Abstract
Purpose
Allergen immunotherapy (AIT), when continued for 3 years, is the only disease-modifying treatment for AR and asthma. Adherence is a key to ensure effectiveness, and poor adherence is a contraindication for AIT. The objective of this study was to evaluate real-world adherence to AIT with subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) preparations in patients allergic to grass or tree pollen. The impact of AIT on the consumption of asthma and rhinitis medication was also analyzed.
Patients and Methods
In this retrospective cohort analysis of a German longitudinal prescription database, the adherence of a grass and tree pollen allergoid was examined and compared to two sublingual AIT tablets/drops. Patients receiving grass or tree allergen-specific immunotherapy prescriptions were compared with non-AIT patients receiving symptomatic allergic rhinitis (AR) and asthma prescriptions. The study endpoints included therapy adherence, AR progression, and asthma progression. Multivariate regression analyses were used to estimate the effects of SCIT or SLIT, adjusting for variables related to demographics and prescriptions.
Results
SCIT adherence was 60.1–61.8% at 2 years and 35.0–37.5% at 3 years for the two allergens. SLIT adherence was distinctly lower (29.5–36.5% and 9.6–18.2%, respectively). Adherence in children was higher compared to adolescents or adults. All products were highly efficacious at reducing symptomatic AR medication consumption. SCIT also reduced asthma medication use for both allergens, whereas for SLIT these results were significant only for grasses but not trees.
Conclusion
Subcutaneous AIT in a real-world setting achieved significantly higher adherence rates compared to sublingual administration. SCIT reduced the use of rhinitis and asthma medication significantly for both allergens, while SLIT reduced the use of rhinitis medication for both allergens and the use of asthma medication for grasses only.
Introduction
Allergic rhinitis (AR) is a common chronic disease with the potential for major impact on a patient’s quality of life (QOL) and the risk of developing asthma.Citation1 Over the last decade, allergic rhinitis has been on the rise,Citation2 and its overall prevalence is expected to further increase in the future.Citation3–Citation5 Like house dust mite allergies, pollen allergies (grasses, trees) have reached prevalence levels of more than 20%.Citation6
AR places a considerable burden on patients, which can be observed as sleep disturbance, decreased productivity, and poor school performance.Citation7–Citation9 Individuals with allergic rhinitis and/or asthma also suffer more frequently from temperature-related exacerbations of health problems than individuals without AR.Citation10
Furthermore, allergic rhinitis patients frequently have asthma or non-specific bronchial hyper-responsiveness, whereas up to 80% of asthma patients also suffer from AR.Citation11 While the direct ecologic association of pollen exposure with asthma prevalence is unclear and has yet to be confirmed,Citation12 treatment of AR may also improve asthma control in children and adolescents.Citation13
The recommended methods for treating allergic rhinitis are as follows: 1) avoiding contact with allergens, 2) symptomatic medications, and 3) allergen-specific immunotherapy.Citation14 Allergen immunotherapy (AIT) is the only treatment for AR and asthma with a disease-modifying effect.Citation15
Continuous immunotherapy for a period of at least 3 years (as recommended by international guidelines) modifies the underlying course of the disease and may achieve long-term remission of symptoms for several years without further need for AIT treatment.Citation16,Citation17
AIT has traditionally been administered by subcutaneous injection (SCIT) of high-dose allergen preparations. SCIT is administered by a physician during recurrent office visits, particularly during the induction phase, and requires post-injection observation. Sublingual preparations (SLIT) can be administered at home following the initial supervised office-administered dose, which may be more convenient for certain patients.
As AIT requires repeated administration of the vaccine for at least 3 years in order to achieve clinical effectiveness, adherence is a major problem, from the perspective of patients, providers, and payers.Citation15,Citation18 However, adherence to AIT is characterized by widely varying discontinuation rates.Citation19–Citation21 Based on controlled and observational studies that have provided adherence information with regard to different treatment times, overall discontinuation rates were approximated from treatment year one to treatment year three for SCIT (22%, 34%, and 26%) and SLIT (42%, 29%, and 27%).Citation22
Data from controlled studies are partially biased, as patients are strictly selected, instructed, and observed. Drop-outs result in further bias, and, as a consequence, data is excluded from the final study results. More reliable data can be derived from real-life studies.Citation19 Senna et al reported that SCIT real-world studies included overall larger populations and longer durations, whereas only one SLIT study featured adherence data for a period of more than 3 years.Citation19
Comparable real-life data for different AIT administration routes are still scarce. Therefore, the objective of this real-world evidence study was both to analyze real-world data for SCIT and SLIT over at least three continuous years of treatment with regard to adherence and to examine the impact on allergic rhinitis and asthma medication prescription as an indicator of persisting symptoms.
Patients and Methods
Overall Study Design
The database used for the current analysis was IMS LRx® (IQVIA, Frankfurt am Main, Germany), which is based on about 60% of German statutory health care prescriptions. The overall analysis period was January 2008 to February 2017.
For the AIT groups, Allergovit® was chosen as the most prescribed SCIT product in the study period for both pollen allergens (grasses, trees), and two tablets (Grazax® and Oralair®) for grasses and two liquids (Staloral® and Sublivac®) for trees were chosen to represent SLIT. For AR, symptomatic medications consisting of nasal corticosteroids (NCS, ATC: R01A1) and antihistamines (ATC: R06A0) were chosen since these represent a fair share of prescription-only medications in Germany (Table S1 in eSupplement). Prescriptions were no longer required for NCS starting in March 2017, so that the study was only conducted including data through February 2017.
The disease definition for AR (Table S2 in eSupplement) was extended for the separate analyses of children alone because OTC medication is reimbursed in such patients in Germany. Of the remaining ATC classes, nasal antiallergic medication (ATC: R01A6) was also included when identifying AR. Furthermore, when analyzing the progression of this disease, ophthalmic products for treating conjunctivitis were added since rhinitis often occurs in association with conjunctivitis. The relevant classes included were ophthalmic corticosteroids (ATC: S01B0, S01C1) and, for the analyses of children, ophthalmic anti-allergic preparations (ATC: S01G1-S01G3).
The identification of asthma (Table S3 in eSupplement) was based on short-acting ß-agonists (SABA, ATC: R03A2, R03A4), inhaled corticosteroids (ICS), either alone (ATC: R03D1) or combined with long-acting beta-agonists (ATC: R03F1), and leukotriene receptor antagonists (ATC: R03J2). The asthma index definition was based on two asthma medication prescriptions since it was intended to record the onset of the disease. The asthma index date was thus defined as the date of the first of two asthma prescriptions in the same or successive grass or early bloomer pollen seasonal cycles.
Datasets and Proxy Clinical Data
The longitudinal prescription database (LRx) collects pharmacy data from data centers where the prescriptions of all German patients with statutory health insurance coverage are processed for reimbursement purposes. Data entries covered patient-specific data over time, including each patient’s anonymized identification number, age, sex, insurance company, and region of living, as well as such prescription information as the prescriber’s specialty, prescription date, and package information.
Analysis Time Periods
Pollen allergies are strictly seasonal, occurring only when the causal agent is flourishing. The main season for early bloomer trees is February through April, while the one for grasses is May through August, with a slight spread around these months. Since trees and grasses only overlap slightly, patients receiving symptomatic medication can be separated based on prescription times. Therefore, it is important to restrict the identifying prescriptions to those in a period when medication would not be taken to relieve symptoms induced by other allergens. To allow for a certain stagger between the main seasons in different years for a given allergen, the grass season was defined as May through September, the period for trees as January through May, with the time since last season being the preseason and both together the seasonal cycle.
The intention was to evaluate the effect of AIT on AR and asthma medication consumption prior to vs. at least 2 years after treatment. Patient history was thus split into a pre-AIT, during AIT, and a follow-up period. For the test groups, the treatment period ranged from the dates of first focus AIT prescription (designated as the “index date” in the time between 10/2009 and 9/2013 for grasses and 6/2009–5/2013 for trees) to the expiry of the last such prescription. The 18 months prior to the index date represented the pre-treatment period, and the entire interval after AIT up to the release of NCS to the OTC market (February 28, 2017) was designated as the follow-up period.
For the control group, the challenge was to select a random segment of a patient’s AR history while avoiding placing the index date at the beginning of this period. The rationale for this was that AIT patients would not normally receive AIT if suffering from light AR and would have had a history of symptomatic AR treatment prior to the index date.
Patients and Inclusion/Exclusion Criteria
The adherence analyses were intended to assess the adherence for all patients thus treated and included patients with low adherence and those who switched between products. Patients were included if they had received a first prescription of a relevant AIT medication between 9/2009 and 8/2013 (grasses) or 6/2010-5/2013 (trees), with no such medication being prescribed in the 730 days prior to the index date. In the case of an AIT product switch, only the first grass or tree product in the patient’s history was assessed. Patients with a single focus prescription in the database were eliminated. For products with a recommended treatment gap (e.g., Oralair®), this was allowed for when looking for treatment breaks to assess adherence and resulted in adding 120 days (8-month treatment according to product information) to the expected duration of the last prescription in each season except the very last prescription in the patient’s history.
For the other analyses, in order to compare the AIT and control patient groups, measures were taken to ensure a period of symptomatic treatment before the index date in both the AIT and the control patients. AIT patients (test groups) were selected based on at least two relevant grass or early bloomer AIT prescriptions in two successive seasonal cycles. Patients in the control group were required to have had three symptomatic AR prescriptions in successive pollen seasons, the second of these marking the index date. For both patient groups and allergens, a gap of at most one seasonal cycle between successive identifying prescriptions was permitted. The AIT patients were also required to have had a symptomatic prescription in the 18 months prior to the index date. All patients were thus selected based on three prescriptions in three to four seasonal cycles.
For the analyses of children only, the requirement for a symptomatic AR prescription prior to the index date was dropped since, due to the increased efficacy of AIT in younger patients, children are more likely to be put on AIT right at the start of their history of (prescription-bound) AR.
Various exclusion criteria were applied to both patient groups. Index dates were only accepted if they fell in the period between 10/2009 and 9/2013 (grasses) or between 6/2009 and 5/2013 (trees) to ensure sufficient database observability before and after the treatment period. AIT patients switching between AIT products for the same allergen or receiving other forms of AIT (e.g., for mites) were excluded, and control patients were not permitted to have even a single AIT prescription of any kind in their history. Patients had to be 5–50 years old on the index date, and patients with perennial asthma (receiving ICS or theophylline-containing medication in three successive 3-month periods) or severe asthma (receiving anti-IgE or anti-IL5 biologicals) were eliminated (summary of the attrition process in Figures S1 and S2 in eSupplement).
Study Endpoints
Study endpoints included the following: (1) adherence defined as the time from the first focus Rx to the expiry of the last focus Rx, (2) days on therapy (DoT) defined as the number of treatment days during the adherence period, (3) AR progression based on the comparison of the annual number of symptomatic rhinitis or conjunctivitis prescriptions post-treatment vs. pre-index date, and (4) asthma progression based on the comparison of the annual number of asthma prescriptions post-index vs. pre-index in patients with asthma at baseline. A treatment gap of more than 90 days between focus prescriptions (expiry of previous prescription to dispensation of next) or a switch to another AIT product for the same allergen resulted in premature termination of the adherence period. The descriptive analysis of adherence was carried out using Kaplan–Meier curves.
Statistical Testing
Adherence to therapy was tested between products and between different age groups within a product by using a Log-rank test. This test shows any differences between at least two curves, so pairwise comparisons were conducted. The resulting p-values were corrected using Bonferroni adjustment to ensure that the overall Type I/Type II error remained at or below 5%.
The progressions of AR and asthma were both tested using Poisson regression with a log link function and a dispersion factor, including confounding variables (age class, sex, region, doctor speciality, N seasons AIT, AA/asthma treatment level pre-index) as covariates. The number of relevant prescriptions in the analysis phase was compared between the groups, correcting for the patient-specific duration of this phase as one of the confounder variables.
SAS 9.4 software was used for statistical testing. All tests were considered statistically significant at the 5% level (p <0.05).
Ethical Statement
German law allows the use of anonymous electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data.
Because patients were only queried as aggregates and no protected health information was available for queries, no IRB approval was required for the use of this database or the completion of this study.
Results
In the adherence/DoT analyses, 16,774 and 11,931 SCIT patients vs. 29,183 and 10,698 SLIT patients were analyzed in the grass and tree groups, respectively. According to the inclusion/exclusion criteria, the patients for the progression analyses represented a subset of patients used for the adherence/DoT analyses that were treated for a longer time. Therefore, in SLIT, 11.3% (grass) or 14.6% (trees) of the patients in the adherence analyses remained for analyses of efficacy parameters; in SCIT, 34.4% (grass) or 52.5% (trees) were available for the analyses.
In the AR/asthma analyses, the equivalent counts were 5775 and 6263 for SCIT, 3293 and 1565 for SLIT, and 90,175 and 82,655 for the control group. The demographic and prescription-related characteristics at the index date for patients in the AR/asthma-related analyses are shown in . AIT patients were younger; male patients and patients treated by specialists were found more often in the AIT than in the control group. The follow‐up periods were very similar for the test and control groups but marginally longer in the AIT groups for grasses and in the control group for trees.
Table 1 Demographic and Prescription‐Related Characteristics of Patients in the SCIT, SLIT, and Control Groups (Patient Counts, Percentages in Parentheses Unless Otherwise Stated)
Adherence
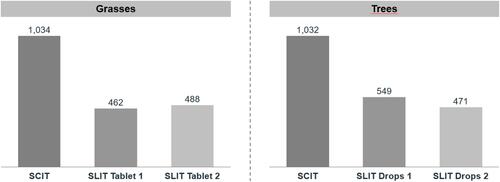
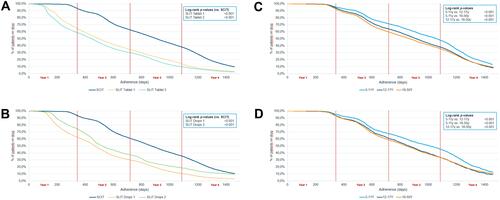
For both pollen types, adherence to SCIT treatment was high throughout the first and into the second year ( and ). Adherence dropped more rapidly after around 500 days, reaching 61.8% and 60.1% by the end of the second and 37.5% and 35.0% by the end of the third year for grasses and trees, respectively. Adherence to SLIT was significantly lower in both grass (29.6–33.7% at 2 years, 9.6–13.4% at 3 years) and tree patients (29.5–36.9% at 2 years, 10.3–18.2% at 3 years) (p<0.001 for all SLIT comparisons vs. SCIT). When SCIT patients were analyzed by age group, children had the highest adherence rates, followed by adolescents, and finally adults (47.0%, 37.7%, and 35.2%, respectively, at 3 years for grasses, 44.6%, 33.0% and 33.0%, respectively, at 3 years for trees) ( and ). The differences were statistically significant over the course of the analysis period (p<0.001 for all paired comparisons). Accordingly, SCIT also showed a considerably greater number of days on therapy than the SLIT forms for both grasses and trees ().
Figure 1 (A) Kaplan–Meier curves for adherence in patients allergic to grass pollen: SCIT (n = 16,774) vs. SLIT tablets (n1 = 11,705; n2 = 17,478), with the results of the log-rank comparisons between the curves. (B) Kaplan–Meier curves for adherence in patients allergic to tree pollen: SCIT (n = 11,931) vs. SLIT (n1 = 8034; n2 = 2664), with the results of the log-rank comparisons between the curves. (C) Kaplan–-Meier curves for adherence in SCIT grass pollen patients by age class (overall n = 14,920; 5–11y n = 2988; 12–17y n = 2966; 18–50y n = 8966), with the results of the log-rank comparisons between the curves. (D) Kaplan–Meier curves for adherence in SCIT tree pollen patients by age class (overall n = 9585; 5–11y n = 1425; 12–17y n = 1252; 18–50y n = 6908), with the results of the log-rank comparisons between the curves.
Progression of Allergic Rhinitis
On average, after adjustment for confounders, patients treated with SCIT required 64.8% (95% CI: 62.2–67.2, p<0.0001) fewer prescriptions for grass pollen allergens and 56.0% (CI: 53.0–58.9, p<0.0001) fewer prescriptions for tree allergens than the respective control groups following the treatment period. The equivalent values for SLIT were 53.6% (CI: 49.2–57.7, p<0.0001) for grass allergens and 46.5% (CI: 39.4–52.9, p<0.0001) for tree allergens. When only children were analyzed, these values were similar for grass allergens (SCIT: 60.7%, CI: 57.2–64.0, p<0.0001; SLIT: 50.8%, CI: 45.4–55.6, p<0.0001) but somewhat lower for tree allergens (SCIT: 42.3%, CI: 36.5–47.6, p<0.0001; SLIT: 36.8%, CI: 26.5–45.7, p<0.0001) ().
Figure 3 Poisson regression analysis of the mean number of allergic rhinitis (AR) medications during the follow-up period for SCIT and SLIT grass and tree products compared with a non-AIT control group (n = 90,175 [grasses] or 82,655 [trees] patients aged 5–50 years; n = 37,059 [grasses] or 25,167 [trees] children aged 5–12 years).
![Figure 3 Poisson regression analysis of the mean number of allergic rhinitis (AR) medications during the follow-up period for SCIT and SLIT grass and tree products compared with a non-AIT control group (n = 90,175 [grasses] or 82,655 [trees] patients aged 5–50 years; n = 37,059 [grasses] or 25,167 [trees] children aged 5–12 years).](/cms/asset/525169f9-4eb4-4266-863a-458a2b6e3c6c/dppa_a_12178462_f0003_c.jpg)
Progression of Asthma
Among patients allergic to grass pollen, both AIT forms reduced the need for asthma medication, though SCIT proved superior to SLIT among all patients (SCIT: 14.0% reduction vs. control, CI: 7.5–20.0, p<0.0001; SLIT: 10.6% reduction vs. control, CI: 1.6–18.9, p=0.0225), as well as in the sub-group of children (SCIT: 27.4% reduction vs. control, CI: 19.9–34.3, p<0.0001; SLIT: 21.0% reduction vs. control, CI: 10.6–30.2, p=0.0002). Among patients allergic to tree pollen, only SCIT patients showed a significant treatment reduction both for all patients (SCIT: 9.3% reduction vs. control, CI: 3.3–14.9, p=0.0027; SLIT: 5.8% increase vs. control, CI: −6.3–19.5, p=0.3662) and in the sub-group of children (SCIT: 14.1% reduction vs. control, CI: 5.3–22.1, p=0.0024; SLIT: 4.0% reduction vs. control, CI: −18.0–12.4, p=0.6116) ().
Figure 4 Poisson regression analysis of the mean number of asthma medications during the follow-up period for SCIT and SLIT grass and tree products compared with a non-AIT control group (n = 23,262 [grasses] or 21,203 [trees] patients aged 5–50 years; n = 10,467 [grasses] or 7331 [trees] children aged 5–12 years).
![Figure 4 Poisson regression analysis of the mean number of asthma medications during the follow-up period for SCIT and SLIT grass and tree products compared with a non-AIT control group (n = 23,262 [grasses] or 21,203 [trees] patients aged 5–50 years; n = 10,467 [grasses] or 7331 [trees] children aged 5–12 years).](/cms/asset/fe54e271-6be2-429f-beea-1b7f9c2f0b04/dppa_a_12178462_f0004_b.jpg)
Discussion
In DBPC trials, AIT using both routes of application (SCIT and SLIT) has been proven to ameliorate symptom scores, medication scores, and combinations of both in patients with allergic diseases (rhinitis, asthma) while on treatment, as well as to deliver long-term clinical benefits that may persist for years after discontinuation of treatment.Citation23 Therefore, AIT is the only disease-modifying therapy for allergen-induced AR.Citation15 This has mainly been shown for grass pollen preparations. Three to 4 years of treatment with an unmodified SCIT preparation resulted in long-term efficacy for 3 years after discontinuation.Citation24 For a grass pollen tablet, long-term effectiveness and reduction in asthma symptoms have been proven in a pediatric population.Citation25 In a controlled trial in children, 3 years of pre-seasonal treatment with an allergoid preparation indicated advantages for the AIT treated patients in a twelve-year follow-up setting.Citation26
This treatment success depends on a therapy length of three continuous years.Citation27,Citation28 Our retrospective cohort analysis of a German longitudinal prescription database is the first to provide comparative real-world evidence on therapy adherence, AR progression, and asthma development in patients receiving SCIT or SLIT and in non-AIT patients suffering from grass and tree pollen-induced AR.
In our RWE analysis, SCIT adherence in Germany was >60% at the end of the second and >35% at the completion of the third year for grasses and early bloomers, respectively. Children exhibited the highest adherence rates, followed by adolescents and adults.
Our data compare favorably with a recent German statutory sick-fund data analysis in children and adolescents (between 7 and 15 years at treatment initiation), which observed a 3-year persistence of 45% for SCIT.Citation29 When contrasted with German prescription sales data from 2007 to 2011 with a total of 66%, 60%, and 42% of patients with pollen SCIT receiving AIT for 1, 2, and 3 years, respectively, our adherence data were only slightly lower. Again, identical to our RWE data, SCIT adherence was higher in children and adolescents (57% and 49%) compared to adults (41%).Citation30 A recent smaller retrospective mono-center study in SCIT patients in Turkey observed an adherence of 65% and a non-persistence of 28% after 1 year. In this specific setting, one-third of the non-persistent patients stated that their discontinuation was due to financial reasons.Citation31 In contrast, AIT treatments are 100% reimbursed by German statutory healthcare funds (including approximately 90% of patients) and private healthcare providers. Therefore, income and socioeconomic status are very unlikely to have influenced prescriptions of or adherence to AIT in this study.
Adherence to SLIT was significantly lower both at 2 years (29.6–33.7%, 29.5–36.9%) and after 3 years (9.6–13.4%, 10.3–18.2%) for grass and early bloomer patients, respectively. Our RWE SLIT data are lower compared to the results of most of the available shorter studies.
A 3-year observational study with subjective reporting of SLIT adherence observed a share of 55% adherent patients after 3 years.Citation32 However, the authors also stated that adherence was at risk to be overestimated due to subjective measurement. Furthermore, results showed a high variation in adherence between clinics due to potential differences between clinics related to interest and literacy to handle AIT patients.Citation32 In a German mono-center study, SLIT patients exhibited a higher dropout rate (39.0%, N=123) than SCIT patients (32.4%, N=207). However, these rates, which comprised a wide range of different allergen extracts in a well-organized practice with private patients only, did not differ significantly.Citation33 Varying adherence rates in other studies comparing SCIT and SLIT applications are also due to different definitions of “adherence” or “persistence”. In our study, adherence was defined as the time from the first focus Rx to the expiry of the last focus Rx. In a study using data of the German IMS Health Disease Analyzer database, Allam et al defined persistence as >1 prescription of the medication in both the second and third treatment years over the three-year follow-up period leading to comparable three-year persistence between both application forms.Citation34
Similar to our results, Wang et al found that 54% of AR patients terminated their treatment within the first year of SLIT. The top reasons for treatment discontinuation included patients not being reachable (25%), ineffectiveness (24%), and length of treatment course (18%).Citation35 Our results are also in line with data from a recent RCT-trial by Scadding et al that covered longer time spans, where only 47% of SLIT participants took more than 90% of the doses over the 2-year period compared with 82% for SCIT.Citation36
Observed adherence rates for SCIT are comparable with other chronic diseases. Approximately 50% of patients with cardiovascular disease have poor adherence to their prescribed medications.Citation37 Since AR is a debilitating but not life-threatening disease, a strong need for further adherence improvement in order to gain the full benefit from AIT is of paramount importance, not only for patients but also for statutory health insurance bodies (SHI).Citation29
According to Baiardini et al, successful strategies for improving adherence should combine different interventions (ie, education, counseling, more convenient care, self-monitoring, reinforcement, and reminders).Citation38 Sanchez observed that patients who were less involved in participative treatment choices had a higher dropout rate for SLIT than those who received subcutaneous AIT. Dropout rates were similar when patients were actively involved in AIT treatment selection.Citation39
Based on German SHI data, Breitkreuz et al suggested switching to the SLIT administration mode despite being technically unable to analyze or publish SLIT adherence rates when providing SCIT results from an AOK SHI-data source.Citation29 According to our results, a presumed better adherence to SLIT cannot be confirmed at all, neither for children and adolescents nor for adults. In contrast, our results showed that SLIT adherence in patients covered by German SHI funds was considerably lower than SCIT adherence, despite adding 120 days for SLIT products when allowing for a “planned treatment gap” from September to December.
In Europe, real-world evidence studies using different prescription databases showed benefits of grass tablet treatments with regard to reduced dispensing of AR and asthma medication,Citation40 slower AR progression, reduced risk of new asthma onset in non-asthmatic patients,Citation41 and slower asthma progression.Citation42
In our RWE analysis, patients treated with SCIT required significantly fewer prescriptions (65%) for grass allergies and fewer prescriptions (56%) for allergies to early bloomers than the respective control groups following AIT. Corresponding values for SLIT were 54% and 47%, respectively. With regard to impact on asthma progression, SCIT trended toward being superior to SLIT among all grass allergy patients, an effect that was even more pronounced in early bloomer allergy patients, among which only those treated with SCIT showed a significant treatment reduction compared to the control group (all patients and children). Effectiveness results were calculated only for those patients who were adherent for at least 2 years, resulting in 11.3%/14.6% of SLIT patients vs. 34.4%/52.5% of SCIT patients who matched this minimum adherence target in the grass or tree treatment groups. Again, despite excluding more uncompliant and unresponsive patients for SLIT products, results for SCIT were better.
In particular, the effect in children contrasted with SLIT study results in a controlled Dutch primary care setting where during a two-year trial in youngsters with a pollen allergy, SLIT was not able to achieve any reduction in medication.Citation43 Furthermore, when compared to SLIT with regard to quality of life, only SCIT could demonstrate a meaningful and statistically significant clinical improvement in QOL in a “real-world” clinical setting, which may also be due to the poor adherence to SLIT.Citation44
Adherence to treatment is necessary for improving the efficacy of treatment in patients with allergic respiratory diseases, reducing healthcare costs, and for minimizing the disease’s burden on a patient’s life.Citation38 Overall, studies examining the pharmacoeconomics of AIT have been able to demonstrate cost savings.Citation45 However, if full treatment success is expected with only three continuous seasonal cycles,Citation27,Citation28 early patient discontinuation considerably impacts the midterm cost-effectiveness of AIT, as the investment in year one or even year two without reaching year three could be considered a lost opportunity and non-recoverable cost. The discontinuation rate for SLIT treatments considerably depends on the number of visits to the physician per year.Citation46 Vita et al showed that SLIT discontinuation in the first and second year was 15% and 18% for those with at least two annual visits, whereas patients with only one visit withdrew in 29% and 41% of all cases.Citation46 Based on the observed longer adherence of SCIT patients, cost-effectiveness comparisons based on real-world evidence probably favor SCIT.Citation22
In particular, RWE studies comprise patient populations that are far more representative than those in RCTs, can have very large sample sizes and can provide information on treatment practices and outcomes in specific populations that are rarely included in DBPC trials, such as children.Citation47 The IMS LRx® database as the underlying data source of this controlled retrospective cohort study comprises prescription data of 60% of all German statutory health insurance patients. Though diagnoses (ICD information) are not available, disease states can be approximated based on disease-specific prescription data.Citation42 Therefore, thanks to an adjustment for age, sex, physician specialty, number of seasons of AIT, AR treatments before AIT treatment, and region, we were able to report representative real-world outcomes of AIT with high external validity.Citation48 Our data cover an analysis of the treatment period as well as a pre-treatment (18 months) and a follow-up period of 2 years, thus providing a complete overview of the relevant AR patient history.
Since the present study used secondary data, some limitations should be mentioned at this point. First, the database used does not provide diagnoses, so the approximation of AR and AA, despite being meticulously specified with guideline-based treatment schemes, may not fully reflect the disease states being studied. This might also have led to a bias of more severe AR cases being included in the SCIT cohort, as in real-world office settings, allergic conditions are occasionally being treated on a trial-and-error basis unless a convincing diagnosis exists.Citation49 However, this selection bias would have discriminated SCIT against non-AIT patients. Second, selection based on published allergy season cycles only may have resulted in a slightly biased patient selection, as patients with overlapping time spans or patients outside this time span may not have been assigned correctly. However, this selection of the time span prevented the inclusion of subjects allergic to house dust mites. Nevertheless, our analysis may have also contained some patients in whom the prime allergy trigger was neither early bloomer pollen nor grass pollen. Finally, due to the database being restricted to reimbursed medications, OTC products are not included in this analysis. Therefore, changes in patients’ full drug consumption behavior could not be analyzed.
Conclusion
Subcutaneous AIT in a real-world setting achieved significantly higher adherence compared to SLIT. SCIT for both allergens (grasses, trees) significantly reduced rhinitis and asthma medication consumption, whereas SLIT reduced rhinitis medication for both and asthma medication for grasses only.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was funded by the Allergopharma GmbH & Co. KG.
Disclosure
C. Vogelberg has received lecture or consulting fees from ALK-Abelló, Allergopharma, AstraZeneca, Boehringer Ingelheim, Bencard Allergy, DBV Technologies, Novartis Pharma, and Sanofi Aventis. M. Jutel has received consulting fees from ALK-Abelló, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Circassia, LETI Pharma, Biomay, HAL Allergy, Astra-Zeneca, GSK, Novartis, Teva, Vectura, UCB, Takeda, Roche, Janssen, Medimmune, and Chiesi. B. Brüggenjürgen reports receiving grants from Allergopharma during this study.
H. Richter reports consultancy fees from IQVIA. The authors have no other relevant affiliations or financial involvements with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Ozdoganoglu T, Songu M, Inancli HM. Quality of life in allergic rhinitis. Ther Adv Respir Dis. 2012;6(1):25–39. doi:10.1177/1753465811424425
- Goksor E, Wennergren G, Vasileiadou S, et al. Increased prevalence of allergic rhinitis in young men in Western Sweden. Eur Res J. 2018;52 Supp:1.
- Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81(12):1440–1446.
- Punekar YS, Sheikh A. Establishing the incidence and prevalence of clinician-diagnosed allergic conditions in children and adolescents using routinely collected data from general practices. Clin Exp Allergy. 2009;39(8):1209–1216. doi:10.1111/j.1365-2222.2009.03248.x
- Calderon MA, Demoly P, Gerth van Wijk R, et al. EAACI: a European declaration on immunotherapy. Designing the future of allergen specific immunotherapy. Clin Transl Allergy. 2012;2(1):20. doi:10.1186/2045-7022-2-20
- Bousquet P-J, Chinn S, Janson C, et al. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European community respiratory health survey I. Allergy. 2007;62(3):301–309. doi:10.1111/j.1398-9995.2006.01293.x
- Bousquet J, Bullinger M, Fayol C, Marquis P, Valentin B, Burtin B. Assessment of quality of life in patients with perennial allergic rhinitis with the French version of the SF-36 health status questionnaire. J Allergy Clin Immunol. 1994;94(2 Pt 1):182–188. doi:10.1053/ai.1994.v94.a54939
- Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004;113(4):663–668. doi:10.1016/j.jaci.2003.12.589
- Bousquet J, Schunemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130(5):1049–1062. doi:10.1016/j.jaci.2012.07.053
- Hyrkas-Palmu H, Ikaheimo TM, Laatikainen T, Jousilahti P, Jaakkola MS, Jaakkola JJK. Cold weather increases respiratory symptoms and functional disability especially among patients with asthma and allergic rhinitis. Sci Rep. 2018;8(1):10131. doi:10.1038/s41598-018-28466-y
- Navarro A, Valero A, Julia B, Quirce S. Coexistence of asthma and allergic rhinitis in adult patients attending allergy clinics: ONEAIR study. J Investig Allergol Clin Immunol. 2008;18(4):233–238.
- Marchetti P, Pesce G, Villani S, et al. Pollen concentrations and prevalence of asthma and allergic rhinitis in Italy: evidence from the GEIRD study. Sci Total Environ. 2017;584–585:1093–1099. doi:10.1016/j.scitotenv.2017.01.168
- de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67(7):582–587. doi:10.1136/thoraxjnl-2011-201168
- Huang FL, Liao EC, Yu SJ. House dust mite allergy: its innate immune response and immunotherapy. Immunobiology. 2018;223(3):300–302. doi:10.1016/j.imbio.2017.10.035
- Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi:10.1111/all.13317
- Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: world allergy organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. doi:10.1186/1939-4551-7-6
- Halken S, Larenas-Linnemann D, Roberts G, et al. EAACI guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28(8):728–745. doi:10.1111/pai.12807
- Manzotti G, Riario-Sforza GG, Dimatteo M, Scolari C, Makri E, Incorvaia C. Comparing the compliance to a short schedule of subcutaneous immunotherapy and to sublingual immunotherapy during three years of treatment. Eur Ann Allergy Clin Immunol. 2016;48(6):224–227.
- Senna G, Caminati M, Lockey RF. Allergen immunotherapy adherence in the real world: how bad is it and how can it be improved? Curr Treat Options Allergy. 2015;2:14. doi:10.1007/s40521-014-0037-6
- Incorvaia C, Mauro M, Leo G, Ridolo E. Adherence to sublingual immunotherapy. Curr Allergy Asthma Rep. 2016;16(2):12. doi:10.1007/s11882-015-0586-1
- Senna G, Ridolo E, Calderon M, Lombardi C, Canonica GW, Passalacqua G. Evidence of adherence to allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9(6):544–548. doi:10.1097/ACI.0b013e328332b8df
- Bruggenjurgen B, Reinhold T. Cost-effectiveness of grass pollen subcutaneous immunotherapy (SCIT) compared to sublingual immunotherapy (SLIT) and symptomatic treatment in Austria, Spain, and Switzerland. J Med Econ. 2018;21(4):374–381. doi:10.1080/13696998.2017.1419959
- Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72(11):1597–1631. doi:10.1111/all.13201
- Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi:10.1056/NEJM199908123410702
- Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141(2):529–538 e513. doi:10.1016/j.jaci.2017.06.014
- Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61(2):198–201. doi:10.1111/j.1398-9995.2006.01011.x
- Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41(9):1177–1200. doi:10.1111/j.1365-2222.2011.03794.x
- Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long-term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. 2018;5(3):275–290. doi:10.1007/s40521-018-0176-2
- Breitkreuz J, Witte C, Zahn T. Saisonale Pollenallergien bei Kindern und Jugendlichen - Inanspruchnahme und Therapiepersistenz der spezifischen Immuntherapie. Monit Versorgungsforschung. 2019;2019(1):7.
- Egert-Schmidt AM, Kolbe JM, Mussler S, Thum-Oltmer S. Patients’ compliance with different administration routes for allergen immunotherapy in Germany. Patient Prefer Adherence. 2014;8:1475–1481. doi:10.2147/PPA.S70326
- Tat T. Adherence to subcutaneous allergen immunotherapy in Southeast Turkey: a real-life study. Med Sci Monit. 2018;24:21. doi:10.12659/MSM.910860
- Kiotseridis H, Arvidsson P, Backer V, Braendholt V, Tunsater A. Adherence and quality of life in adults and children during 3-years of SLIT treatment with grazax-a real life study. NPJ Prim Care Respir Med. 2018;28(1):4. doi:10.1038/s41533-018-0072-z
- Lemberg ML, Berk T, Shah-Hosseini K, Kasche EM, Mosges R. Sublingual versus subcutaneous immunotherapy: patient adherence at a large German allergy center. Patient Prefer Adherence. 2017;11:63–70. doi:10.2147/PPA.S122948
- Allam JP, Andreasen JN, Mette J, Serup-Hansen N, Wustenberg EG. Comparison of allergy immunotherapy medication persistence with a sublingual immunotherapy tablet versus subcutaneous immunotherapy in Germany. J Allergy Clin Immunol. 2018;141(5):1898–1901 e1895. doi:10.1016/j.jaci.2017.12.999
- Wang T, Li Y, Wang F, Zhou C. Nonadherence to sublingual immunotherapy in allergic rhinitis: a real-life analysis. Int Forum Allergy Rhinol. 2017;7(4):389–392. doi:10.1002/alr.21909
- Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317(6):615–625. doi:10.1001/jama.2016.21040
- Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis. 2013;55(6):590–600. doi:10.1016/j.pcad.2013.02.001
- Baiardini I, Novakova S, Mihaicuta S, Oguzulgen IK, Canonica GW. Adherence to treatment in allergic respiratory diseases. Expert Rev Respir Med. 2018;13:1–10.
- Sanchez J. Adherence to allergen immunotherapy improves when patients choose the route of administration: subcutaneous or sublingual. Allergol Immunopathol (Madr). 2015;43(5):436–441. doi:10.1016/j.aller.2014.04.011
- Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: a retrospective database study in France. Allergy. 2019;74(7):1317–1326. doi:10.1111/all.13705
- Devillier P, Wahn U, Zielen S, Heinrich J. Grass pollen sublingual immunotherapy tablets provide long-term relief of grass pollen-associated allergic rhinitis and reduce the risk of asthma: findings from a retrospective, real-world database subanalysis. Expert Rev Clin Immunol. 2017;13(12):1199–1206. doi:10.1080/1744666X.2017.1398082
- Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. 2018;73(1):165–177. doi:10.1111/all.13213
- Röder E, Berger MY, Hop WCJ, Bernsen RMD, de Groot H, Gerth van Wijk R. Sublingual immunotherapy with grass pollen is not effective in symptomatic youngsters in primary care. J Allergy Clin Immunol. 2007;119(4):892–898. doi:10.1016/j.jaci.2006.12.651
- Schwanke T, Carragee E, Bremberg M, Reisacher WR. Quality-of-life outcomes in patients who underwent subcutaneous immunotherapy and sublingual immunotherapy in a real-world clinical setting. Am J Rhinol Allergy. 2017;31(5):310–316. doi:10.2500/ajra.2017.31.4465
- Wallace D. Allergen immunotherapy: efficacy, persistence, consistency, and cost effectiveness. RUDN J Med. 2018;22(3):14. doi:10.22363/2313-0245-2018-22-3-288-301
- Vita D, Caminiti L, Ruggeri P, Pajno GB. Sublingual immunotherapy: adherence based on timing and monitoring control visits. Allergy. 2010;65(5):668–669. doi:10.1111/j.1398-9995.2009.02223.x
- Camm AJ, Fox KAA. Strengths and weaknesses of ‘real-world’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart. 2018;5(1):e000788. doi:10.1136/openhrt-2018-000788
- Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force report–part I. Value Health. 2009;12(8):1044–1052. doi:10.1111/j.1524-4733.2009.00600.x
- Himmel W, Hummers-Pradier E, Schümann H, Kochen MM. The predictive value of asthma medications to identify individuals with asthma–a study in German general practices. Br J Gen Pract. 2001;51(472):879.