; 95% CI=1.21–1.69; P<0.001).
Objective 2 – association between adherence and viral suppression
Overall, 18 studies (five RCTsCitation42–Citation46 and 13 observational studies)Citation5,Citation32,Citation47–Citation57 investigated the association between adherence thresholds and viral suppression (), of which 13 (72%) found that higher levels of adherence were associated with greater viral suppression.Citation5,Citation32,Citation42,Citation44,Citation47,Citation50–Citation57 Owing to high variation in the adherence thresholds and viral outcomes reported in the identified studies, a meta-analysis addressing this objective was not performed. One study using MPR to measure adherence, however, found that patients optimally adherent to ART were three times more likely than non-adherent patients to be virology-cally suppressed.Citation32
Table 3 Characteristics of studies identified by the systematic literature review that assess the effect of treatment adherence on viral suppression (objective 2)
Of the 11 studies that used 95% as the adherence threshold, seven (64%) found that adherence was strongly associated with viral suppression.Citation5,Citation42,Citation44,Citation47,Citation50,Citation52,Citation57 All three studies using 90% as a threshold showed improved virologic outcomes with adherence,Citation32,Citation53,Citation55 and two studies found high levels of viral suppression when adherence was ≥80%Citation40 and >75%.Citation35 Martin et alCitation55 reported mixed virologic results with <70% adherence, resulting in virologic failure in 100% of patients prescribed unboosted PI-based regimens, 50% virologic failure in those receiving boosted PI-based regimens, and 34.5% virologic failure in individuals on NNRTI-based regimens.
Five studies demonstrated a positive effect of self-reported adherence on viral outcome. The results of two suggested that higher (≥95%) adherence improved virologic outcomes,Citation5,Citation47 while three found that suboptimal adherence (self-reported non-adherenceCitation50 or <95% adherence)Citation42,Citation44 was associated with virologic failure. The remaining studies used a variety of other methods to assess adherence, including therapeutic drug monitoring,Citation52 a simplified medication adherence questionnaire,Citation52,Citation56 pill counts,Citation53,Citation55 pharmacy refills,Citation55 and the antiviral medication adherence form.Citation53 All studies demonstrated a positive association between treatment adherence and virologic outcomes.
Of the 18 identified studies reporting the association between adherence and viral suppression, five (28%) had mixed outcomes or did not find an effect of adherence on virologic outcomes.Citation43,Citation45,Citation46,Citation48,Citation49 In two studies, adherence improved treatment outcomes with some regimens but not with others. For example, Nelson et alCitation45 found that suboptimal adherence (<95%) to darunavir/ritonavir was not associated with virologic response, whereas virologic response to lopinavir/ritanovir improved with better adherence (≥95%). Similarly, Viswanathan et alCitation48 demonstrated that adherence was associated with greater viral suppression in patients using PI-based regimens, but not in those receiving NNRTI-based regimens (≥95%). In the remaining three studies, adherence was not predictive of virologic outcomes.Citation43,Citation46,Citation49
Objective 3 – association between STR vs MTR use and viral suppression
Overall, five studies investigated the association between STR vs MTR use and viral suppression ().Citation3,Citation5,Citation32,Citation58,Citation59 Chakraborty et alCitation58 examined viral load trends in population subgroups by assessing a state-wide surveillance dataset, and showed that there was a more rapid decrease in viral load among people using STRs than in those using MTRs. Hanna et alCitation5 reported that STR use was significantly associated with virologic suppression. The remaining studies found no significant difference between the effect of STRs and that of MTRs on virologic outcomes.Citation3,Citation32,Citation59 Of note, one of these studies found no significant effect of STRs compared with MTRs on achievement of >90% adherence.Citation32
Table 4 Characteristics of studies identified by the systematic literature review that assessed the effect of use of STR vs MTR on viral suppression (objective 3)
Discussion
To our knowledge, this systematic literature review provides the most current summary of published literature assessing associations between STR vs MTR use and ART adherence and the impact of adherence on virologic outcome. The results of this study suggest that STR regimens are related to better adherence in real-world, observational settings. We identified many observational studies investigating the association between STR vs MTR use and adherence in observational settings (objective 1) and between adherence and viral suppression in observational settings and RCTs (objective 2); however, few studies assessing the association between observational STR vs MTR use and viral suppression were found (objective 3). Although the studies identified included patients on a variety of ART regimens, there was a paucity of available data on INSTI regimens, which are now recommended. Only one study specifically included patients receiving an INSTI (elvitegravir).Citation5 The remaining studies that were included consisted of combinations of patients receiving NRTIs, NNRTIs, and PIs.
A meta-analysis of objective 1 using data from eight observational studies demonstrated that STRs are significantly associated with a 63%–72% higher level of adherence (at the ≥95% adherence threshold) than MTRs. The results of this systematic literature review and meta-analysis assessing the association between STR vs MTR use and adherence in observational settings (objective 1) are consistent with those of previous studies that demonstrated significant benefits of STRs compared with MTRs. These findings, together with those from a meta-analysis by Clay et alCitation21 that assessed adherence and clinical and cost outcomes (reporting a 2.37-fold adherence advantage of STRs over MTRs) confirm the importance of STRs in optimizing clinical outcomes. Our study extends these earlier findings by using specific thresholds of adherence rather than the relative adherence in patients on STRs vs MTRs.Citation21 The current study also included a greater number of publications and patients (eight studies involving 30,470 patients compared with four studies involving 1,224 patients), leading to more robust meta-analyses. Furthermore, the larger sample size allowed for in-depth sensitivity analyses to account for factors that could have confounded the observed associations. Studies from the EU and USA were assessed in a sensitivity analysis owing to the differences in healthcare provision in these regions.Citation60 Additionally, because we were unable to confirm whether once-daily dosing was used in all the included studies, we performed a sensitivity analysis to clarify the impact of confirmation of once-daily dosing, since reducing dosage frequency to once-daily has been shown to be related to higher treatment adherence.Citation61 Sensitivity analysis was also used to investigate the impact of different methods of adherence measurement (MPR vs PDC). The results were robust to these sensitivity analyses, and to meta-regression analyses assessing the effect of sex, age, race, and study period.
This study was also further able to incorporate RCT data and to examine different levels of adherence thresholds (objective 2). Importantly, thresholds of >90% and >95% were consistently associated with viral suppression, but findings did not remain consistent when lower thresholds were investigated (<90%). Of the identified studies, results of two suggested that ≥75% adherence may be sufficient to achieve viral suppression.Citation35,Citation40 One of these studies included patients receiving NNRTI-based regimens,Citation41 and the other included patients on NRTI-based regimens.Citation46 The positive effects of lower (<80%) levels of adherence on viral suppression observed in these studies are consistent with findings from a small pilot study of a “five-on, two-off” treatment schedule demonstrating viral suppression with ~70% adherence.Citation62 Additionally, a systematic review with meta-analysis attempted to disentangle this relationship further.Citation22 In this review, adherence of 80%–90% appeared to be adequate for viral suppression, suggesting that some types of regimens may be more forgiving than others, owing to higher efficacy and lower toxicity (in newer regimens),Citation63 and unique pharmacological properties such as longer half-life.Citation64 Results of the meta-analysis by Bezabhe et alCitation22 suggests that the level of adherence required for sustaining viral suppression may be lower than has previously been thought; however, data are inconclusive as to the minimum level necessary to ensure durable virologic suppression, and the relationship between adherence and virologic failure was impacted by study design and study region.
The majority of studies identified in our searches used higher adherence thresholds (≥95%) than those suggested to be necessary for sustaining viral suppression in the Bezabhe study.Citation22 In that review, however, virologic failure was variably defined (<100 copies/mL vs 500 copies/mL and <1,000 copies/mL), and the likelihood of viral suppression differed for various adherence thresholds, with greater ORs for higher thresholds. Moreover, thresholds of adherence varied among their reported studies (from 100% to 80%),Citation22 and the effect of adherence on viral suppression was significantly higher, with lower cut-offs for virologic failure (<100 copies/mL vs 500 copies/mL and 1,000 copies/mL).Citation22 Thus, it is crucial for future research to use well-defined and clinically meaningful thresholds to better compare studies.
Only five studies comparing the effect of STR and MTR use on virologic outcomes were found (objective 3),Citation3,Citation5,Citation32,Citation58,Citation59 three of which demonstrated no significant differences in viral suppression for patients using STRs compared with those using MTRsCitation3,Citation32,Citation59 (the other two showed better viro-logic outcomes with STRsCitation5,Citation58). Thus, despite several studies showing that STRs are associated with greater adherence than MTRs, the impact of this improvement on virologic suppression has yet to be conclusively demonstrated. In the Clay et alCitation21 meta-analysis that included only three studies, viral suppression at 48 weeks was significantly greater in patients using STRs than in those using MTRs (P=0.0003).
The low number of studies and the differing results suggest that further research is needed to determine whether adherence to STRs translates into improved virologic and clinical outcomes. Although a limited number of studies have demonstrated that STRs are associated with lower rates of disease progression and mortality compared with MTRs,Citation9,Citation65,Citation66 it is crucial that future research addresses this gap in our understanding of how pill burden impacts viral suppression so that the needs of patients are better addressed.
As PLWH age and experience a higher burden of comorbidities in addition to HIV,Citation25 it is crucial that they are helped to manage comorbidities and coordination of multiple therapies. Aside from pill burden, other potential barriers to adherence, such as inadequate housing, food insecurity, substance use, and psychiatric disorders including depression, should be addressed to better support PLWH.Citation67 To improve treatment adherence in PLWH, it is critical to take multiple approaches, such as minimizing socio-structural barriers, improving management of health systems, strengthening patient–provider relationships, and working with patients to ensure that regimens are acceptable, feasible, and have minimal side-effects.
Despite the robust findings, our systematic literature review and meta-analyses are not without limitations. The studies reporting associations between STR vs MTR use and real-world adherence were non-randomized, and may be confounded by patient differences and medication characteristics between treatment groups. While the lack of ran-domization limits the internal validity, the approach taken in the current study is more applicable to real-world settings in which patients face barriers to treatment adherence and have less support and oversight than is present in a controlled setting. In most studies, adherence was calculated according to pharmacy records and reimbursement claims data, which may not reflect true adherence. The results of this meta-analysis, however, are generally consistent with those of sensitivity analyses involving identified studies using the more accurate PDC approach and those using MPR.
Although the results of a substantial number of studies suggest that STRs are associated with higher adherence to therapy than MTRs and show an association between adherence and viral suppression, only a small number of studies directly evaluated the association between STR use and clinical outcomes, and few data are available on INSTI regimens, which are now recommended.
Conclusion
This systematic review addresses an important gap in the HIV adherence literature, providing a quantitative analysis of the effect of STRs vs MTRs on the achievement of 90% and 95% adherence. The analysis re-affirms the significant association between the use of STRs and adherence, and the positive impact of adherence on virologic outcome. However, future research is still necessary to measure the association between specific adherence thresholds and specific levels of viral suppression. Although the existing literature shows similar treatment outcomes when adherence is defined using 95% and 90% thresholds, virologic outcomes have been mixed with thresholds below 90%. Overall, our systematic review and meta-analysis provides a quantitative estimate of the benefits of STRs over MTRs on adherence and virologic outcomes, and highlights the need for research assessing the impact of pill burden on viral suppression to meet patients’ needs more effectively.
Author contributions
FA: manuscript review and final approval. OE: data inclusion assessment; data extraction; data analysis and interpretation; manuscript draft; manuscript review and final approval. SS: data inclusion assessment; data extraction; data analysis and interpretation; manuscript draft; manuscript review and final approval. GC: manuscript draft; manuscript review and final approval. ACB: concept and design; manuscript draft; manuscript review and final approval. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Abbreviations
| ABC-3TC | = | abacavir and lamivudine |
| ACTG | = | AIDS Clinical Trials Group |
| AIDS | = | acquired immune deficiency syndrome |
| AMAF | = | antiviral medication adherence form |
| ART | = | antiretroviral therapy |
| EFV | = | efavirenz |
| EU | = | European Union |
| FTC | = | emtricitabine |
| HAART | = | highly active antiretroviral therapy |
| HIV | = | human immunodeficiency virus |
| HIV-1 | = | type 1 HIV |
| HRQoL | = | health-related quality of life |
| INSTI | = | integrase strand transfer inhibitor |
| M-MASRI | = | Modified Medication Adherence Self-Report Inventory |
| MPR | = | medication possession ratio |
| MTR | = | multiple-tablet regimen |
| NA | = | not applicable |
| NNRTI | = | non-nucleoside reverse transcriptase inhibitor |
| NRTI | = | nucleoside reverse transcriptase inhibitor |
| NVP | = | nevi-rapine |
| OR | = | odds ratio |
| PBMC | = | peripheral blood mononuclear cell |
| PDC | = | proportion of days covered |
| PI | = | protease inhibitor |
| PLWH | = | people living with human immunodeficiency virus |
| PPD | = | pills per day |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QD | = | once daily |
| RCT | = | randomized controlled trial |
| RPV | = | rilpivirine |
| SMAQ | = | simplified medication adherence questionnaire |
| SPNS | = | Special Project of National Significance |
| STR | = | single-tablet regimen |
| TDF | = | tenofovir disoproxil fumarate |
| TDM | = | therapeutic drug monitoring |
| VAS | = | visual analog scale |
| WIHS | = | Women’s Interagency HIV Study |
Acknowledgments
The authors acknowledge the help of Reza Oskrochi who assisted with data analysis and interpretation. This study was funded by Gilead Sciences.
Supplementary materials
Table S1 MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946–present; search conducted on September 14, 2016Table Footnotea
Table S2 Eligibility criteria
Disclosure
Obaro Evuarherhe, Gemma Carter and Sophie Shina are employees of Oxford PharmaGenesis Ltd, Oxford UK, which was funded by Gilead Sciences. Anne Christine Beaubrun is an employee of Gilead Sciences, Foster City, CA, USA. The authors report no other conflicts of interest in this work.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents: guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescentsDepartment of Health and Human Services [updated July 14, 2016]. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdfAccessed March 23, 2017
- AstutiNMaggioloFSingle-tablet regimens in HIV therapyInfect Dis Ther2014311725134808
- DejesusEYoungBMorales-RamirezJOSimplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified anti-retroviral therapy in virologically suppressed HIV-1-infected patientsJ Acquir Immune Defic Syndr20095116317419357529
- GlassTRDe GeestSHirschelBSelf-reported non-adherence to antiretroviral therapy repeatedly assessed by two questions predicts treatment failure in virologically suppressed patientsAntivir Ther2008137785
- HannaDBHessolNAGolubETIncrease in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected womenJ Acquir Immune Defic Syndr20146558759624326606
- ParientiJJBangsbergDRVerdonRGardnerEMBetter adherence with once-daily antiretroviral regimens: a meta-analysisClin Infect Dis20094848448819140758
- World Health Organization (WHO)Adherence to long-term therapiesEvidence for action12003 Available from: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdfAccessed October 31, 2016
- WilsonDPLawMGGrulichAECooperDAKaldorJMRelation between HIV viral load and infectiousness: a model-based analysisLancet200837231432018657710
- HoggRSYipBChanKO’ShaughnessyMMontanerJNonadherence to triple-combination ART is predictive of AIDS progression and death in HIV+ men and womenPresented at the XIII International AIDS ConferenceJuly 9–14; 2000Durban, South Africa Abstract TuOrB419
- KitahataMMReedSDDillinghamPWPharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and deathInt J STD AIDS20041580381015601486
- YehiaBRMehtaJMCiuffetelliDAntiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adultsClin Infect Dis20125559359922610923
- MannheimerSBMattsJTelzakEQuality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherenceAIDS Care200517102215832830
- Panel on treatment of HIV-infected pregnant women and prevention of perinatal transmissionRecommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States [updated October 26, 2016] Available from: http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdfAccessed April 30, 2012
- GardnerEMMaraviMERietmeijerCDavidsonAJBurmanWJThe association of adherence to antiretroviral therapy with healthcare utilization and costs for medical careAppl Health Econ Health Policy2008614515519231907
- LeisegangRClearySHislopMEarly and late direct costs in a Southern African antiretroviral treatment programme: a retrospective cohort analysisPLoS Med20096e100018919956658
- GuptaAKArshadSPoulterNRCompliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysisHypertension20105539940720026768
- O’ConnorJLGardnerEMMannheimerSBFactors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trialJ Infect Dis2013208404923204161
- SweetDKZhongYZhuoDSignorovitchJReal-world adherence among patients receiving single versus multiple tablet regimens for HIV-1 infection, and associations between adherence and viral suppression: a systematic literature review and meta-analysisPresented at the 20th International AIDS ConferenceJuly 20–25; 2014Melbourne, Australia Abstract MOPE054
- UthmanOAParientiDWDowdyDWRegimen simplification in HIV infection toward once-daily dosing and fixed-dose combinations: a meta-analysis and sequential analysis of randomized controlled trialsPoster presented at the XIX International AIDS ConferenceJuly 22–27; 2012Washington, DC, USA Poster TUPE096
- PatersonDLSwindellsSMohrJAdherence to protease inhibitor therapy and outcomes in patients with HIV infectionAnn Intern Med2000133213010877736
- ClayPGNagSGrahamCMNarayananSMeta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimensMedicine (Baltimore)201594e167726496277
- BezabheWMChalmersLBereznickiLRPetersonGMAdherence to antiretroviral therapy and virologic failure: a meta-analysisMedicine (Baltimore)201695e336127082595
- Del CarmenTFreemanRSieglerELSinghHA qualitative analysis of inpatient geriatric consults in the aging HIV populationInnovation in Aging20171Suppl 1835
- Martin-IguacelRLlibreJMFriis-MollerNRisk of cardiovascular disease in an aging HIV population: where are we now?Curr HIV/ AIDS Rep20151237538726423407
- NachegaJBHsuAJUthmanOASpinewineAPhamPAAntiret-roviral therapy adherence and drug-drug interactions in the aging HIV populationAIDS201226Suppl 1S39S5322781176
- CherrySBBennerJSHusseinMATangSSNicholMBThe clinical and economic burden of nonadherence with antihypertensive and lipid-lowering therapy in hypertensive patientsValue Health20091248949718783393
- van OnzenoortHAMengerFENeefCVerberkWJKroonAAde LeeuwPWvan der KuyPHParticipation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort studyHypertension20115857357821825228
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials198671771883802833
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ200332755756012958120
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ19973156296349310563
- TennantSJHesterEKCaulderCRLuZKBookstaverPBAdherence among rural HIV-infected patients in the deep south: a comparison between single-tablet and multi-tablet once-daily regimensJ Int Assoc Provid AIDS Care201514647125331217
- CohenCJMeyersJLDavisKLAssociation between daily antiret-roviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIVBMJ Open20133e003028
- CookeCELeeHYXingSAdherence to antiretroviral therapy in managed care members in the United States: a retrospective claims analysisJ Manag Care Pharm201420869224372462
- KaufTLDavisKLEarnshawSRDavisEASpillover adherence effects of fixed-dose combination HIV therapyPatient Prefer Adherence2012615516422399848
- LangnessJCookPFGillJBoggsRNetsanetNComparison of adherence rates for antiretroviral, blood pressure, or mental health medications for HIV-positive patients at an academic medical center outpatient pharmacyJ Manag Care Spec Pharm20142080981425062074
- RogatoFMartinezCRodriguez SagradoMAAdherence in HIV-positive patients treated with single tablet regimens versus recommended once daily multi-pill regimen in clinical practice: findings from the international iSTRAP studyPresented at the 21st International AIDS ConferenceJuly 18–22; 2016Durban, South Africa Abstract THPEB072
- RaffiFYazdanpanahYFagnaniFLaurendeauCLafumaAGourmelenJPersistence and adherence to single-tablet regimens in HIV treatment: a cohort study from the French National Healthcare Insurance DatabaseJ Antimicrob Chemother2015702121212825904729
- SaxPEMeyersJLMugaveroMDavisKLAdherence to antiret-roviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United StatesPLoS One20127e3159122384040
- SuttonSSHardinJWBramleyTJD’SouzaAOBennettCLSingle-versus multiple-tablet HIV regimens: adherence and hospitalization risksAm J Manag Care20162224224827143289
- TanejaCJudayTGertzogLAdherence and persistence with non-nucleoside reverse transcriptase inhibitor-based antiretroviral regimensExpert Opin Pharmacother2012132111211822970926
- CohenCJMolinaJMCassettiIWeek 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III random-ized trialsAIDS20132793995023211772
- JosephsonFAnderssonMCFlamholcLThe relation between treatment outcome and efavirenz, atazanavir or lopinavir exposure in the NORTHIV trial of treatment-naive HIV-1 infected patientsEur J Clin Pharmacol20106634935719967342
- LathouwersEDe MeyerSDierynckIVirological characterization of patients failing darunavir/ritonavir or lopinavir/ritonavir treatment in the ARTEMIS study: 96-week analysisAntivir Ther2011169910821311113
- NelsonMGirardPMDemasiRSuboptimal adherence to daruna-vir/ritonavir has minimal effect on efficacy compared with lopinavir/ ritonavir in treatment-naive, HIV-infected patients: 96 week ARTEMIS dataJ Antimicrob Chemother2010651505150920498120
- SaxPEDeJesusEMillsACo-formulated elvitegravir, cobi-cistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeksLancet20123792439244822748591
- BaxiSMGreenblattRMBacchettiPNevirapine concentration in hair samples is a strong predictor of virologic suppression in a prospective cohort of HIV-infected patientsPLoS One201510e012910026053176
- ViswanathanSJusticeACAlexanderGCAdherence and HIV RNA suppression in the current era of highly active antiretroviral therapyJ Acquir Immune Defic Syndr20156949349825886923
- WilkinsELCohenCJTrottierBPatient-reported outcomes in the single-tablet regimen (STaR) trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/teno-fovir disoproxil fumarate in antiretroviral treatment-naive adults infected with HIV-1 through 48 weeks of treatmentAIDS Care20162840140826489045
- BonoraSCalcagnoAViganoOEfficacy, tolerability and viro-logical consequences of long-term use of unboosted atazanavir plus 2 NRTIs in HIV-infected patientsCurr HIV Res20141233934625106410
- DragovicGSalemovicDRaninJNikolicJKusicJJevtovicDClinical and immunologic outcomes of HAART-treated HIV-infected women in resource constrain settings: the Belgrade studyWomen Health201454354724555810
- Hernandez ArroyoMJCabrera FigueroaSESepulveda CorreaRImpact of a pharmaceutical care program on clinical evolution and antiretroviral treatment adherence: a 5-year studyPatient Prefer Adherence2013772973923983457
- JayaweeraDDejesusENguyenKLGrimmKButcherDSeekinsDWVirologic suppression, treatment adherence, and improved quality of life on a once-daily efavirenz-based regimen in treatment-Naive HIV-1-infected patients over 96 weeksHIV Clin Trials20091037538420133268
- JulianFSMartinPEricksonSRValidation of the special projects of national significance adherence tool in HIV/AIDS patientsAnn Pharmacother2010441003100920442352
- MartinMDel CachoECodinaCRelationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort studyAIDS Res Hum Retroviruses2008241263126818834323
- PodzamczerDRozasNDomingoPACTG-HIV symptoms changes in patients switched to RPV/FTC/TDF due to previous intolerance to CART. Interim analysis of the PRO-STR studyJ Int AIDS Soc2014174 Suppl 31981425397558
- Torres-CornejoABenmarzouk-HidalgoOJGutierrez-ValenciaACellular HIV reservoir replenishment is not affected by blip or intermittent viremia episodes during darunavir/ritonavir monotherapyAIDS20142820120824361681
- ChakrabortyHWeissmanSDuffusWAHIV community viral load trends in South CarolinaInt J STD AIDS20163131
- DeJesusERockstrohJKHenryKCo-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trialLancet20123792429243822748590
- RiceTRosenauPUnruhLYBarnesAJSaltmanRBvan GinnekenEUnited States of America: health system reviewHealth Syst Transit2013151431
- SrivastavaKAroraAKatariaACappelleriJCSadoskyAPetersonAMImpact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysisPatient Prefer Adherence2013741943423737662
- CohenCJColsonAESheble-HallAGMcLaughlinKAMorseGDPilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) studyHIV Clin Trials20078192317434845
- Antiretroviral Therapy Cohort CollaborationSurvival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studiesLancet HIV20174e349e35628501495
- TrancartSCharreauIMarchouBPresence of lamivudine or emtricitabine is associated with reduced emergence of nonnucleoside reverse transcriptase inhibitor mutations in an efavirenz-based intermittent antiretroviral treatment regimenAntimicrob Agents Chemother2012561655165722203586
- BangsbergDRRaglandKMonkADeeksSGA single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed peopleAIDS2010242835284021045636
- ReisACPrista GuerraMLencastreLTreatment adherence, quality of life and clinical variables in HIV/AIDS infectionJ Int AIDS Soc200013Suppl 4119
- SaagMSBensonCAGandhiRTAntiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA PanelJAMA201832037939630043070