Abstract
Background
Medication-induced oral hyperpigmentation is an oral condition that impacts patients’ quality of life and has been linked to many systemic therapeutic agents. The exact pathogenesis of tissue pigmentation varies greatly and is not completely known. This systematic review aimed to present data on the causal association between medications and the development of oral/mucosal pigmentation as an adverse drug reaction.
Methods
A systematic review and analysis of literature were conducted using the following databases: PubMed, Science Direct, ProQuest, Web of Science, and Scopus. The systematic review included original articles written in English and published between January 1982 and June 2020. Following the PRISMA statement, eligible articles were systematically reviewed, and data were extracted from eligible studies and analyzed.
Results
A total of 235 articles were identified, of which 57 met the inclusion criteria and were included in this review. The mean age of included patients was 46.2±16.38 years (range: 10–90 years) with a male to female ratio of 1:1.45. Oral mucosal hyperpigmentation was reported following the use of several classes of medications such as antiviral (eg, zidovudine), antibiotic (eg, minocycline), antimalarial (eg, chloroquine), anti-fungal (eg, ketoconazole), antileprotic (eg, clofazimine), antihypertensive (eg, amlodipine), chemotherapeutic, and antineoplastic drugs. The risk of developing oral pigmentation was significantly higher with antimalarial medications, antibiotics, antineoplastic and chemotherapeutic agents. Medication-induced oral hyperpigmentation was most frequent among women and in the hard palate.
Conclusion
Future research is warranted to better understand the pathogenesis and risk factors for medication-induced oral hyperpigmentation in order to reassure patients during prescription and management.
Introduction
The World Health Organization (WHO) defines an adverse drug reaction (ADR) as an unintended and noxious response to a drug that occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or modification of physiological function.Citation1
An ADR in the oral cavity could present as xerostomia, lichenoid reaction/lichen planus, aphthous-like ulcers, bullous disorders, pigmentation, fibrovascular hyperplasia, epithelial keratosis, dysesthesia, osteonecrosis of the jaws, and/or angioedema.Citation2
Medication-induced oral hyperpigmentation (MIOH) is an ADR that may develop rapidly after taking an associated drug once or for several continuous days or years.Citation3 Clinically, MIOH presents as localized or generalized zones of blue to ill-defined black pigmentation affecting any site in the oral cavity, most commonly the gingiva, tongue, and buccal mucosa.Citation4,Citation5 Detailed patient medical history and information regarding clinical manifestations facilitate reaching a proper diagnosis.Citation2 While the exact mechanism of MIOH is still unclear, several mechanisms have been proposed, including an increase in the number of melanocytes in tissues, melanin production, and/or deposition/accumulation of medication metabolites.Citation6
Management of MIOH varies and includes, but is not limited to, the discontinuation of the offending medication, switching to another drug, or laser photoablation.Citation2,Citation3 The oral pigmentation is usually involved in ADRs as common as cutaneous involvement; the dentist should therefore be informed regarding medications that cause oral ADRs to differentiate them from other etiologies and disorders.
There is currently a dearth of evidence-based literature to prove the relation between medications and oral hyperpigmentation; most published reports thus far are based on individual case studies or repeated observations. This review aimed to address this gap in the literature by systematically reviewing different aspects of MIOH and providing evidence of the causal relationship between medicinal drugs and subsequent ADRs presenting as mucosal pigmentation. We believe that the findings of this review will help familiarize dentists with this oral condition and thus provide patients with proper evaluation, diagnosis, and health management.
Materials and Methods
Protocol and Registration
The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42018087955) and followed the PRISMA statement guidelines.Citation7 All study steps were conducted in accordance with the Cochrane Handbook for Systematic Review of Interventions.Citation8
Inclusion and Exclusion Criteria
The inclusion criteria for this systematic review were as follows: 1) English language studies published between January 1982 and June 2020; 2) original studies on the usage of a medication or medication class associated with MIOH; and 3) observational cohort studies, cross-sectional studies, case reports, and case series. We excluded animal studies, review articles, abstracts, and trials with outcomes irrelevant to our study or with data that were not reliable for extraction.
Literature Search
A computer-based literature search was carried out using five authentic electronic databases: Medline (PubMed), Science Direct, Scopus, ProQuest, and Web of Science. The following keywords were applied in the search strategy: (“Alkylating drugs” OR “Cyclophosphamide” OR “Antiarrhythmic drugs” OR “Amiodarone” OR “Anti-depressant drugs” OR “Imipramine” OR “Antifungal” OR “Ketoconazole” OR “Antileprotic drugs” OR “Clofazimine” OR “Antimalarial drugs” OR “Amodiaquine” OR “Chloroquine” OR “Hydroxychloroquine” OR “Quinacrine” OR “Quinidine” OR “Antimetabolites” OR “5 – Fluorouracil” OR “Antineoplastic drugs” OR “Cisplatin” OR “Hydroxyurea” OR “Antipsychotic drugs” OR “Chlorpromazine” OR “Phenothiazine” OR “Antiviral” OR “Zidovudine” OR “Contraceptives” OR “Oral contraceptives” OR “Cytotoxic antibiotic drugs” OR “Bleomycin” OR “Doxorubicin” OR “drug induced” OR “adverse drug effect” OR “adverse drug events” OR “adverse drug reaction” OR “drugs use” OR “medication use” OR “Other drugs” OR “Busulfan” OR “Desipramine” OR “Gold compounds” OR “Imatinib” OR “Minocycline” OR “Phenolphthalein” OR “Tegafur”) AND (“Oral hyper-pigmentation” OR “oral auriasis” OR “oral discoloration” OR “oral melanosis” OR “oral pigmentation” OR “oral staining” OR “oral tanning”).
Selection of Studies
Two authors (NB and MB) reviewed eligible studies in a two-phase stepwise manner, including screening of abstracts for eligibility and retrieval of full texts for final inclusion in our study.
Data Extraction
Data extraction from eligible studies was performed by two authors (NB and MB) independently using an online data extraction form that included study design, outcome, population, medical history relevant to patients, social history, sample size, and medication (type, dose, and duration of intake). In addition, the risk of bias assessment for each domain was conducted in order to establish transparency of the review. Cases of dispute between reviewers during data extraction were resolved upon consulting a third reviewer (SM).
Quality Assessment
We used the tool proposed by Murad et alCitation9 for critical appraisal and quality assessment of the risk of bias in case reports and case series within our study. This tool uses the domains of selection, ascertainment, causality, and reporting for risk of bias assessment. Additionally, we used the Newcastle-Ottawa scale (NOS) for assessment of bias in the included cohort and cross-sectional studies.Citation10 NOS relies on selection, comparability, and outcome domains for risk of bias revision. Each study’s risk of bias was classified as low, moderate, or high.
Statistical Analysis
Statistical analysis for included studies was performed using IBM SPSS Statistics for Windows (Version 24.0. Armonk, NY: IBM Corp). Continuous variables were presented as means and standard deviations, categorical data were presented as frequencies and percentages, and p-values less than 0.05 were considered statistically significant.
Results
Search Results
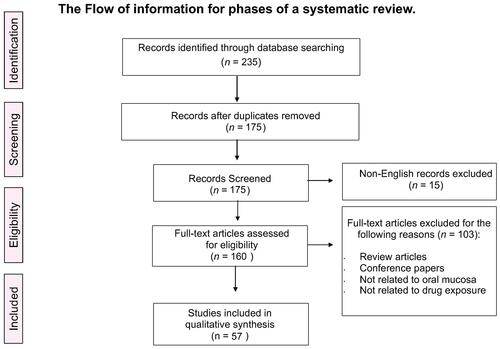
We searched five reliable databases: PubMed, Scopus, Science Direct, ProQuest, and Web of Science. Our search using pre-specified keywords yielded a total of 235 citation records. After removing duplicate studies, 175 unique records remained to be screened based on our intended inclusion and exclusion criteria. Title and abstract screening led to the exclusion of 15 studies because they were not written in the English language. Further screening was applied to the remaining studies. We excluded 103 full-text articles because they were either review articles or conference papers, or did not involve drug exposure or drug-induced hyperpigmentation in the oral cavity. Ultimately, 57 studies were eligible for our systematic review and statistical analysis.Citation4,Citation5,Citation12–Citation64 depicts a study flow diagram for the selection process following the PRISMA guidelines.Citation7
Figure 1 A flow diagram of the study selection process following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Notes: Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.J Clin Epidemiol. 2009;62:10.Citation7
Study Characteristics and Outcomes
We extracted data pertaining to a total of 268 patients involved in the selected studies. The mean age was 46.2±16.38 years (range: 10–90 years) and the male to female ratio was 1:1.44. Data on the characteristics of the enrolled studies are shown in . Hyperpigmentation of the oral mucosa associated with antibiotic use was reported in 24 cases. The most affected sites were the gingiva, palate, and tongue. In total, 22 cases (91.7%) were associated with minocycline used for facial acne vulgaris at a dosage of 100–200 mg daily.Citation4,Citation12,Citation13,Citation15,Citation17,Citation18,Citation23,Citation27–Citation30,Citation34,Citation37,Citation44,Citation65 The overall treatment duration ranged from 2 weeks to 11 years. Clinical characteristics included a bluish to grayish discoloration with variable size and site. Few cases reported cutaneous, tooth, or bone pigmentation. Additionally, linezolid and clindamycin use were reported in two other cases (4.1%).Citation38,Citation64
Table 1 Summary of Characteristics of Included Patients
Antimalarial medications were linked to MIOH in 68 cases affecting the palate, gingiva, and lips. Quinacrine hydrochloride,Citation47,Citation63,Citation66 hydroxychloroquine,Citation18,Citation39 quinidine sulfate,Citation35,Citation41 Daraclor,Citation32 amodiaquine,Citation42 and chloroquine compoundCitation31,Citation33,Citation39,Citation45,Citation60 were the most commonly-reported offending medications. Oral melanosis occurred with different doses, in various populations, within fluctuating periods. The lesions varied in size, color, and involvement of skin, teeth, and/or bone (Supplementary Table 1).
Oral hyperpigmentation induced by chemotherapeutic and antineoplastic agents was reported in 30 and 125 patients, respectively. The most prevalent agent was imatinibCitation18,Citation21,Citation22,Citation24,Citation36,Citation46,Citation48–Citation55,Citation57,Citation62 other agents included capecitabine,Citation16 cyclophosphamide,Citation25,Citation40 5-fluorouracil,Citation43 irinotecan,Citation26 and hydroxyurea.Citation11 The risk of oral pigmentation in association with imatinib therapy increased as the duration of intake increased (an average of 7 years) and occurred most commonly in the hard palate.Citation59 Other affected sites included the gingiva and tonsils.Citation53,Citation57
Zidovudine, an antiviral agent, was associated with melanotic macules in the buccal mucosa and lips in 14 cases.Citation5 Other reported medications included golimumabCitation18 and cortisoneCitation64 (immunosuppressants), ketoconazole (an anti-fungal agent),Citation64 amlodipine (an antihypertensive agent),Citation14 retigabine (an antiepileptic agent),Citation56 and clofazimine (an antileprotic agent).Citation64 All baseline data of enrolled patients in the included studies are presented in Supplementary Table 1.
Risk of Bias Assessment
The majority of the included studies were case reports and case series,Citation4,Citation5,Citation11–Citation19,Citation21–Citation32,Citation35–Citation42,Citation44–Citation46,Citation48,Citation54,Citation58,Citation62–Citation66 most of which achieved a moderate quality level based on Murad et al’s method.Citation9 The NOS was used for the assessment of bias in the included cohort and cross-sectional studies. Included cohort studies achieved a moderate level of quality,Citation5,Citation11,Citation28,Citation40,Citation60 while the cross-sectional study achieved a high level of quality.Citation39,Citation59 The quality of each included study is summarized in Supplementary Table 2–4.
Discussion
In this systematic review, we aimed to provide evidence of the causal relation between medicinal drugs and subsequent ADRs presenting as oral/mucosal pigmentation and highlight the characterization of MIOH. Clinically, medication- and non-MIOH may share the same clinical features with no specific criteria for differentiation. To differentiate them, a thorough investigation should be conducted to consider the site, color, and duration of the lesion, patient’s medical and social history, and medications used.Citation66
The current literature describes oral/mucosa hyperpigmentation as an adverse event due to the usage of several medications such as antibiotic (eg, minocycline), antiviral (eg, zidovudine), antimalarial (eg, chloroquine), chemotherapeutic, antineoplastic, anti-fungal drugs (eg, ketoconazole), antileprotic (eg, clofazimine), and antihypertensive drugs (eg, amlodipine). The pathophysiology of MIOH is still poorly understood and could differ based on the type of medication. For instance, one proposed theory of the underlying mechanisms focuses on the precipitation of minocycline metabolites in addition to a colored quinone derived from the aromatic ring.Citation67 However, imatinib is reported to have caused hyperpigmentation of unknown mechanisms and hypopigmentation in in-vitro studies due to the inhibition of the c-KIT pathway, which is usually involved in melanocyte development and regulation. Other agents in the same antineoplastic drug category as ipilimumab have shown yet another mechanism of reaction involving inhibition of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and subsequent immune system activation.Citation68
The most common category of medication associated with oral pigmentation was found to be Antineoplastic. Imatinib-associated hyperpigmentation was described by several studies that reported treatment duration as a major risk factor leading to larger and darker lesions.Citation59,Citation68,Citation69 In addition, antimalarial was the second most common category caused MIOH. A long duration (up to 15 years) of use of antimalarial drugs was observed to be associated with severe blue-black and gray diffused lesions in the hard palate with cutaneous involvement.Citation31,Citation35,Citation42 An important development of used hydroxychloroquine in the treatment or prophylaxis of Coronavirus Disease-2019 (COVID-19) addresses the need for further analysis and follow up of MIOH in this population.Citation70
Minocycline, which is the most common antibiotic agent associated with MIOH, was reported to be associated with a diffuse bluish-gray discoloration affecting any site in the oral cavity within a relatively short duration around one month of use.Citation4,Citation28 Bone pigmentation was only associated with the use of minocycline, and teeth pigmentation was seen in patients who consumed either minocycline or imatinib.Citation17,Citation19,Citation27,Citation34,Citation53
Several antiviral agents were also linked to MIOH. Notably, Ficarra et al reported 14 cases of hyperpigmentation of the buccal mucosa and lips associated with zidovudine. This cohort studyCitation5 included human immunodeficiency virus (HIV)-seropositive patients who were administered zidovudine. At the two-year follow-up, 14 (out of 217) HIV patients had developed pigmented lesions in the oral mucosa. Similarly, Almeida et alCitation71 conducted a systematic review and meta-analysis comparing a group of patients on highly active antiretroviral therapy (HAART) to a non-HAART group in terms of oral lesions. They analyzed data from five studies and reported that 78 out of 724 (10.77%) patients who received HAART ultimately developed MIOH compared to 37 (8.5%) patients of the non-HAART group, with a risk ratio of 1.65 (95% Confidence Interval: 1.16–2.32).Citation71 This could be explained by the augmented melanin production in the epithelium associated with the increased release of the melanocyte-stimulating hormone as a result of the systemic ketoconazole and zidovudine therapies.Citation72 Few cases of hyperpigmentation induced by antihypertensive drugs have been reported.Citation14
Thus far, no malignant transformation potential or serious complication with MIOH has been reported and no treatment is required. However, follow-up for the lesions is still advisable for the timely detection of any changes. Discontinuation of minocycline, imatinib, and hydroxychloroquine yielded persistence of pigmentation, not disappearance,Citation17,Citation18,Citation39,Citation52,Citation55,Citation57 while few reports did document regression of pigmentation after discontinuation of medication.Citation16,Citation18,Citation58 In some cases, MIOH raises aesthetic concerns for patients based on the location of the lesions in the oral cavity. Few articles have reported the removal of minocycline‐induced pigmentation by Q‐switched Nd: YAG (neodymium-doped yttrium aluminum garnet), ruby, or alexandrite lasers.Citation18,Citation65 The best management strategies for MIOH should depend on proper diagnosis by a specialist and comprehensive patient education. Therefore, oral and maxillofacial specialists should be well-informed of all medications that can induce pigmentation in the oral mucosa in order to reassure patients about this unexpected hyperpigmentation.
This systematic review had several limitations. First, the included studies had a lower evidence level because the majority were case reports (class III evidence). Second, the heterogeneity of the study designs and outcome measures of the included studies affect the comparison of outcomes. Third, a correlation analysis between the risk factors, patient characteristics, and outcomes of the included studies was not feasible due to the lack of raw data.
Conclusion
In conclusion, there was a significant association between oral/mucosal hyperpigmentation among middle-aged adults and prescribed medications, with most of the cases being caused by the use of antineoplastic, antimalarial and antibiotic agents. The practitioners and dentists should be aware of medications associated with hyperpigmentation to reassure the patients and monitor the potential side effects. Future research is required to better understand the risk factors, patients at risk and pathogenesis of MIOH to assist in patient management.
Disclosure
The authors report no conflicts of interest for this work.
References
- WHO. International drug monitoring: the role of national centres. Rep WHO Meet. 1972;1–25.
- Yuan A, Woo S-B. Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(1):35–47. doi:10.1016/j.oooo.2014.09.009
- Abdollahi M, Radfar M. A review of drug-induced oral reactions. J Contemp Dent Pract. 2003;4(1):10–31. doi:10.5005/jcdp-4-1-10
- Meyerson MA, Cohen PR, Hymes SR. Lingual hyperpigmentation associated with minocycline therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 1995;79(2):180–184. doi:10.1016/S1079-2104(05)80279-3
- Ficarra G, Shillitoe EJ, Adler-Storthz K, et al. Oral melanotic macules in patients infected with human immunodeficiency virus. Oral Surg Oral Med Oral Pathol. 1990;70(6):748–755. doi:10.1016/0030-4220(90)90014-J
- Eisen D. Disorders of pigmentation in the oral cavity. Clin Dermatol. 2000;18(5):579–587. doi:10.1016/S0738-081X(00)00148-6
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi:10.1016/j.jclinepi.2009.06.006
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5. 2008. doi:10.1002/9780470712184
- Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi:10.1136/bmjebm-2017-110853
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. 2016.
- Kumar B, Saraswat A, Kaur I. Mucocutaneous adverse effects of hydroxyurea: a prospective study of 30 psoriasis patients. Clin Exp Dermatol. 2002;27(1):8–13. doi:10.1046/j.0307-6938.2001.00947.x
- Fendrich P, Brooke RI. An unusual case of oral pigmentation. Oral Surg Oral Med Oral Pathol. 1984;58(3):288–289. doi:10.1016/0030-4220(84)90056-2
- Johnston S. Feeling blue? Minocycline-induced staining of the teeth, oral mucosa, sclerae and ears - A case report. Br Dent J. 2013;215(2):71–73. doi:10.1038/sj.bdj.2013.682
- Erbagci Z. Amlodipine associated hyperpigmentation. Saudi Med J. 2004;25(1):103–105.
- Treister NS, Magalnick D, Bin WS. Oral mucosal pigmentation secondary to minocycline therapy: report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(6):718–725. doi:10.1016/j.tripleo.2003.11.006
- Vasudevan B. An unusual case of capecitabine hyperpigmentation: is hyperpigmentation a part of hand-foot syndrome or a separate entity. Indian J Pharmacol. 2010;42(5):326–328. doi:10.4103/0253-7613.70401
- Siller GM, Tod MA, Savage NW. Minocycline-induced oral pigmentation. J Am Acad Dermatol. 1994;30(2):350–354. doi:10.1016/S0190-9622(94)70038-9
- Tosios KI, Kalogirou EM, Sklavounou A. Drug-associated hyperpigmentation of the oral mucosa: report of four cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(3):e54–e66. doi:10.1016/j.oooo.2017.10.006
- Salman RA, Salman DG, Glickman RS. Minocycline induced pigmentation of the oral cavity. J Oral Med. 1985;40(3):154–157.
- Renald Perusse RM. Oral Pigmentation induced by premarin. Laval Univ Sch Dent. 1991;48:61–64.
- Lyne A, Creedon A, Bailey BMW. Mucosal pigmentation of the hard palate in a patient taking imatinib. BMJ Case Rep. 2015;2015(apr16 1):1–3. doi:10.1136/bcr-2015-209335
- Romeo U, Palaia G, Fantozzi PJ, Tenore G, Bosco D, Rare A. Case of melanosis of the hard palate mucosa in a patient with chronic myeloid leukemia. Case Rep Dent. 2015;2015:9–12. doi:10.1155/2015/817094
- Patel K, Cheshire D, Vance A. Oral and systemic effects of prolonged minocycline therapy. Br Dent J. 1998;185(185):560. doi:10.1038/sj.bdj.4809867
- Resende RG, Teixeira RGL, Vasconcelos FDO, Silva MEDS, Abreu MHG, Gomez RS. Imatinib-associated hyperpigmentation of the palate in post-HSCT patient. J Craniomaxillofac Surg. 2012;40(5):e140–e143. doi:10.1016/j.jcms.2011.07.010
- Parvaei P, Mortazavi H, Baharvand M. Severe pigmentation of oral mucosa, skin and nails due to breast cancer chemotherapy – case report. Dent Med Probl. 2015;52(3):363–365.
- Nestor LA, Flint S, Galvin S. Unusual case of hyperpigmentation secondary to irinotecan. BMJ Case Rep. 2016;2016:1–2. doi:10.1136/bcr-2016-217545
- Odell EW, Hodgson RP, Haskell R. Oral presentation of minocycline-induced black bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol. 1995;79(4):459–461. doi:10.1016/S1079-2104(05)80128-3
- Ozog DM, Gogstetter DS, Scott G, Gaspari AA. Minocycline-induced hyperpigmentation in patients with pemphigus and pemphigoid. Arch Dermatol. 2000;136(9):1133–1138. doi:10.1001/archderm.136.9.1133
- Cale AE, Freedman PD, Lumerman H. Pigmentation of the jawbones and teeth secondary to minocycline hydrochloride therapy. J Periodontol. 1988;59(2):112–114. doi:10.1902/jop.1988.59.2.112
- Morrow GL, Abbott RL. Minocycline-induced scleral, dental, and dermal pigmentation. Am J Ophthalmol. 1998;125(3):396–397. doi:10.1016/S0002-9394(99)80156-1
- de Filho MRM, da Silva CAD, da Dourado MR, de Pires MBO, Pêgo SPB, de Freitas EM. Palate hyperpigmentation caused by prolonged use of the anti-malarial chloroquine. Head Neck Pathol. 2012;6(1):48–50. doi:10.1007/s12105-011-0288-5
- Levy H. Chloroquine-induced pIgmentatIon. S Afr Med J. 1982;62(December1981):1981–1983.
- De Andrade BAB, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases. J Cutan Pathol. 2013;40(9):833–838. doi:10.1111/cup.12182
- Beehner ME, Houston GD, Young JD. Oral pigmentation secondary to minocycline therapy. J Oral Maxillofac Surg. 1986;44(7):582–584. doi:10.1016/S0278-2391(86)80104-5
- Birek C, Ph D. Short communications & case reports associated two cases of oral pigmentation with quinidine therapy. Oral Surg Oral Med Oral Pathol. 1988;66(66):59–61. doi:10.1016/0030-4220(88)90067-9
- Bombeccari GP, Garagiola U, Pallotti F, et al. Hyperpigmentation of the hard palate mucosa in a patient with chronic myeloid leukaemia taking imatinib. Maxillofac Plast Reconstr Surg. 2017;39(1). doi:10.1186/s40902-017-0136-y
- Buddula A. Staining of palatal torus secondary to long term minocycline therapy. J Indian Soc Periodontol. 2009;13(1):48. doi:10.4103/0972-124x.51896
- Balaji G, Maharani B, Ravichandran V, Parthasarathi T. Linezolid induced black hairy tongue. Indian J Pharmacol. 2014;46(6):653–655. doi:10.4103/0253-7613.144942
- Bahloul E, Jallouli M, Garbaa S, et al. Hydroxychloroquine-induced hyperpigmentation in systemic diseases: prevalence, clinical features and risk factors: a cross-sectional study of 41 cases. Lupus. 2017;26(12):1304–1308. doi:10.1177/0961203317700486
- Acharya S, Pai KM, Bhat S, Mamatha B, Bejadi V, Acharya S. Oral changes in patients undergoing chemotherapy for breast cancer. Indian J Dent Res. 2017;28(3):261–268. doi:10.4103/ijdr.IJDR_379_16
- Mahler R, Sissons W, Watters K. Pigmentation induced by quinidine therapy. Arch Dermatol. 1986;122(9):1062–1064. doi:10.1001/archderm.1986.01660210112031
- Watson IB, MacDonald DG. Amodioquine induced oral pigmentation ‐ a light and electron microscopic study. J Oral Pathol Med. 1974;3(1):16–21. doi:10.1111/j.1600-0714.1974.tb01694.x
- Co ML, Esteban MJ. Lingual hyperpigmentation after 5-fluorouracil chemotherapy. BMJ Case Rep. 2017;2017:1–2. doi:10.1136/bcr-2017-219806
- Ayangco L, Sheridan PJ. Minocycline-induced staining of torus palatinus and alveolar bone. J Periodontol. 2003;74(5):669–671. doi:10.1902/jop.2003.74.5.669
- de Andrade BAB, Padron-Alvarado NA, Muñoz-Campos EM. Hyperpigmentation of hard palate induced by chloroquine therapy. J Clin Exp Dent. 2017;9(12):e1487–e1491. doi:10.4317/jced.54387
- Wong M, Sade S, Gilbert M, Klie HBE. Oral melanosis after tyrosine kinase inhibition with imatinib for chronic myelogenous leukemia: report of a case and review of the literature. Dermatol Online J. 2011;17(5).
- López-Jornet P, Pons-Fuster A. Pigmented oral mucosa due to quinacrine. N Y State Dent J. 2011;77(6):49–51.
- Mattsson U, Halbritter S, Mörner Serikoff E, Christerson L, Warfvinge G. Oral pigmentation in the hard palate associated with imatinib mesylate therapy: a report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(5):e12–e16. doi:10.1016/j.tripleo.2010.11.006
- Di Tullio F, Mandel VD, Scotti R, Padalino C, Pellacani G. Imatinib-induced diffuse hyperpigmentation of the oral mucosa, the skin, and the nails in a patient affected by chronic myeloid leukemia: report of a case and review of the literature. Int J Dermatol. 2018;57(7):784–790. doi:10.1111/ijd.13931
- Song HSKH, Kang HY. Imatinib mesylate-induced hyperpigmentation of the nose and palate. Ann Dermatol. 2014;26(4):532–533. doi:10.5021/ad.2014.26.4.532
- Roeker LEWA, Wolanskyj AP. Imatinib-associated melanosis of the palate. Am J Hematol. 2014;89(5):564. doi:10.1002/ajh.23589
- Khoo TL, Catalano A, Supple S, et al. Hyperpigmentation of the hard palate associated with imatinib therapy for chronic myeloid leukemia with a genetic variation in the proto-oncogene c-KIT. Leuk Lymphoma. 2013;54(1):186–188. doi:10.3109/10428194.2012.702904
- Singh NBS, Bakhshi S. Imatinib-induced dental hyperpigmentation in childhood chronic myeloid leukemia. J Pediatr Hematol Oncol. 2007;29(3):208–209. doi:10.1097/MPH.0b013e318033a76c
- Li CC, Malik SM, Blaeser BF, et al. Mucosal pigmentation caused by imatinib: report of three cases. Head Neck Pathol. 2012;6(2):290–295. doi:10.1007/s12105-011-0325-4
- Lewis DM. Diffuse pigmentation of the palate. J Okla Dent Assoc. 2009;100:24–25.
- Beacher NG, Brodie MJ, Goodall C. A case report: retigabine induced oral mucosal dyspigmentation of the hard palate. BMC Oral Health. 2015;15(1):1–5. doi:10.1186/s12903-015-0102-y
- Steele JC, Triantafyllou A, Rajlawat BPFE, Field EA. Oral mucosal hyperpigmentation and horizontal melanonychia caused by imatinib. Clin Exp Dermatol. 2012;37(4):432–433. doi:10.1111/j.1365-2230.2011.04196.x
- Dixon DR, Yassin A. Oral effects and early implant survival results after imatinib discontinuation therapy for chronic myelogenous leukemia: a case report. J Clin Periodontol. 2017;7(3):115–120. doi:10.1902/cap.2016.160050
- Oliveira SR, de Azevedo Branco LG, Rocha AL, et al. Association of oral mucosa hyperpigmentation with imatinib mesylate use: a cross-sectional study and a systematic literature review. Clin Oral Investig. 2019;23(12):4371–4382. doi:10.1007/s00784-019-02886-0
- Chacón-Dulcey V, López-Labady J, Villarroel-Dorrego M, et al. Oral manifestations associated with antimalarial therapy in patients with systemic lupus erythematosus. Lupus. 2020;29(7):761–766. doi:10.1177/0961203320922620
- Kristensson JH, Sander BB, von Euler-chelpin M, Lynge E. Predictors of non-participation in cervical screening in Denmark. Cancer Epidemiol. 2014;38(2):174–180. doi:10.1016/j.canep.2013.12.007
- Mascitti M, Luconi E, Togni L, Barlattani A, Santarelli A. Imatinib-related hyperpigmentation of oral mucosa: case report and literature review. J Dent Sci. 2019;14(3):335–337. doi:10.1016/j.jds.2019.02.005
- Lerman MA, Karimbux N, Guze KA, Bin WS. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):8–12. doi:10.1016/j.tripleo.2008.07.022
- Langford A, Pohle HD, Gelderblom H, Zhang X, Reichart PA. Oral hyperpigmentation in HIV-infected patients. Oral Surg Oral Med Oral Pathol. 1989;67(3):301–307. doi:10.1016/0030-4220(89)90360-5
- LaPorta VN, Nikitakis NG, Sindler AJ, Reynolds MA. Minocycline-associated intra-oral soft-tissue pigmentation: clinicopathologic correlations and review. J Clin Periodontol. 2005;32(2):119–122. doi:10.1111/j.1600-051X.2005.00646.x
- Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189–194. doi:10.1067/moe.2000.106340
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat. 2009;17(2):123–126.
- Dai J, Belum VR, Wu S, Sibaud V, Lacouture ME. Pigmentary changes in patients treated with targeted anticancer agents: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(5):902–910.e2. doi:10.1016/j.jaad.2017.06.044
- Mcpherson T, Sherman V, Turner R. Imatinib-associated hyperpigmentation, a side effect that should be recognized. J Eur Acad Dermatol Venereol. 2009;23(1):82–83. doi:10.1111/j.1468-3083.2008.02706.x
- Eljaaly K, Alireza KH, Alshehri SA-TJ, Al-Tawfiq JA. Hydroxychloroquine safety: a meta-analysis of randomized controlled trials. Travel Med Infect Dis. 2020;36:101812. doi:10.1016/j.tmaid.2020.101812
- de Almeida VL, Lima IFP, Ziegelmann PK, Paranhos LR, de Matos FR. Impact of highly active antiretroviral therapy on the prevalence of oral lesions in HIV-positive patients: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2017;46(11):1497–1504. doi:10.1016/j.ijom.2017.06.008
- Hamza OJ, Matee MI, Simon EN, et al. Oral manifestations of HIV infection in children and adults receiving highly active anti-retroviral therapy [HAART] in Dar es Salaam, Tanzania. BMC Oral Health. 2006;6(1):12. doi:10.1186/1472-6831-6-12
