Abstract
Purpose
To describe (1) the clinical profiles and the patterns of use of long-acting injectable (LAI) antipsychotics in patients with schizophrenia at risk of nonadherence with oral antipsychotics, and in those who started treatment with LAI antipsychotics, (2) health care resource utilization and associated costs.
Patients and methods
A total of 597 outpatients with schizophrenia at risk of nonadherence, according to the psychiatrist’s clinical judgment, were recruited at 59 centers in a noninterventional prospective observational study of 1-year follow-up when their treatment was modified. In a post hoc analysis, the profiles of patients starting LAI or continuing with oral antipsychotics were described, and descriptive analyses of treatments, health resource utilization, and direct costs were performed in those who started an LAI antipsychotic.
Results
Therapy modifications involved the antipsychotic medications in 84.8% of patients, mostly because of insufficient efficacy of prior regimen. Ninety-two (15.4%) patients started an LAI antipsychotic at recruitment. Of these, only 13 (14.1%) were prescribed with first-generation antipsychotics. During 1 year, 16.3% of patients who started and 14.9% of patients who did not start an LAI antipsychotic at recruitment relapsed, contrasting with the 20.9% who had been hospitalized only within the prior 6 months. After 1 year, 74.3% of patients who started an LAI antipsychotic continued concomitant treatment with oral antipsychotics. The mean (median) total direct health care cost per patient per month during the study year among the patients starting any LAI antipsychotic at baseline was €1,407 (€897.7). Medication costs (including oral and LAI antipsychotics and concomitant medication) represented almost 44%, whereas nonmedication costs accounted for more than 55% of the mean total direct health care costs.
Conclusion
LAI antipsychotics were infrequently prescribed in spite of a psychiatrist-perceived risk of nonadherence to oral antipsychotics. Mean medication costs were lower than nonmedication costs.
Introduction
Deviation from maintenance antipsychotic therapy remains a recurrent problem in the treatment of schizophrenia,Citation1 represents a major difficulty in the management of the disease, and jeopardizes the achievement of relevant clinical outcomes.Citation2–Citation5 Long-acting injectable (LAI) antipsychotics were developed specifically to promote adherence and enhance relapse prevention.Citation6 Despite 50 years of clinical experience, the knowledge base for examining their potential gains over oral antipsychotics remains inconclusiveCitation7–Citation10 and their use is confined to those patients who have suffered multiple relapses within the context of repeated episodes of nonadherence with antipsychotic medication.Citation11
Among other factors, the limited availability of second-generation LAI formulationsCitation12 and their high purchase costsCitation13 have been commonly cited as reasons hampering LAI antipsychotic prescription. Currently, when the spectrum of second-generation LAI antipsychotics is growing after years of experience with risperidone as the single novel agent available, updated data on the clinical use of LAI antipsychotics, and the associated health care costs, are welcome.
The present paper reports the results of a naturalistic prospective research done in a cohort of outpatients with schizophrenia whose treatment was modified because of a physician-perceived risk of nonadherence to oral antipsychotic therapy. Although this study put emphasis on evaluating the impact of therapeutic modifications on clinical outcomes in these patients, it also featured the secondary objective of providing comprehensive observational data on how LAI antipsychotics are initiated, what health care resources are utilized, and the total direct health care costs incurred by patients with schizophrenia switched to LAI antipsychotics. This article reports and comments the post hoc analyses and results related to this secondary objective.
Material and methods
Design and patients
This article concerns a post hoc exploratory analysis of the data collected in a 1-year prospective observational study of outpatients with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria who were considered to be at risk of nonadherence to oral antipsychotic medication by their treating psychiatrists. The primary objective was to evaluate the time to relapse in these patients undergoing any modification of their therapy,Citation14 but this study also aimed to assess how LAI antipsychotics are started and used. The aforementioned post hoc analyses involved the description of the resources utilized and the direct health care costs of patients as a result of therapeutic challenges related to adherence issues after starting treatment with LAI antipsychotics. Results of these analyses are also provided.
Patients were recruited on the basis of a modification of their therapy related to a psychiatrist-perceived risk of nonadherence to oral antipsychotic medications, according to their best clinical judgment. To homogenize their criteria, psychiatrists were asked to identify one or more of the following four features related to nonadherence in each patient: (1) poor insight, defined as a general total score ≥4 on the Scale to Assess Unawareness of Illness in Mental Disorders (SUMD);Citation15 (2) a negative attitude toward pharmacotherapy, defined as a score <0 in the 10-item Drug Attitude Inventory (DAI-10);Citation16 (3) documented history of nonadherence; or (4) lack of efficacy (documented clinical instability in the prior 6 months) or inadequate tolerability of current treatment. Therapy modifications could be either a dose adjustment; change or addition in antipsychotic pharmacotherapy; any modification in the use of other selected concomitant psychotropic drugs commonly prescribed to patients with schizophrenia (anticholinergic, antidepressant, anxiolytic, hypnotic, and/or mood stabilizing agents); or the initiation, change, or removal of nonpharmacologic therapies (day center attendance, outpatient support services, rehabilitation, psychotherapy, psychoeducation, and others). Among the changes in antipsychotic medication, the start of an LAI formulation was a possibility. Patients already on LAI antipsychotics were excluded from the study, as it focused on how these medications are started and used afterwards. Therapeutic decisions were not altered due to study participation and were taken at the entire discretion of the treating physician and the patient.
Recruitment took place in 59 mental health community centers in Spain from February to May, 2008. To ensure sample representativity, the number of centers was proportional to the updated regional census. All participants provided written informed consent and the study was approved by an ethical review board following applicable laws and regulations in Spain.
Treatment patterns and clinical evaluations
After the initial assessment, patients were evaluated again after approximately 3, 6, and 12 months, coinciding with their routine follow-up visits, and when they relapsed or withdrew from the study. The initial assessment included detailed data about therapy modifications for each of the three components mentioned, including the reasons suggested by treating psychiatrists. Information on several known potential risk factors for non-adherence and relapse was collected during a semistructured interview, including sociodemographic data, and psychiatric familial/personal history and comorbidities (). A battery of clinical instruments was also administered. Among others, these included the Premorbid Adjustment Scale,Citation17 the Clinical Global Impression scale-Severity (CGI-S),Citation18 the DAI-10, and the SUMD. Total scores below and above 0 in the DAI-10 denoted negative and positive attitudes towards medication, respectively, and patients with a general score ≥4 on the first three items of the SUMD were considered to have a poor level of insight. At follow-up visits, the investigators updated the information on patients’ therapy and again administered the clinical instruments. Relapse was defined as the occurrence, at any time during follow-up, of either (1) worsening of psychiatric symptoms that led to a patient’s hospitalization or withdrawal from study, or (2) an increase equal to or greater than 1 point in the CGI-S that resulted in a score ≥4.
Table 1 Subjects’ baseline characteristics according to prescription of long-acting antipsychotics
Patients’ patterns of antipsychotic prescription were evaluated to identify those who started treatment with an LAI antipsychotic. The aspects of LAI antipsychotic use evaluated included the proportion of patients concurrently treated with any oral antipsychotic, the duration of this concurrent use pattern, and doses and frequencies of treatment with each antipsychotic drug formulated as LAI.
Resource utilization and cost
Health care resource utilization was evaluated at each assessment point of the study. Structured abstraction forms were handled to participating psychiatrists to aid in this task. The information collected included the following three cost components: (1) resources: hospitalizations (psychiatric hospitals and general hospitals), emergency room, psychiatric outpatient consultations, other specialized outpatient consultations, visits to primary care services, skilled nursing facilities; (2) medications: antipsychotics and other psychotropic drugs; and (3) nonpharmacologic therapies: institutional support, assertive community treatment/case management, psychoeducation, psychotherapy, rehabilitation, and management programs for severe mental illness. Nonmedical (informal) direct costs were disregarded. To estimate costs, average unit costs published for Spain,Citation19 updated to reflect 2010 rates, were used for each of the resources ascertained; drug acquisition costs were assimilated to the weighted average retail price (plus value-added tax) per mg in the indication of schizophrenia and were calculated from the 2010 official registered prices.Citation20 Another published source was used for the costs of nonpharmacologic therapies,Citation21 which was supplemented with a Spanish database.Citation22
Data analysis
All data were analyzed descriptively and used the observations available at each time point (missing data were not imputed). This involved the calculation of descriptive statistics (means, medians, standard deviations, interquartile ranges, numbers, and frequencies) for study variables. Patients’ baseline characteristics stratified by prescription of LAI antipsychotics were calculated in the whole sample. The doses of each LAI antipsychotic were described throughout the study for patients who started an LAI antipsychotic at baseline. The Kaplan–Meier method was used to describe the time to relapse (in all patients) and the duration of concurrent use of oral antipsychotics with LAI antipsychotics. The Kaplan–Meier method allowed for the estimation of the duration of concurrent use of oral antipsychotics since the start of LAI antipsychotics, regardless of whether it was at baseline or during the study. Utilization of health care resources and mean direct health care cost components were assessed during the study year and expressed as mean and median averaged costs per month per patient for the 92 patients who started an LAI antipsychotic at baseline.
No sample size calculations were made in advance for these analyses. The sample size of the study was calculated at 607 patients, to have sufficient power to analyze the primary objective concerning the time to relapse.
Results
Patient disposition, characteristics, and therapy modifications at baseline
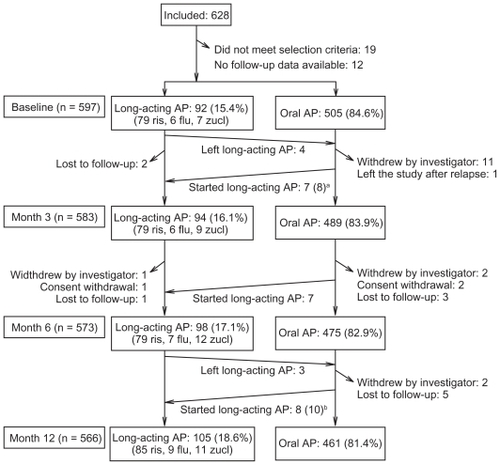
Six hundred and twenty-eight patients were recruited. Thirty-one were not analyzed because of the reasons provided in . The remaining 597 formed the study cohort; 566 patients completed the study.
Figure 1 Patients’ disposition throughout the study.
Abbreviations: AP, antipsychotics; flu, fluphenazine; ris, risperidone; zucl, zuclopenthixol.
provides the descriptive statistics of patients’ characteristics. They showed an unfavorable clinical profile, featuring severe psychopathology (CGI-S mean: 4.3, higher scores indicating more severity, scoring range: 1–7), frequent substance/alcohol use (30.2%) and hospitalizations (20.9% within the prior 6 months), poor insight (SUMD total: 6.0, higher scores indicating poorer insight, scoring range: 0–9), and quality of life (EQ-5D mean quality of life: 58.5, higher scores indicating better quality of life, scoring range: 0–100). Some of these characteristics, particularly patients’ subjective responses (attitudes) toward medications as measured with the DAI-10, differed between patients who started and did not start an LAI antipsychotic at baseline ().
Antipsychotic drugs were modified in 506 patients (84.8%), nonpharmacologic therapies were modified in 190 patients (31.8%), and concomitant psychotropic medications were modified in 92 patients (15.4%). The most common reasons alleged for modifying antipsychotic drugs were lack of efficacy (64.8%), tolerability issues (14.2%), and prior history of nonadherence (13.8%). Nonpharmacologic therapies were modified because of insufficient effectiveness (49.0%), lack of insight (32.1%), and documented nonadherence (11.6%).
Effectiveness and relapse
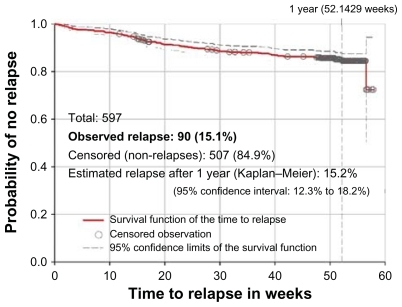
During the study year, the CGI-S scores improved by 0.7 points on average from baseline. In this period, 55 (9.2%) patients were hospitalized and 90 (15.1%) relapsed. The survival distribution function of the time to relapse () shows that relapses occurred at a constant pace throughout follow-up. The estimated cumulative incidence of relapse during 1 year, according to the Kaplan–Meier method was 15.2%. This incidence seemed lower than the proportion of patients who had been hospitalized only in the preceding 6 months (20.9%, ). Relapse affected 16.3% of patients who started and 14.9% of patients who did not start an LAI antipsychotic at baseline.
Use of antipsychotics
LAI antipsychotics were started at baseline in 92 out of 597 (15.4%) patients. The chosen agent was risperidone in most cases (79 patients, 85.9%), followed by fluphenazine (6 patients, 6.5%), and zuclopenthixol (7 patients, 7.6%). During the study year, 25 patients started (15 risperidone, 4 fluphenazine, and 6 zuclopenthixol) and seven discontinued LAI antipsychotics (). Only eight out of these 25 patients (32.0%) started the LAI antipsychotic within 1 month after a relapse. Of 31 patients who withdrew prematurely, five were on LAI treatment, giving a total of 105 out of 566 patients (18.6%) on LAI antipsychotics by the study end. Throughout the study, the mean modal dose of LAI risperidone was 50 mg every 2 weeks, and about one quarter of patients received higher doses (). The most common dosing regimens of LAI fluphenazine and zuclopenthixol were 25 mg every 3 weeks and 200 mg every 3 weeks, respectively.
Figure 3 Distribution of the doses of LAI risperidone at each study assessment among patients treated with this medication.
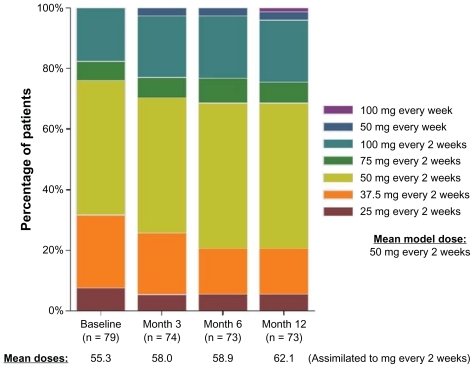
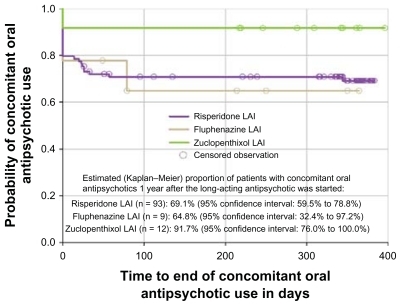
At baseline, 73 out of 92 patients who started an LAI antipsychotic (79.3%) maintained any concomitant oral antipsychotic drug (62 out of 79 patients started on LAI risperidone, four out of six patients started on LAI fluphenazine, and seven out of seven patients started on LAI zuclopenthixol). This proportion was quite similar 1 year after (78 out of 105 patients on LAI antipsychotics, 74.3%). The 1-year Kaplan–Meier estimates of concurrent use of oral antipsychotics were of 69.1% with LAI risperidone, 64.8% with LAI fluphenazine, and 91.7% with LAI zuclopenthixol (). Together with LAI risperidone started at baseline, the oral formulations of risperidone, olanzapine, quetiapine, and aripiprazole (accounting for more than 90% of concurrent oral antipsychotic medication) were given at median doses of 6 mg/day, 20 mg/day, 1200 mg/day, and 15 mg/day, respectively, throughout the study. Risperidone and olanzapine were also the most common oral agents used concomitantly with LAI fluphenazine and LAI zuclopenthixol, although at lower median doses (4 mg/day of risperidone and 10 mg/day of olanzapine).
Health resource utilization and direct health care costs
provides detailed results and the unit costs applied to resource utilization data to calculate direct costs per patient. The mean (median) total health care cost per patient per month during the study year among the 92 patients who started any LAI antipsychotic at baseline was €1407 (€897.7); this was greater among patients treated with LAI risperidone (€1487 [€998.4]) than with LAI fluphenazine (€938.3 [€147.1]), or LAI zuclopenthixol (€904.3 [€391.3]).
Table 2 Resource utilization and direct health care costs according to prescription of long-acting antipsychotics at baseline
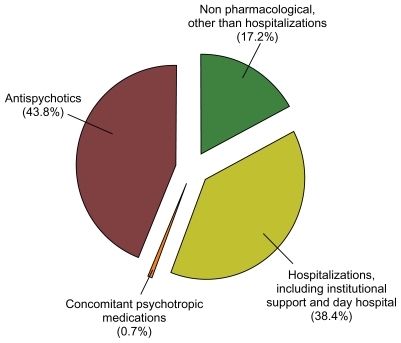
Medication costs represented 44.4% of mean total direct health care costs. LAI risperidone acquisition costs represented 39.4% of the mean total direct costs incurred by patients treated with this LAI formulation. Those from LAI fluphenazine or LAI zuclopenthixol accounted for no more than 1.2% of the mean total direct costs, whereas oral supplementation costs accounted for 6.4%, 10.8%, and 33.6% of the mean total direct costs incurred by patients on LAI risperidone, LAI fluphenazine, and LAI zuclopenthixol, respectively.
Nonmedication costs represented 55.6% of mean total direct health care costs. Inpatient (general and psychiatric hospitalizations) and outpatient hospitalizations (including institutional support and day hospital stays) costs represented 38.4% of the mean total direct costs (, ). Specifically, institutional support and day hospital stays represented a relevant contribution to mean total direct costs: €263.5 (€0) and €208.1 (€0), despite costs being incurred by only 7 and 18 patients, respectively. However, and despite their high unit costs, inpatient hospitalizations did not account for relevant costs because of their low incidence throughout the study year (10 out of 92 patients, 10.9%). provides a detailed breakdown of resource utilization data and costs.
Discussion
Patients included in this study showed an unfavorable clinical profile, including factors for bad prognosis that have been consistently associated with nonadherence. Importantly, the intervention made at baseline was associated with improved effectiveness, as denoted by the 9.2% of patients hospitalized during 1-year follow-up, compared with 20.9% of patients who had been hospitalized only within the prior 6 months. Although prestudy relapse incidence was not recorded, it is highly probable, based on the comparison of the pre-posthospitalization rates, that the relapse incidence during the prior year was well above the 15.1% observed during follow-up. Given the unfavorable profile and the selection procedure, prescription of LAI antipsychotics at baseline (15.4%) was lower than anticipated. More than 9 in 10 of these LAI prescriptions were of risperidone (the only second-generation agent commercially available as LAI when the data were collected), oral antipsychotic supplementation was maintained during 1 year in more than two thirds of patients, and the doses of both oral and LAI antipsychotics were close to their maximum-labeled doses and, therefore, associated with relevant costs. The information available on nonpharmacologic therapies, and health care resource utilization, indicates that the nonmedication direct costs of patients who started therapy with LAI antipsychotics in the year after the initial prescription were substantial, representing 55.6% of the mean total direct health care costs. Many of these facts are consistent with the relevant literature and have clinical implications.
The prescription of LAI antipsychotics has been considered too low in many studies investigating their use to date,Citation11–Citation13,Citation23 which is in agreement with the low utilization in this study. Together with the challenging clinical profile of the patients evaluated, these findings support the generalized view among psychiatrists that long-acting antipsychotics should be reserved for a small subgroup of patients and used only after a patient has repeatedly demonstrated difficulty adhering to an oral regimen and has had several relapses.Citation11,Citation24,Citation25 The cited studies also included nonadherent patients and, although they were performed in different cultural settings, the proportion of patients changed to long-acting antipsychotics was also low and similar to the 15.4% observed in this study (10.6%Citation25 and 17.6%Citation11). In this vein, the high doses of LAI antipsychotics and the extensive use of oral antipsychotic supplementation suggest a restrictive use pattern reserved for the patients hardest to treat.Citation26 This circumstance might also explain the doses employed; of note, more than one quarter of patients treated with LAI risperidone were above the maximum recommended dose of 50 mg every 2 weeks.Citation27
Strikingly, the authors observed similar relapse rates in patients receiving LAI antipsychotics than in patients treated with oral antipsychotics. Some explanations can be cited for this somewhat paradoxical finding. For instance, LAI-treated patients could be in a worse condition than non-LAI patients, but they did not relapse more as they benefited from LAI antipsychotics. Alternatively, nonpharmacologic therapies might also have been effective in preventing relapse in some patients on oral antipsychotics. Importantly, whatever the reason, this study shows that even in the most challenging patients, relapse risk reduction is still clinically feasible if the strategies best suited for each individual are used.
The widespread concurrent use of oral supplementation may also have a clinical reading, in particular considering that risperidone was the agent used in nearly all cases, whose delayed onset of action is known to be inconvenient.Citation28 Because oral supplementation therapy should be maintained for at least 3 weeks after starting long-acting risperidone,Citation27,Citation29 and because it was reserved for the most troublesome cases, the treating psychiatrists would refrain from ceasing oral therapy once a few weeks had elapsed since the switching, for fear of clinical destabilization of their more challenging patients,Citation30 leading to a prolonged polypharmacy. In contrast, a much lower concurrent use of oral antipsychotics with long-acting risperidone has recently been reported after 2 years in the electronic Schizophrenia Treatment Adherence Registry (e-STAR).Citation31 Possible reasons for this divergence include differences in disease severity, selection criteria, and LAI risperidone dosing. Patients in the e-STAR were not selected on the basis of nonadherence risk; some were receiving LAI risperidone before recruitment, and started and maintained it at lower doses than in the present study. It remains to be elucidated whether prolonged oral antipsychotic supplementation will be less common when other LAI second-generation antipsychotics that do not require an initial overlap with prior oral therapy are available. The prominent use of risperidone over first-generation LAI antipsychotics also supports the opinion that the unavailability of other second-generation LAI antipsychotics plays a role in their low utilization,Citation32,Citation33 as psychiatrists tend to prime the potential advantages of second-generation antipsychotics over the benefits of long-acting formulations.Citation34
Few patients started LAI antipsychotics immediately after a relapse. Consistently, psychiatrists questioned about their attitudes toward long-acting antipsychotics, stated that these are not an appropriate option after a relapse,Citation13 or for first-episode patients.Citation12,Citation13,Citation26 This study has focused on patients undergoing changes of their therapeutic strategy related to nonadherence risk and it cannot address the relevant question of when is the optimal moment for starting long-acting antipsychotic therapy. There are reports suggesting that patients with experience of long-acting antipsychotics have a good opinion on them,Citation35,Citation36 yet potential biases of patients established on LAI antipsychotics may distort this judgment.Citation32,Citation37 Future research should focus on the use of LAI antipsychotics in stable patients with a favorable disease course, as these have been recently suggested as potential candidates in a survey of psychiatrists’ opinions.Citation26
One reason alleged to avoid LAI antipsychotics is that they are associated with high treatment costs.Citation13 While not opposing this belief, the monthly direct health care cost per patient in this study was substantially higher than the reported average costs of schizophrenia in Spain.Citation19 However, the present study selected a sample of challenging patients who probably require more resources than the average patient with schizophrenia and, furthermore, we did not account for direct nonmedical costs, which in the cited study represented as much as 47% of total direct costs. Interestingly, such cost analysis was done prior to licensing of LAI risperidone. In an even more recent report, LAI risperidone proved to be a cost-effective strategy despite the higher drug acquisition costs, because of the considerable reduction in hospital stays.Citation38 Relapse is an important predictor of treatment costs for patients with schizophrenia. In the US Schizophrenia Care and Assessment Program (US-SCAP) study, the total direct health care costs of patients without any relapse were lower, but the costs of patients with multiple relapses more than doubled those reported by us.Citation39
This research has some limitations. It is based on post hoc analyses and the results should be regarded as exploratory. Comparisons of the outcomes of oral and long-acting second generation antipsychotics is a research priorityCitation7 but this study cannot provide comparative data as the patients were not randomized to either treatment option. The small number of patients treated with either LAI fluphenazine or LAI zuclopenthixol limits the accuracy of their results, which has precluded a detailed explanation for these patients, compared with that made for patients treated with LAI risperidone. This study did not address some concerns, which in addition to a delayed onset of action, surround the use of long-acting antipsychotics: poor definition of the dose-response relationship, facilities and skills required for their storage and administration, the experience of coercion, the clinical circumstances other than nonadherence and relapse that grant the use of these formulations, or how should the reverse switch (from long-acting to oral) can best be accomplished. Furthermore, this study focused on direct costs and did not therefore collect data on indirect costs, which are substantial in this pathology. Citation19 Future research should address these uncertainties and explore the role of long-acting antipsychotics for the maintenance of patients with good levels of insight, who are immersed in the recovery process.
Conclusion
This study provides current data on the clinical and economic outcomes of patients with schizophrenia at risk of nonadherence in clinical practice in Spain during the 12 months of the study period. It has shown that diminishing relapse in patients with long-standing schizophrenia involving therapeutic challenges related to nonadherence is feasible. It has also confirmed that long-acting antipsychotics are infrequently used. Most LAI antipsychotic prescriptions were of risperidone, the only atypical antipsychotic available as an LAI when the study was conducted. According to the observed mean total direct health care costs during the study period, medication costs were lower than nonmedication costs, the latter representing more than 55% of the mean total direct health care costs.
Acknowledgment
The authors thank Jesús Villoria from Medicxact who provided medical writing services for drafting and submitting this manuscript.
Disclosure
Miguel Bernardo has participated as a speaker and member of the advisory boards of, and has received grant/research support and honoraria from, Bristol-Myers-Squibb, Eli Lilly, Janssen-Cilag, Mylan, Organon, and Pfizer. Luis San has received grant/research support, received honoraria from, and participated as a speaker and is on the advisory boards of AstraZeneca, Bristol-Myers-Squib, Eli Lilly, Pfizer, Janssen, and Wyeth. José M Olivares has received honoraria from Janssen-Cilag, Lundbeck, AstraZeneca, Eli Lilly, Bristol-Myers-Squibb, Sanofi-Aventis, and Pfizer and has participated in advisory boards organized by Eli Lilly, Janssen-Cilag, and AstraZeneca. Antonio Ciudad, María Álvarez, Tatiana Dilla, Pepa Polavieja, and Inmaculada Gilaberte are full-time employees of Lilly, SA, an affiliate of Eli Lilly and Company.
References
- LacroJPDunnLBDolderCRLeckbandSGJesteDVPrevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literatureJ Clin Psychiatry2002631089290912416599
- Ascher-SvanumHFariesDEZhuBErnstFRSwartzMSSwansonJWMedication adherence and long-term functional outcomes in the treatment of schizophrenia in usual careJ Clin Psychiatry200667345346016649833
- LawMRSoumeraiSBRoss-DegnanDAdamsASA longitudinal study of medication nonadherence and hospitalization risk in schizophreniaJ Clin Psychiatry2008691475318312037
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of Intervention Effectiveness (CATIE) InvestigatorsEffectiveness of antipsychotic drugs in patients with chronic schizophrenia [published correction appears in N Engl J Med. 2010;363(11):1092–1093]N Engl J Med2005353121209122316172203
- MueserKTMcGurkSRSchizophreniaLancet200436394262063207215207959
- DavisJMMatalonLWatanabeMDBlakeLMetalonLDepot antipsychotic drugs. Place in therapyDrugs19944757417737520856
- PatelMXTaylorMDavidASAntipsychotic long-acting injections: mind the gapBr J Psychiatry Suppl200952S1419880911
- RosenheckRAKrystalJHLewRCSP555 Research GroupLong-acting risperidone and oral antipsychotics in unstable schizophreniaN Engl J Med2011364984285121366475
- LeuchtCHeresSKaneJMKisslingWDavisJMLeuchtSOral versus depot antipsychotic drugs for schizophrenia-A critical systematic review and meta-analysis of randomised long-term trialsSchizophr Res20111271–3839221257294
- TiihonenJHaukkaJTaylorMHaddadPMPatelMXKorhonenPA nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophreniaAm J Psychiatry2011168660360921362741
- WestJCMarcusSCWilkJCountisLMRegierDAOlfsonMUse of depot antipsychotic medications for medication nonadherence in schizophreniaSchizophr Bull2008345995100118093962
- HeresSReichhartTHamannJMendelRLeuchtSKisslingWPsychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophreniaEur Psychiatry201126529730120570493
- HeresSHamannJKisslingWLeuchtSAttitudes of psychiatrists toward antipsychotic depot medicationJ Clin Psychiatry200667121948195317194274
- CiudadASanLBernardoMRelapse and therapeutic interventions in a 1-year observational cohort study of nonadherent outpatients with schizophreniaProg Neuropsychopharmacol Biol Psychiatry2011
- AmadorXFFlaumMAndreasenNCAwareness of illness in schizophrenia and schizoaffective and mood disordersArch Gen Psychiatry199451108268367944872
- HoganTPAwadAGEastwoodRA self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validityPsychol Med19831311771836133297
- Cannon-SpoorHEPotkinSGWyattRJMeasurement of premorbid adjustment in chronic schizophreniaSchizophr Bull1982834704847134891
- Clinical global impressionGuyWECDEU Assessment Manual for Psychopharmacology, RevisedRockville MDNational Institute for Mental Health1976217222
- Oliva-MorenoJLópez-BastidaJOsuna-GuerreroRMontejo-GonzálezALDuque-GonzálezBThe costs of schizophrenia in SpainEur J Health Econ20067318218816850333
- Colleges of Pharmacists Spanish CouncilOfficial medicines catalog https://botplusweb.portalfarma.com/Accessed December 15, 2010
- Vázquez-PoloFJNegrínMCabasésJMSánchezEHaroJMSalvador-CarullaLAn analysis of costs of treating schizophrenia in Spain: a hierarchical Bayesian approachJ Ment Health Policy Econ20058315316516278503
- Oblikue Consulting SLOblikue Consulting eSALUD – Healthcare database on costs2008 http://oblikue.com/bddcostesAccessed November 24, 2010
- ShiLAscher-SvanumHZhuBFariesDMontgomeryWMarderSRCharacteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophreniaPsychiatr Serv200758448248817412849
- KeithSJKaneJMTurnerMConleyRRNasrallahHAAcademic highlights: guidelines for the use of long-acting injectable atypical antipsychoticsJ Clin Psychiatry200465112013114744181
- KelinKBrnabicAJNewtonRBaseline characteristics and initial treatment decisions for patients with schizophrenia at risk of treatment nonadherencePatient Prefer Adherence2010430131120859457
- HeresSHamannJMendelRIdentifying the profile of optimal candidates for antipsychotic depot therapy A cluster analysisProg Neuropsychopharmacol Biol Psychiatry20083281987199318948163
- Janssen-CilagRisperdal-ConstaSummary of Product Characteristics http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Risperdal_Consta/human_referral_000027.jsp&murl=menus/regulations/regulations.jspAccessed February 16, 2011
- EerdekensMVan HoveIRemmerieBMannaertEPharmacokinetics and tolerability of long-acting risperidone in schizophreniaSchizophr Res20047019110015246468
- TaylorDPsychopharmacology and adverse effects of antipsychotic long-acting injections: a reviewBr J Psychiatry200919552S1319
- TaylorDMirSMaceSWhiskeyECo-prescribing of atypical and typical antipsychotics – prescribing sequence and documented outcomePsychiatr Bull2002265170172
- PeuskensJOlivaresJMPecenakJTreatment retention with risperidone long-acting injection: 24-month results from the Electronic Schizophrenia Treatment Adherence Registry (e-STAR) in six countriesCurr Med Res Opin201026350150920014981
- PatelMXDe ZoysaNBernadtMDavidADepot and oral antipsychotics: patient preferences and attitudes are not the same thingJ Psychopharmacol200923778979618583438
- PatelMXNikolaouVDavidASPsychiatrists’ attitudes to maintenance medication for patients with schizophreniaPsychol Med2003331838912537039
- PatelMXDavidASWhy aren’t depot antipsychotics prescribed more often and what can be done about it?Adv Psychiatr Treat2005113203211
- CaroliFRaymondetPIzardIPlasJGallBDelgadoAOpinions of French patients with schizophrenia regarding injectable medicationPatient Prefer Adherence2011516517121573047
- WaddellLTaylorMAttitudes of patients and mental health staff to antipsychotic long-acting injections: systematic reviewBr J Psychiatry Suppl200952S435019880916
- BurnsTKnowledge about antipsychotic long-acting injections: bridging that gapBr J Psychiatry Suppl200952S5619880917
- OlivaresJMRodriguez-MartinezABuronJAAlonso-EscolanoDRodriguez-MoralesACost-effectiveness analysis of switching antipsychotic medication to long-acting injectable risperidone in patients with schizophrenia: a 12- and 24-month follow-up from the e-STAR database in SpainAppl Health Econ Health Policy200861415318774869
- Ascher-SvanumHZhuBFariesDEThe cost of relapse and the predictors of relapse in the treatment of schizophreniaBMC Psychiatry201010220059765