Abstract
Purpose
Treatment continuation is considered an important measure of antipsychotic effectiveness in schizophrenia, reflecting the medication’s efficacy, safety, and tolerability from both patients’ and clinicians’ perspectives. This study identified characteristics of patients with schizophrenia who continue olanzapine therapy for a 1-year period in Japan.
Methods
In a large (N = 1850), prospective, observational study, Japanese patients with schizophrenia who initiated treatment with olanzapine were followed for 1 year. Baseline characteristics were compared using t-tests and chi-square tests. Stepwise logistic regression was used to identify independent baseline predictors of treatment continuation.
Results
Most patients (68.2%) continued with olanzapine therapy for the full 1-year study period, with an average duration of 265.5 ± 119.4 days. At baseline, patients who continued were significantly more likely to be male, older, and inpatients; have longer illness duration, higher negative and cognitive symptoms, better health-related quality of life, and prior anticholinergic use. Continuers were significantly less likely to engage in social activities, live independently, work for pay, or have prior antidepressant use. Continuers showed significantly greater early (3-month) improvement in global symptom severity. Logistic regression found that continuation was significantly predicted by longer illness duration, lower positive symptoms, higher negative symptoms, and better health-related quality of life.
Conclusions
In this large naturalistic study in Japan, most patients with schizophrenia stayed on olanzapine therapy for the full 1-year study period. Treatment completion with olanzapine was independently predicted by longer illness duration, lower positive symptoms, higher negative symptoms, and better health-related quality of life.
Introduction
Schizophrenia is a chronic and disabling mental illness that is associated with cognitive, behavioral, social, and occupational impairments.Citation1 The primary recommended treatment for the acute symptoms of schizophrenia is antipsychotic medication.Citation2,Citation3 When assessing outcomes from treatment with antipsychotic medication, a variety of domains have been studied, including the core symptoms of schizophrenia,Citation4–Citation6 functional outcomes,Citation7,Citation8 cognition,Citation9,Citation10 and health care resource use.Citation11,Citation12 However, a simple measure of treatment effectiveness that is relevant to different stakeholders and broadly reflects meaningful outcomes in usual clinical care is needed.
One overall measure of effectiveness for antipsychotic treatment outcome in schizophrenia is time to all-cause medication discontinuation, a measure that captures the medication’s efficacy, safety, and tolerability from both patients’ and physicians’ perspectives.Citation13,Citation14 The Clinical Antipsychotic Trials of Intervention Effectiveness, a large, independent, publicly funded, randomized clinical trial in the USA, used time to all-cause medication discontinuation as its primary outcome variable.Citation13,Citation15 Similarly, the European First Episode Schizophrenia Trial also used this as the study’s primary outcome variable.Citation16 Patients who continue with an anti-psychotic longer have been shown to have better clinical and functional outcomes and a reduced risk of relapse and hospitalization.Citation12,Citation13,Citation17,Citation18
Multiple studies have demonstrated that medication discontinuation is sensitive to differences in outcomes between antipsychotics. Olanzapine therapy in particular, has shown significantly lower rates of discontinuation than ziprasidone,Citation13,Citation15,Citation19–Citation22 quetiapine,Citation13,Citation15,Citation21,Citation23–Citation29 risperidone,Citation11–Citation13,Citation19,Citation24,Citation26,Citation30–Citation37 aripiprazole,Citation4,Citation27,Citation38 as well as typical antipsychotics.Citation7,Citation9–Citation12,Citation13,Citation16,Citation19,Citation24,Citation26,Citation35,Citation39–Citation44
Few studies have attempted to identify baseline characteristics of patients who stay longer on therapy in Japan. Previous studies outside of Japan have identified predictors of antipsychotic continuation in schizophrenia such as male gender,Citation28,Citation45,Citation46 older age,Citation13,Citation25,Citation28,Citation47,Citation48 fewer psychiatric hospitalizations,Citation28 lack of substance-use disorder,Citation47,Citation49,Citation50 better therapeutic alliance,Citation49 greater reduction in symptoms,Citation17,Citation18 and greater improvements in health-related quality of life (HRQOL).Citation17,Citation18 Whether these findings generalize to the Japanese health care system, which utilizes inpatient treatment relatively frequently,Citation51,Citation52 is unclear. Using data from a 1-year naturalistic observational study of patients with schizophrenia in Japan, this study aimed to identify baseline characteristics that differentiate patients who completed 1 year of olanzapine therapy from patients who discontinued olanzapine therapy.
Methods
Sample selection
The data for this study came from the Olanzapine Post Marketing Surveillance (OPMS) study. OPMS was a large multicenter naturalistic 1-year study that took place in Japan and consisted of 1850 patients who met the study entry criteria. To be eligible to take part in the study, participants had to have been diagnosed with schizophrenia and to have initiated treatment with olanzapine. The diagnosis for schizophrenia was based on criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders published by the American Psychiatric Association.Citation1 During this naturalistic study, all treatment decisions, including the decision to initiate olanzapine, were left to the discretion of the treating physician. Study enrollment started in November 2003 and was completed in July 2004. The follow-up period for the study continued for 1 year after enrollment or until the patient discontinued the treatment with olanzapine. Data were collected at the baseline, 3-month, 6-month, and 12-month visits. The procedures were approved by the internal review boards at each of the participating institutions. Informed consent for this observational study was acquired based on the requirements at each institution.
Measures and definitions
The primary dependent variable in this study was a dichotomous variable indicating if a patient continued with or discontinued olanzapine before the end of the 1-year study. Patients who left the study were classified as discontinuing olanzapine.
In this observational study, simple noninvasive measures were chosen to limit interference with usual clinical practice. Patient symptomatology was measured using the Clinical Global Impression-Schizophrenia (CGI-SCH) scale. The CGI-SCH scale consists of ratings for positive, negative, cognitive, and depressive symptoms as well as a global severity rating.Citation53 The ratings for the CGI-SCH scale are anchored and range from no symptoms (0) to severe symptoms (6).Citation53 Assessment of the concurrent validity between the CGI-SCH and the Positive and Negative Syndrome Scale found that the validity coefficients ranged from 0.61 for depressive symptoms to 0.86 for positive symptoms, with the remaining coefficients ranging from 0.75–0.80.Citation54
Consistent with prior research, treatment response was operationally defined based on the CGI-SCH Global Severity rating.Citation55 For patients with a baseline score between 4 and 6 points, treatment response was defined as a 2-point improvement. For patients with a baseline score between 1 and 3, treatment response was defined as a 1-point improvement.
HRQOL was assessed using the European Quality of Life 5-Dimensions Visual Analog Scale (EQ-5D VAS).Citation56 The EQ-5D VAS ranges from 0 to 100 and has demonstrated moderate concurrent validity (ranging from 32 to 62) with the various scales of the World Health Organization Quality of Life – Brief instrument.Citation57
Prior medication use was coded with indicator variables (Yes/No) for each of the following medication classes: antipsychotics, anticholinergics, antidepressants, anxiolytics/ hypnotics, mood stabilizers, or other. The presence of a baseline medical comorbidity was coded based on the presence of any of the following conditions: hypertension, hyperlipidemia, hepatic dysfunction, renal dysfunction, or other. Participation in social activities was coded if the patient indicated they had participated in any social activities in the past 4 weeks. Working for pay was coded if the patient indicated working with income at baseline. Finally, living independently was coded if the patient indicated living independently at baseline rather than being hospitalized, homeless, living communally with persons caring for the patient, or living as a dependent family member.
Statistical methods
Differences in baseline patient characteristics were compared using t-tests for continuous variables and chi-square tests for categorical variables. Stepwise logistic regression was used to identify independent baseline predictors of continuing olanzapine therapy. lists all of the baseline predictor variables available to the stepwise logistic regression model. However, the CGI-SCH Global Severity scale was excluded from the stepwise logistic regression due to multicolinearity with the subscales. The significance level was set at α = 0.05 and all analyses were completed using SAS 9.1.3 (SAS Institute Inc, Cary, NC).
Table 1 Baseline univariate characteristics
Results
Sample description
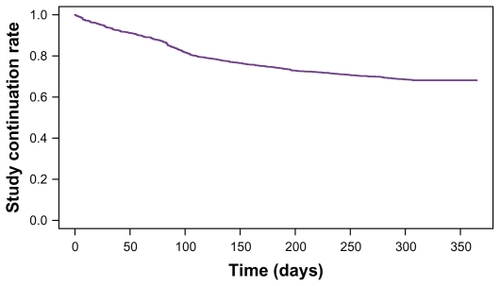
The OPMS study registered and enrolled 1949 patients. This analysis utilized the 1850 (94.9%) patients who met all study entry criteria: 27 were excluded for contract or registration violations, 20 had no case report forms, 49 did not return after the initial visit, and three did not initiate treatment with olanzapine. The baseline characteristics of this sample can be seen in . For the entire sample, the average age was 44.8 ± 15.5 years, 53.2% were male, 43.2% initiated olanzapine in an outpatient setting, and the average duration of illness was 18.3 ± 14.7 years. Most participants (68.2%; 1262/1850) continued the olanzapine treatment for the full 1-year study, while 31.8%; (588/1850) discontinued the study at some point (see ). The mean duration of olanzapine therapy during the 1-year study treatment was 265.5 ± 119.4 days.
Univariate comparisons between discontinuers and continuers
contrasts the baseline characteristics of patients who completed the 1-year study with olanzapine (continuers) with those who discontinued olanzapine treatment prior to the end of the study (discontinuers). Significant differences were observed in multiple demographics, clinical status variables, symptom measures, functional measures, and prior medication use variables (see ).
Multivariate comparisons between discontinuers and continuers
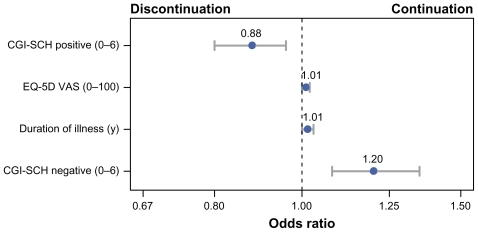
Stepwise logistic regression was used to identify independent predictors of continuation. The odds ratios and confidence intervals for the final version of the stepwise logistic regression model are presented in . Overall, the model was modestly accurate in differentiating patients who would continue or discontinue treatment with olanzapine (c-statistic = 0.635). The c-statistic indicated that the model could accurately classify a randomly selected continuer from a randomly selected discontinuer 63.5% of the time. Continuation was independently predicted by lower positive symptoms, higher negative symptoms, longer illness duration, and better HRQOL. All of the significant predictors were continuous variables; therefore, the odds ratios represent the change in odds of continuation for every one-unit increase in the predictor. For example, for every year that a patient had been diagnosed with schizophrenia, the odds of continuing with olanzapine increased by a factor of 1.01.
Figure 2 Significant predictors of treatment continuation in the stepwise logistic regression analysis. This figure presents the odds ratios and 95% confidence intervals for each of the predictors of continuation in the final stepwise logistic regression model. Each predictor in the final model was a continuous variable; therefore, the odds ratios represent the change in odds of continuation for every one-unit increase in the predictor variable.
Finally, early change in CGI-SCH Global Severity, defined as the difference between baseline and the first visit (3 months), was compared between the continuers and discontinuers. Patients who continued with olanzapine treatment experienced greater improvements (decreases) in CGI-SCH Global Severity compared to those who discontinued olanzapine therapy (−0.69 vs −0.53; P = 001).
Discussion
In this large naturalistic observational study of patients with schizophrenia in Japan, most patients (68.2%) continued olanzapine therapy for the full 1-year study, with an average time to discontinuation of 265.5 days. A large number of baseline characteristics predicted the continuation in the univariate analyses, but only a few were found to be independent predictors in the multivariate analysis. In the stepwise logistic regression model, higher EQ-5D VAS scores, longer duration of illness, higher CGI-SCH negative scores, and lower CGI-SCH positive scores were significant predictors of treatment continuation. The olanzapine continuers had significantly greater early improvements in CGI-SCH Global Severity scores than the discontinuers.
The findings in the current study are consistent with some past schizophrenia research with outpatients outside of Japan. In the univariate analysis, older ageCitation13,Citation25,Citation27,Citation47,Citation48 and male genderCitation28,Citation44,Citation46 predicted antipsychotic continuation. The primary findings in the multivariate analysis were also consistent with previous research on predictors of medication continuation: longer duration of illness,Citation45 higher baseline HRQOL,Citation58 higher baseline negative symptoms,Citation58 and lower baseline positive symptomsCitation59 have all been identified as predictors of antipsychotic continuation. Interestingly, in one study, lower baseline positive symptoms were reported to predict discontinuation rather than continuation.Citation48 Inconsistencies in predictors of continuation may be a result of differences in study methodologies, outcome variables, the inclusion of inpatients, study population, or health care systems.
In addition to the identified patients’ baseline characteristics, poorer efficacy of antipsychotic medications has also been linked to the discontinuation of treatment. In fact, past research has consistently identified lack of efficacy as being one of the primary reasons for antipsychotic discontinuation.Citation13,Citation17,Citation21,Citation26,Citation45,Citation60–Citation63 Consistent with prior research, secondary analysis in the present study found that patients who continued antipsychotic treatment had greater reductions in symptoms.
Limitations
Although a large number of predictors were used in this analysis, some potentially important predictors of antipsychotic persistence were not included in this study. A lower level of substance abuse has been frequently demonstrated as being an important predictor of antipsychotic persistence.Citation47,Citation49,Citation50,Citation63–Citation67 Additionally, insight into illnessCitation67 as well as positive attitudes toward medicationCitation49,Citation68 have been identified as being potentially important in the predictors of antipsychotic persistence. The primary focus of this analysis was on baseline predictors of later antipsychotic persistence. Changes in metabolic and other tolerability parameters were not included in the analyses and may be important predictors of antipsychotic persistence. Furthermore, the OPMS study focused only on olanzapine-treated patients with schizophrenia in Japan, so the findings may not generalize to other antipsychotics or geographic locations.
Conclusion
Consistent with prior research, patients who continued olanzapine therapy were more likely to experience significant improvements in clinical outcomes compared to those who discontinued treatment.Citation18,Citation69 In addition, patients with certain baseline characteristics appear to be more likely to continue with olanzapine treatment for a longer period of time. Stepwise logistic regression revealed that, compared to discontinuers, those who continued with olanzapine therapy had significantly longer illness duration, lower baseline positive symptoms, higher baseline negative symptoms, and higher baseline HRQOL. Identifying these characteristics has clinical implications in usual care in Japan, as they may be useful in early detection of schizophrenia patients who could benefit from targeted interventions aimed at improving their persistence with medication, thus increasing the chance for better long-term treatment outcomes.
Acknowledgments
Funding for this study was provided by Eli Lilly and Company. Technical writing support was provided by Michael Stensland of Agile Outcomes Research Inc, Rochester, MN, and Susan Dennett of Strategic Health Outcomes Inc, Carmel, IN.
Disclosure
Wenyu Ye is a full-time employee of Lilly Suzhou Pharmaceutical Co, Shanghai, People’s Republic of China. Haya Ascher-Svanum is a full-time employee of Eli Lilly and Company, Indianapolis, IN, USA. Jennifer A Flynn and Yuka Tanji are full-time employees of Eli Lilly Japan, KK, Kobe, Japan. Michihiro Takahashi is a consultant for Eli Lilly Japan, KK. All authors are minor stockholders in Eli Lilly and Company.
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders DSM-IV-TR4th edWashington, DCAmerican Psychiatric Association2000
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia, second editionAm J Psychiatry20041612 Suppl15615000267
- FalkaiPWobrockTLiebermanJWorld Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 1: acute treatment of schizophreniaWorld J Biol Psychiatry20056313219116173147
- FleischhackerWWMcQuadeRDMarcusRNA double-blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophreniaBiol Psychiatry200965651051718986646
- GurejeOMilesWKeksNOlanzapine vs risperidone in the management of schizophrenia: a randomized double-blind trial in Australia and New ZealandSchizophr Res2003612–330331412729882
- TollefsonGDBeasleyCMJrTranPVOlanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trialAm J Psychiatry199715444574659090331
- JonesPBBarnesTRDaviesLRandomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1)Arch Gen Psychiatry200663101079108717015810
- PerlickDARosenheckRAKaczynskiRBinghamSCollinsJAssociation of symptomatology and cognitive deficits to functional capacity in schizophreniaSchizophr Res2008991–319219917851042
- KeefeRSYoungCARockSLOne-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophreniaSchizophr Res200681111516202565
- PurdonSEJonesBDStipENeuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidolArch Gen Psychiatry200057324925810711911
- GibsonPJDamlerRJacksonEAWilderTRamseyJLThe impact of olanzapine, risperidone, or haloperidol on the cost of schizophrenia care in a medicaid populationValue Health200471223514720128
- TunisSLFariesDENyhuisAWCost-effectiveness of olanzapine as first-line treatment for schizophrenia: results from a randomized, open-label, 1-year trialValue Health200692778916626411
- LiebermanJAStroupTSMcEvoyJPEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med2005353121209122316172203
- StroupTSMcEvoyJPSwartzMSTheNational Institute of Mental HealthClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol developmentSchizophr Bull2003291153112908658
- StroupTSLiebermanJAMcEvoyJPEffectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychoticAm J Psychiatry2006163461162216585435
- KahnRSFleischhackerWWBoterHEffectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trialLancet200837196181085109718374841
- Liu-SeifertHOsuntokunOOFeldmanPDFactors associated with adherence to treatment with olanzapine and other atypical antipsychotic medications in patients with schizophreniaCompr Psychiatry
- DavisSMStroupTSKochGGTime to all-cause treatment discontinuation as the primary outcome in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia StudyStat Biopharm Res201132253265
- BeasleyCMJrStaufferVLLiu-SeifertHAll-cause treatment discontinuation in schizophrenia during treatment with olanzapine relative to other antipsychotics: an integrated analysisJ Clin Psychopharmacol200727325225817502771
- BreierABergPHThakoreJHOlanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophreniaAm J Psychiatry2005162101879188716199834
- KinonBJLiu-SeifertHAdamsDHCitromeLDifferential rates of treatment discontinuation in clinical trials as a measure of treatment effectiveness for olanzapine and comparator atypical antipsychotics for schizophreniaJ Clin Psychopharmacol200626663263717110821
- StromBLEngSMFaichGComparative mortality associated with ziprasidone and olanzapine in real-world use among 18,154 patients with schizophrenia: The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC)Am J Psychiatry2011168219320121041245
- DeberdtWLipkovichIHeinlothANDouble-blind, randomized trial comparing efficacy and safety of continuing olanzapine versus switching to quetiapine in overweight or obese patients with schizophrenia or schizoaffective disorderTher Clin Risk Manag20084471372019209252
- DossenbachMPecenakJSzulcALong-term antipsychotic monotherapy for schizophrenia: disease burden and comparative outcomes for patients treated with olanzapine, quetiapine, risperidone, or haloperidol monotherapy in a pan-continental observational studyJ Clin Psychiatry200869121901191519012820
- GianfrancescoFRajagopalanKSajatovicMWangRHTreatment adherence among patients with schizophrenia treated with atypical and typical antipsychoticsPsychiatry Res20061442–317718917010448
- HaroJMSuarezDNovickDThree-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies resultsEur Neuropsychopharmacol200717423524417137759
- HattaKSatoKHamakawaHEffectiveness of second-generation antipsychotics with acute-phase schizophreniaSchizophr Res20091131495519553086
- MullinsCDObeidatNACuffelBJNaradzayJLoebelADRisk of discontinuation of atypical antipsychotic agents in the treatment of schizophreniaSchizophr Res2008981–381517596914
- NewcomerJWRatnerREErikssonJWA 24-week, multicenter, open-label, randomized study to compare changes in glucose metabolism in patients with schizophrenia receiving treatment with olanzapine, quetiapine, or risperidoneJ Clin Psychiatry200970448749919358783
- CooperDMoisanJGaudetMAbdousBGrégoireJPAmbulatory use of olanzapine and risperidone: a population-based study on persistence and the use of concomitant therapy in the treatment of schizophreniaCan J Psychiatry2005501490190816494259
- KilziehNTodd-StenbergJAKennedyAWoodAETappAMTime to discontinuation and self-discontinuation of olanzapine and risperidone in patients with schizophrenia in a naturalistic outpatient settingJ Clin Psychopharmacol2008281747718204345
- RascatiKLJohnsrudMTCrismonMLLageMJBarberBLOlanzapine versus risperidone in the treatment of schizophrenia: a comparison of costs among Texas Medicaid recipientsPharmacoeconomics2003211068369712828491
- RenXSQianSLeeAFHerzLMillerDRKazisLETreatment persistence: a comparison among patients with schizophrenia who were initiated on atypical antipsychotic agentsJ Clin Pharm Ther2006311576516476121
- StroupTSLiebermanJAMcEvoyJPEffectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: a CATIE studyAm J Psychiatry2007164341542717329466
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry2011168660360921362741
- YamakawaYTerashimaYTanoueYA survey to examine patterns of prescribing atypical antipsychotic drugs for outpatients with schizophreniaJpn J Clin Psychopharmacol20101311631176 Japanese
- ZhaoZA retrospective economic evaluation of olanzapine versus risperidone in the treatment of schizophreniaManag Care Interface2002152758111875967
- KernRSGreenMFCornblattBAThe neurocognitive effects of aripiprazole: an open-label comparison with olanzapinePsychopharmacology (Berl)2006187331232016810506
- Ascher-SvanumHZhuBFariesDLandbloomRSwartzMSwansonJTime to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophreniaBMC Psychiatry20066816504026
- GerraGDi PettaGD’AmoreACombination of olanzapine with opioid-agonists in the treatment of heroin-addicted patients affected by comorbid schizophrenia spectrum disordersClin Neuropharmacol200730312713517545747
- IshigookaJInadaTMiuraSOlanzapine versus haloperidol in the treatment of patients with chronic schizophrenia: results of the Japan multicenter, double-blind olanzapine trialPsychiatry Clin Neurosci200155440341411442893
- LiebermanJATollefsonGTohenMComparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidolAm J Psychiatry200316081396140412900300
- MarshallTSMcCombsJSStafkey-MaileyDImpact of patient selection criteria and treatment history on comparisons of alternative therapies: a case study of atypical antipsychoticsValue Health200912447348018798808
- TiihonenJWahlbeckKLönnqvistJEffectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up studyBMJ2006333756122416825203
- HodgsonRBelgamwarRAl-tawarahYMacKenzieGThe use of atypical antipsychotics in the treatment of schizophrenia in North StaffordshireHum Psychopharmacol200520214114715651052
- JungSHKimWHChoiHJFactors affecting treatment discontinuation and treatment outcome in patients with schizophrenia in Korea: 10-year follow-up studyPsychiatry Investig2011812229
- KreyenbuhlJSladeEPMedoffDRTime to discontinuation of first- and second-generation antipsychotic medications in the treatment of schizophreniaSchizophr Res20111311–312713221576008
- GaebelWRiesbeckMvon WilmsdorffMDrug attitude as predictor for effectiveness in first-episode schizophrenia: results of an open randomized trial (EUFEST)Eur Neuropsychopharmacol201020531031620202800
- TunisSLFariesDEStenslandMDHayDPKinonBJAn examination of factors affecting persistence with initial antipsychotic treatment in patients with schizophreniaCurr Med Res Opin20072319710417257471
- ChangCLTzengDSLungFWTreatment effectiveness and adherence in patients with schizophrenia treated with risperidone long-acting injectionPsychiatry Res20101801161920488552
- ShinfukuNTanCHPharmacotherapy for schizophrenic inpatients in East Asia – changes and challengesInt Rev Psychiatry200820546046819012132
- OshimaIMinoYInomataYHow many long-stay schizophrenia patients can be discharged in Japan?Psychiatry Clin Neurosci2007611717717239042
- HaroJMKamathSAOchoaSThe Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophreniaActa Psychiatr Scand Suppl2003416162312755850
- KaySRFiszbeinAOplerLAThe positive and negative syndrome scale (PANSS) for schizophreniaSchizophr Bull19871322612763616518
- HaroJMEdgellETNovickDEffectiveness of antipsychotic treatment for schizophrenia: 6-month results of the Pan-European Schizophrenia Outpatient Health Outcomes (SOHO) studyActa Psychiatr Scand2005111322023115701107
- EuroQol – a new facility for the measurement of health-related quality of lifeThe EuroQol GroupHealth Policy199016319920810109801
- KönigHHRoickCAngermeyerMCValidity of the EQ-5D in assessing and valuing health status in patients with schizophrenic, schizotypal or delusional disordersEur Psychiatry200722317718717142014
- NyhuisAWFariesDEAscher-SvanumHStaufferVLKinonBJPredictors of switching antipsychotic medications in the treatment of schizophreniaBMC Psychiatry2010107520920179
- HaroJMNovickDSuarezDRocaMAntipsychotic treatment discontinuation in previously untreated patients with schizophrenia: 36-month results from the SOHO studyJ Psychiatr Res200943326527318644606
- KahnRFleischhackerWWBoterHEffectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trialLancet200837196181085109718374841
- KinonBJLipkovichIEdwardsSBA 24-week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptomsJ Clin Psychopharmacol200626215716216633144
- Liu-SeifertHAdamsDHKinonBJDiscontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugsBMC Med200532116375765
- PerkinsDOGuHWeidenPJPredictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter studyJ Clin Psychiatry200869110611318312044
- Ascher-SvanumHNyhuisAFariesDHeilerLKinonBTreatment discontinuation following randomization to open-label olanzapine, risperidone or typical antipsychotics during a one-year treatment for schizophreniaClin Schizophr Relat Psychoses200823226234
- GilmerTPDolderCRFolsomDPMastinWJesteDVAntipsychotic polypharmacy trends among Medicaid beneficiaries with schizophrenia in San Diego County, 1999–2004Psychiatr Serv20075871007101017602020
- NovickDHaroJMSuarezDPerezVDittmannRWHaddadPMPredictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophreniaPsychiatry Res20101762–310911320185182
- ValensteinMGanoczyDMcCarthyJFMyra KimHLeeTABlowFCAntipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective reviewJ Clin Psychiatry200667101542155017107245
- LacroJPDunnLBDolderCRLeckbandSGJesteDVPrevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literatureJ Clin Psychiatry2002631089290912416599
- DunayevichEAscher-SvanumHZhaoFLonger time to antipsychotic treatment discontinuation for any cause is associated with better functional outcomes for patients with schizophrenia, schizophreniform disorder, or schizoaffective disorderJ Clin Psychiatry20076881163117117854239