Abstract
Imidafenacin is an antimuscarinic agent with high affinity for the M3 and M1 muscarinic receptor subtypes and low affinity for the M2 subtype, and is used to treat overactive bladder. Several animal studies have demonstrated that imidafenacin has organ selectivity for the bladder over the salivary glands, colon, heart, and brain. In Phase I studies in humans, the approximately 2.9-hour elimination half-life of imidafenacin was shorter than that of other antimuscarinics such as tolterodine and solifenacin. Imidafenacin was approved for clinical use in overactive bladder in Japan in 2007 after a randomized, double-blind, placebo-controlled Phase II study and a propiverine-controlled Phase III study conducted in Japanese patients demonstrated that imidafenacin 0.1 mg twice daily was clinically effective for treating overactive bladder and was not inferior to propiverine for reduction of episodes of incontinence, with a better safety profile than propiverine. Several short-term clinical studies have demonstrated that imidafenacin also improves sleep disorders, nocturia, and nocturia-related quality of life. In addition, it is speculated that addon therapy with imidafenacin is beneficial for men with benign prostatic hyperplasia whose overactive bladder symptoms are not controlled by alpha-1 adrenoceptor antagonists. No cognitive impairment or influence of imidafenacin on the QTc interval has been observed. Although there have been very few relevant long-term clinical studies, the available information suggests the long-term efficacy, safety, and tolerability of imidafenacin, with less frequent severe adverse events, such as dry mouth and constipation. In addition, imidafenacin can be used safely for a long time even for cognitively vulnerable elderly patients with symptoms of overactive bladder. Thus, it is highly likely that imidafenacin is safe, efficacious, and tolerable to control symptoms of overactive bladder even over the long term. However, it remains unknown if the practical effectiveness of imidafenacin is applicable to ethnic groups other than Japanese.
Introduction
Overactive bladder (OAB) is a clinical diagnosis defined by the International Continence Society as the presence of urinary urgency, usually accompanied by frequency and nocturia, with or without urge incontinence, in the absence of a urinary tract infection or other obvious pathology.Citation1,Citation2 OAB has a considerable impact on patient quality of life, although it does not affect survival. A nationwide survey conducted in JapanCitation3 demonstrated that the estimated prevalence of OAB was 12.4% in the general population over the age of 40 years. The study showed that 11.2% and 53.0% of subjects, respectively, reported “an impact” or “a slight impact” on their quality of life related to symptoms of OAB, through impairment of mental health, vitality, physical activity, home life, and work. Given that the actual number of patients with OAB has been increasing in parallel with advancement of our aging society, an appropriate treatment strategy should be established as soon as possible.
Treatment of OAB consists of behavioral therapies, such as bladder training, bladder control strategies, pelvic floor muscle training, fluid management, and medical therapy.Citation4–Citation6 Oral antimuscarinic agents are the mainstay of current medical management, although a beta 3-adrenoceptor agonist, mirabegron, has been introduced to treat OAB.Citation7 Several antimuscarinic agents are clinically available, including darifenacin, fesoterodine, imidafenacin, oxybutynin, propiverine, solifenacin, and tolterodine. Although an extensive review of randomized trials showed no evidence for differential efficacy of these agents, adverse event profiles for dry mouth and constipation vary between medications since each antimuscarinic agent has distinct features in terms of affinity for muscarinic receptor subtypes, organ selectivity, and pharmacokinetics. These differences may result in differences in efficacy and safety profiles in clinical practice, especially in elderly patients with various comorbidities.
Imidafenacin, which was developed by Kyorin Pharmaceutical Company (Tokyo, Japan) is an antimuscarinic agent used to treat OAB and has high affinity for the M3 and M1 muscarinic receptor subtypes and low affinity for the M2 subtype.Citation8,Citation9 In addition, it shows organ selectivity for the bladder over the salivary glands. Imidafenacin was approved for clinical use in the treatment of OAB in Japan in 2007 after a randomized, double-blind, placebo-controlled Phase II trialCitation10 and a propiverine-controlled Phase III trialCitation11 in Japanese patients demonstrated that 0.1 mg twice daily was a clinically appropriate dose of imidafenacin for treating OAB and was not inferior to propiverine for reduction of incontinence episodes, with a better safety profile than propiverine.
Although the Japanese clinical guidelines for OABCitation4 make a grade A recommendation for use of imidafenacin in OAB after publication of the results of the Phase IICitation10 and Phase III studies,Citation11 imidafenacin is still only available in Japan. Because of the limited number of reports on imidafenacin published in the English language, its clinical characteristics are still not widely recognized. In this article, the short-term and long-term efficacy and safety of imidafenacin for OAB are reviewed based on the English literature as well as several Japanese reports demonstrating its distinct clinical characteristics. The English and Japanese literature comprising PubMed and the Japanese Medical Abstract Society Web (version 5) until November, 2012 was searched using “imidafenacin” as the keyword, with further manual searches of reference lists.
Pharmacological characteristics of imidafenacin
Imidafenacin (KRP-197/ONO-8025), 4-(2-methyl 1-H-imidazol-1-yl)-2, 2, diphenyl butanamide) is an antimuscarinic agent with high affinity for the M3 and M1 muscarinic receptor subtypes and low affinity for the M2 subtype. In vitro research has shown that imidafenacin inhibits rat and human urinary bladder smooth muscle contraction by mediating antagonism of the M3 muscarinic receptor subtype and regulating acetylcholine release by mediating the prejunctional facilitatory M1 subtype.Citation8,Citation12 In addition, because imidafenacin has the highest relative potency (8.8), calculated as the ID50 of salivary secretion divided by the ID30 of distention-induced rhythmic bladder contraction in conscious rats, among propiverine (0.9), tolterodine (5.0), oxybutynin (1.4), and darifenacin (1.4), the drug has organ selectivity for the bladder over the salivary gland.Citation9 Yamazaki et al reported that the relative bladder selectivity of imidafenacin, solifenacin, and tolterodine was 15-fold, 1.7-fold, and, 2.5-fold higher than for the salivary gland; 150-fold, 1.9-fold, and 9.2-fold higher than for the colon; and 50-fold, 12-fold, and 4.6-fold higher than for the heart, respectively, compared with propiverine in a rat study.Citation13 Pharmacokinetic data have demonstrated that imidafenacin administered orally distributes predominantly to the bladder and exerts a more selective and longer-lasting effect on the bladder than on other tissues such as the submaxillary gland, colon, and brain.Citation14 Yamada et alCitation14 also speculated that imidafenacin excreted in urine may be transferred directly from urine to the bladder tissue by simple diffusion and contribute to the selective and long-lasting binding of bladder muscarinic receptors in rats. In addition, it is known that the urothelium is one of the targets of antimuscarinics via reduction of bladder tone.Citation15 Recently, Yokoyama et al demonstrated that imidafenacin inhibited ATP production in the urothelium and improved detrusor overactivity in rats with cerebral infarction through afferent C-fiber suppression.Citation16 Nishijima et al also showed that the inhibitory effect of imidafenacin might be partly due to the blocking of an increase of ATP release in the rat bladder epithelium.Citation17
The M1 muscarinic receptor subtype in the central nervous system is involved in cognitive functions, including learning and memory.Citation18 Although it is speculated that M1 inhibition by imidafenacin may impair cognitive function, imidafenacin 10 mg/kg, which is a 60-fold higher dose than that for distention-induced rhythmic bladder contraction (0.17 mg/kg ID30) did not increase the escape latencies of rats in a Morris maze.Citation9 Intravenous injection of imidafenacin at pharmacological doses of 0.01–0.1 mg/kg did not decrease the binding potential of [11C](+)3-MPB in the rat central cortex and corpus striatum on positron emission tomography.Citation19 In a positron emission tomography study using conscious monkeys,Citation20 although oral administration of imidafenacin at therapeutic doses occupied muscarinic receptors in the cortices and brain stem to some extent, it did not induce discernible cognitive impairment evaluated by the titration version of the delayed matching to sample task. Thus, in animal studies, it seems that it is hard for imidafenacin to penetrate the blood-brain barrier because of its moderate polarity and low lipophilicity.Citation19
Pharmacokinetics of imidafenacin
The pharmacokinetics of imidafenacin has been evaluated mostly in Japanese subjects, with the exception of a few reports.Citation21–Citation27 In brief, when a 0.1 mg imidafenacin tablet was orally administered to 12 healthy male Japanese subjects (mean age 23 years, mean body weight 63.6 kg) in the fasted state, the median time taken to reach peak plasma concentration (Tmax) < mean peak plasma level (Cmax), mean area under the concentration-time curve from zero to infinity (AUC0–∞), and mean elimination half-life (T1/2) were 1.5 hours, 471 pg/mL, 2400 pg · hour/mL, and 2.9 hours, respectively.Citation24 These pharmacokinetic parameters were comparable with those in 14 healthy white male subjects (mean age 32 years, mean body weight 80.6 kg) who received a single oral dose of imidafenacin 0.1 mg, in whom the median Tmax, mean Cmax, mean AUC0–∞, and mean T1/2 were 1.0 hour, 416 pg/mL, 2060 pg · hour/mL, and 3.0 hours, respectively.Citation26 There were no differences in these parameters between a conventional and orally disintegrating tablet.Citation25 Thus, the elimination half-life of imidafenacin is shorter than for other antimuscarinics, including fesoterodine (7–8 hours), darifenacin (7–20 hours), solifenacin (45–68 hours), and tolterodine (7–18 hours).Citation5 There are no obvious pharmacokinetic differences between nonelderly and elderly males; the median Tmax, mean Cmax, mean AUC0–∞, and mean T1/2 were 1.0 hour, 399 pg/mL, 1980 pg · hour/mL, and 3.2 hours, respectively, after a single oral dose of imidafenacin 0.1 mg in six healthy elderly Japanese males aged 65 years and older.Citation23,Citation24 On the other hand, a population pharmacokinetic analysis of 547 subjects (90 healthy individuals and 457 patients with OAB) in eight clinical trials in Japan showed that oral clearance was decreased with advancing age.Citation28 A recent update of the data for the population pharmacokinetic analysis showed no clear relationship between the plasma concentration of imidafenacin and QTc.Citation29 Imidafenacin is predominantly metabolized by cytochrome P450 3A4 and uridine 5′-diphospho-glucuronosyltransferase 1A4, and less than 10% of the dose is excreted unchanged in urine.Citation27,Citation30
Dose-finding and randomized placebo-controlled studies in Japan
After the Phase I trial described aboveCitation21–Citation24,Citation26,Citation27, a Phase II clinical study was conducted to determine the efficacy, safety/tolerability, and dose-response relationship of imidafenacin in Japanese patients with OAB.Citation10 Men and women aged ≥ 20 years with OAB, defined as urinary incontinence (≥5 episodes/week), frequency of micturition (≥8 voids/day), and urgency (≥one episode/day) were included. After a 2-week, single-blind, run-in period, eligible patients were randomized in equal numbers to receive double-blind treatment with imidafenacin 0.05, 0.1, or 0.25 mg or placebo twice daily. Voiding diaries were completed over 7 consecutive days during the run-in period (baseline) and once every 4 weeks for 7 days during the 12-week treatment period. Of a total of 562 patients enrolled, 401 were randomized to treatment with imidafenacin 0.1 mg/day (n = 99), 0.2 mg/day (n = 100), 0.5 mg/day (n = 101), or placebo (n = 101). Of these patients, 45 (11.2%) discontinued treatment before completion of the study (7.1%, 7.0%, 24.8%, and 5.9% in the 0.1, 0.2, 0.5 mg/day, and placebo groups, respectively). After 12 weeks of treatment, the primary efficacy endpoint (percentage change in number of incontinence episodes per week) was −42.86%, −59.81%, −71.61%, and −82.19% in the placebo, 0.1 mg/day (P = 0.0906 versus placebo), 0.2 mg/day (P = 0.0010 versus placebo), and 0.5 mg/day (P < 0.0001 versus placebo) groups, respectively. The incidence of dry mouth in the imidafenacin groups increased in a dose-dependent manner. Although the percentage of patients receiving 0.5 mg/day who discontinued treatment due to dry mouth was high (8.9%), that for 0.1 mg/day (1.0%) and 0.2 mg/day (0%) was comparable with placebo (0%). Thus, considering the balance between efficacy and safety, imidafenacin 0.1 mg twice daily (0.2 mg/day) is recommended as a clinically appropriate dose.
Next, a randomized, double-blind, Phase III clinical trial was conducted to compare the short-term efficacy and tolerability of imidafenacin 0.1 mg twice daily with that of 20 mg of propiverine once daily and placebo in patients with OAB.Citation11 Inclusion and exclusion criteria were the same as for the Phase II study, as were the study protocol and primary efficacy endpoint.Citation10 Of the total of 1166 patients enrolled, 781 were allocated to treatment with imidafenacin (n = 324), propiverine (n = 310), or placebo (n = 147). Of these patients, 70 (9.0%) discontinued treatment before completion of the study (7.1%, 10.0%, and 10.9% in the imidafenacin, propiverine, and placebo groups, respectively). After 12 weeks of treatment, a significantly large percentage change in number of incontinence episodes per week was observed in the imidafenacin group compared with the placebo group based on the full analysis set population (). The noninferiority of imidafenacin compared with propiverine in reducing the number of incontinence episodes per week was confirmed, based on the per protocol set population (P = 0.0014, noninferiority margin 14.5%). Secondary efficacy endpoints, including number of urgency incontinence episodes per week (P < 0.0001), micturitions per day (P = 0.0112), urgency episodes per day (P = 0.0002), and urine volume voided per micturition (P = 0.0075) were also significantly improved in the imidafenacin group compared with the placebo group. The incidence of adverse events with imidafenacin was significantly lower than with propiverine (72.9% versus 81.7%, P = 0.0101). Some degree of dry mouth was observed in 31.5% and 39.9% of patients on imidafenacin and propiverine, respectively (P = 0.0302). The incidence of moderate-to-severe dry mouth for imidafenacin was significantly lower than for propiverine (5.0% versus 9.2%, P = 0.0433). The mean QTc interval showed no change with imidafenacin, but increased significantly with propiverine (P < 0.0001). Therefore, imidafenacin at a dose of 0.1 mg twice daily was not inferior to propiverine with regard to reduction in number of incontinence episodes, and was well tolerated for the treatment of OAB symptoms.
Table 1 Changes in incontinence episodes as a primary endpoint in a randomized, double-blind, placebo-controlled and propiverine-controlled trial of imidafenacin in Japan
Other short-term Japanese studies of imidafenacin
There is a report of the subjective efficacy of imidafenacin being observed as early as 3 days after administration of the drug in 19 patients with OAB evaluated by the question “To what extent did you feel the effects of this medicine?” every day for 2 weeks.Citation31 Mitsuhashi et al reported that 0.1 mg of imidafenacin twice daily significantly increased the maximum cystometric capacity from 202 ± 103 mL at baseline to 279 ± 120 mL at 4 weeks (P = 0.023) in 18 patients with overactive bladder.Citation32 Although detrusor overactivity during the filling phase was observed in seven patients at baseline, it disappeared in five after treatment. Sakakibara et al also reported that maximum cystometric capacity was increased, from 223 mL at baseline to 266 mL at 12 weeks (P < 0.05), by 0.1 mg of imidafenacin twice daily in 35 patients with OAB of neurogenic origin.Citation33 The efficacy of imidafenacin has been observed for treatment of stress urinary incontinence in female patients,Citation34 in whom the number of episodes of stress urinary incontinence per day decreased significantly from 1.3 ± 1.2 times at baseline to 0.6 ± 0.7 times at 12 weeks (P < 0.05).
There are several studies demonstrating the efficacy of imidafenacin for nocturia and sleep disorders in patients with OAB. The multi-institutional EPOCH study demonstrated that nocturia shown in a frequency volume chart was significantly decreased from 2.5 ± 1.3 to 2.0 ± 1.3 times (P < 0.001) by imidafenacin 0.1 mg twice daily for 8 weeks in 118 patients ≥ 50 years with OAB, defined as the frequency of micturition (≥8 voids/day), urgency (≥one episode/day), and nocturia (≥twice).Citation35 At baseline, 66 patients (55.9%) were above 5.5 (cutoff value) on the Pittsburg Sleep Quality Index (PSQI) and 20 subjects (16.9%) were above 11 (cutoff value) on the Epworth Sleepiness Scale. Thus, a substantial number of these elderly patients with OAB suffered from sleep disorders. The PSQI and the Epworth Sleepiness Scale were significantly decreased from 9.1 ± 3.3 to 6.7 ± 3.1 (P < 0.001) and from 14.3 ± 4.0 to 8.9 ± 5.3 (P < 0.001), respectively, by imidafenacin. There were significant correlations between changes in nocturia on the frequency volume chart and the PSQI global score (r = 0.407) and Epworth Sleepiness Scale score (r = 0.624). Thus, sleep disorders are associated with nocturia, and both sleep disorders and nocturia were improved by imidafenacin in patients with OAB.
Further, the EVOLUTION study evaluated the effect of imidafenacin on nocturia-related quality of life and hours of undisturbed sleep.Citation36 One hundred and sixty-five patients aged ≥ 20 years with urgency (≥ one episode/week) and nocturia (≥twice) were enrolled in this multi-institutional study. Imidafenacin 0.1 mg was given twice daily for 8 weeks. Nocturia-related quality of life was evaluated by the Japanese version of the Nocturia Quality-of-Life questionnaire, which has been linguistically validated.Citation37,Citation38 Nocturia shown by the frequency volume chart decreased from 3.7 times at baseline to 2.8 times at 8 weeks (P < 0.001). The PSQI global score and hours of undisturbed sleep were improved and prolonged from 6.7 ± 3.4 to 4.6 ± 3.1 (P < 0.001) and from 2.6 ± 1.1 to 3.8 ± 1.8 (P < 0.001), respectively, by imidafenacin. The Nocturia Quality-of-Life questionnaire showed significant improvement in total score (65.1 ± 20.2 versus 84.0 ± 16.8, P < 0.001), sleep/energy domain score (69.4 ± 22.8 versus 85.7 ± 16.7, P < 0.001), bother/concern domain score (62.5 ± 22.7 versus 82.2 ± 21.1, P < 0.001), and quality of life score (5.7 ± 3.0 versus 8.1 ± 2.3, P < 0.001). A correlation was found between nocturia on the frequency volume chart and Nocturia Quality-of-Life questionnaire (r = −0.407). Thus, both sleep disorders and nocturia-related quality of life were improved by imidafenacin in patients with OAB and nocturia. Wada et al further demonstrated that imidafenacin for 8 weeks improved the PSQI, especially subjective sleep quality, sleep latency, and daytime dysfunction, in 26 patients with sleep disorders at baseline.Citation39 Interestingly, they observed that imidafenacin significantly reduced the nocturnal polyuria index from 0.48 ± 0.11 to 0.43 ± 0.14 (P < 0.05) as a result of a significant decrease in nocturnal urine volume from 888 ± 286 mL to 795 ± 294 mL (P < 0.05) in 40 patients with nocturnal polyuria, although the mechanism involved remains unknown. Kadekawa et al evaluated the effect of dose escalation of imidafenacin to ameliorate nocturia. Sixty patients with OAB received imidafenacin 0.1 mg once daily before going to sleep.Citation40 Of these patients, 21 who had a suboptimal response to imidafenacin (defined as a quality of life score ≥ 3), or opted to escalate their dose (even their QOL score = 2), their night-time frequency was unchanged at 4 weeks (3.8 ± 1.5 times versus 3.6 ± 1.8 times). Escalation of imidafenacin to 0.2 mg once daily before sleeping for an additional 4 weeks resulted in a significant decrease in night-time frequency to 2.8 ± 1.4 times (P = 0.001).
Recent clinical guidelines for benign prostatic hyperplasia and male lower urinary tract symptomsCitation41,Citation42 recommend addition of antimuscarinic agents for patients whose storage symptoms persist after initial treatment with alpha-1 blockers, based on the results of several randomized clinical trials. However, information concerning the efficacy and safety of combination therapy using alpha-1 blockers and imidafenacin is limited.Citation43–Citation46 The randomized, open-label, parallel-group, multicenter ADDITION study was conducted to assess the efficacy and safety of imidafenacin + tamsulosin versus tamsulosin alone in patients aged 50 years or older with OAB/benign prostatic hyperplasia.Citation43 In total, 308 patients with benign prostatic hyperplasia who had urgency (≥one episode/week) and an overactive bladder symptom score (OABSS) ≥3 points after taking tamsulosin for 8 or more weeks were enrolled and randomly allocated to a combination of imidafenacin 0.1 mg twice daily + tamsulosin 0.2 mg/day or tamsulosin 0.2 mg/day alone. After 12 weeks of treatment, total OABSS significantly improved in the combination group compared with the tamsulosin alone group (−4.4 versus −2.1, P < 0.05). There were no serious adverse events in either group and no clinical changes in postvoid residual volume. Thus, addon therapy with imidafenacin is beneficial for men with persistent OAB symptoms despite continued alpha-1 blocker therapy.
In a subanalysis of the EVOLUTION study, treatment with imidafenacin 0.1 mg twice daily for 12 weeks significantly improved daytime frequency, night-time frequency, urgency, urge incontinence on the frequency volume chart, OABSS, nocturia-related quality of life, and sleep disorders associated with nocturia equally in both genders (67 men and 83 women).Citation47 There are no reports published in the English language evaluating the efficacy and safety of imidafenacin in patients who terminated previous antimuscarinic therapy because of lack of efficacy or adverse events. With regard to the short-term influence of imidafenacin on cognitive function, Sakakibara et al reported that imidafenacin 0.1 mg twice daily for 12 weeks did not cause impairment of cognitive function evaluated by the Mini-Mental State Examination (MMSE, used to screen for dementia), the Frontal Assessment Battery (used to assess frontal lobe function), and the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) in 62 elderly patients (mean age 70 years) with OAB of neurogenic origin.Citation33 At baseline and at 12 weeks, the average MMSE (normal range ≥ 24), Frontal Assessment Battery (normal range ≥ 15), and ADAS-cog (normal range < 10) were 21.8 and 22.1, 10.7 and 11.1, and 14.8 and 14.4, respectively; none of these changes were statistically significant.
Long-term efficacy and safety of imidafenacin
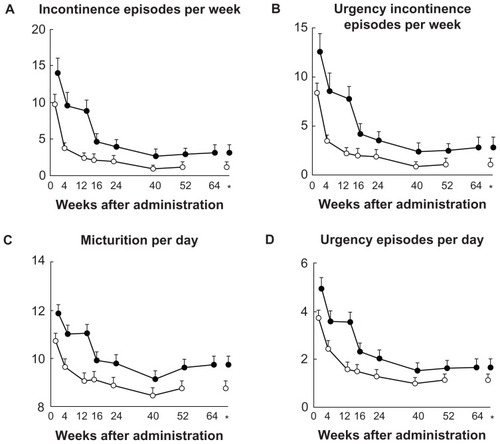
There are very few reports demonstrating the long-term efficacy and safety of imidafenacin.Citation48–Citation51 Homma and Yamaguchi have reported the results of a 52-week, open-label, uncontrolled study conducted at 74 centers in Japan.Citation48 Men and women aged ≥ 20 years who had OAB symptoms with urgency incontinence (at least one episode/week), frequency of micturition (≥8 micturitions/day), and urgency (at least one episode/day) were included in the study. Eligible patients received imidafenacin 0.1 mg twice daily. No dose adjustment was allowed during the study. A total of 478 patients (108 men and 370 women, mean age 59.7 years) received imidafenacin. Of these, 376 (78.7%) completed the 52-week treatment program, whereas 102 patients discontinued due to adverse events in 49 (10.3%), an adverse drug reaction in 27 (5.6%), and lack of efficacy in 13 (2.7%). Imidafenacin was well tolerated even in the long term. Dry mouth, the most frequent adverse event, was reported in 40.2%. Severe, moderate, and mild dry mouth was observed in 0.6%, 6.3%, and 33.3%, respectively. Although constipation was reported in 14.4% of patients, it was not severe. Compared with short-term treatment, long-term therapy did not produce an increase in the frequency of adverse events.Citation11 There were no significant increases in mean correct QTc interval from baseline. Nor were there any clinically relevant changes in mean values for vital signs, laboratory test parameters, or postvoid residual volume. Percent changes from baseline in incontinence episodes per week, urgency incontinence episodes per week, micturitions per day, and urgency episodes per day were significantly decreased (). The percentage of patients whose incontinence episodes completely disappeared increased over time. There were also significant reductions from baseline in all domains of the King’s Health Questionnaire.Citation52 Thus, imidafenacin is expected to be useful for the long-term treatment of symptoms of chronic OAB.
Table 2 Changes from baseline in the efficacy endpoints during 52 weeks of imidafenacin treatment in a long-term, open-label, uncontrolled study in Japan (per protocol set, n = 364)
Another multicenter, open-label study conducted in Japan investigated the safety, tolerability, and efficacy of long-term treatment with imidafenacin 0.2 mg twice daily for 52 weeks in OAB patients with an insufficient effect of imidafenacin 0.1 mg twice daily at 12 weeks.Citation49 Imidafenacin 0.1 mg twice daily was given to 435 patients (71 men and 364 women, mean age 57.5 years) with urgency incontinence (at least one episode/week), frequency of micturition (≥8 micturitions/day), and urgency (at least one episode/day). Of these patients, 182 (41.8%) showed insufficient effects at 12 weeks and received an increased imidafenacin dose of 0.2 mg twice daily. One hundred and fifty-three patients (84.1%) completed an additional 52 weeks of this treatment. Of the 209 patients who continued on the original dose of imidafenacin (0.1 mg twice daily) after 12 weeks, 185 (88.5%) completed an additional 40 weeks of treatment.
Treatment with imidafenacin 0.2 mg twice daily was safe and well tolerated. This dose increase further reduced the numbers of incontinence episodes per week, urgency incontinence episodes per week, micturitions per day, and urgency episodes per day (). Dry mouth and constipation were reported by 26.5% and 9.9% of patients in the 0.1 mg twice daily arm and by 53.3% and 18.7% of patients in the 0.2 mg twice daily arm, respectively. Although the incidences of dry mouth and constipation were twice as high in the 0.2 mg twice daily arm, the majority of these events were mild in severity. Thus, a dose increase to 0.2 mg twice daily can be considered for patients with OAB who are not satisfied with the effect of a standard imidafenacin dose (0.1 mg twice daily).
Figure 1 Changes from baseline in the efficacy endpoints during 52 to 64 weeks of imidafenacin treatment.
Zaitsu et al have reported the results of a 52-week, prospective, randomized, comparative study to evaluate the efficacy and tolerability of two antimuscarinics, ie, imidafenacin and solifenacin.Citation50 Forty-one patients aged 50–79 years with a score for urinary urgency of ≥2 points and a total OABSS ≥ 3 points were enrolled in the study and randomly allocated to receive imidafenacin 0.1 mg twice daily (n = 21) or solifenacin 5 mg once daily (n = 20). Seventeen (80.9%) and 18 (90.0%) of these patients continued imidafenacin and solifenacin, respectively after 12 weeks. The long-term outcome at 52 weeks was compared between 11 patients in the imidafenacin group and 14 in the solifenacin group. Although there was no significant difference in the incidence of dry mouth between imidafenacin and solifenacin (71.4% versus 90.0%, P = 0.2379), dry mouth in the imidafenacin group was significantly less severe than in the solifenacin group (P = 0.0092). In addition, constipation was less frequently reported in the imidafenacin group than in the solifenacin group (14.3% versus 65.0%, P = 0.0013). There were no significant differences in efficacy evaluated by the OABSS (4.3 ± 2.8 versus 5.1 ± 2.1, P = 0.6384) or quality of life evaluated by King’s Health Questionnaire at 52 weeks between the imidafenacin and solifenacin groups. Thus, they concluded that imidafenacin was preferable to solifenacin from the perspective of safety.
The influence of imidafenacin on the long-term cognitive function of OAB patients who had mild cognitive impairment at baseline was prospectively evaluated in a multicenter study in Japan.Citation51 Given that mild cognitive impairment that meets the criteria of complaint of defective memory, normal activities of daily living, normal general cognitive function, abnormal memory function for age, and absence of dementia is a transitional state between normal cognition and dementia, it is likely that subjects with mild cognitive impairment will develop dementia in the future.Citation53 Of 65 OAB patients with mild cognitive impairment at baseline (29 men and 36 women, mean age 76.0 years), only three (4.6%) developed dementia during treatment with imidafenacin 0.1 mg twice daily (annual conversion rate 5.9% per year). Although there was no control arm in this study, it is speculated that this annual conversion rate in OAB patients receiving imidafenacin treatment is comparable with that in the general population, ie, 5%–10%, reported in a meta-analysis.Citation54 Of these 65 patients, 12 were followed for 48 weeks. There was no significant change in MMSE score, being 24.6 ± 2.4 at baseline, 25.0 ± 2.4 at 12 to 24 weeks, and 24.5 ± 2.6 at 48 weeks. Thus, imidafenacin was used safely for a long time, even in cognitively vulnerable patients with OAB symptoms.
Conclusion
To increase adherence with drugs, education and encouragement are necessary to persevere and take the drugs as prescribed.Citation5 However, in general, discontinuation of antimuscarinics for several reasons, such as symptomatic improvement, insufficient efficacy, and adverse events, is widely observed during long-term follow-up in clinical practice.Citation55 In other words, most patients fail to continue the same medication in the long term. Thus, we need information on the long-term efficacy and safety as well as discontinuation rates, and the reasons for discontinuation not only in clinical trials but also in actual clinical practice should be provided. Imidafenacin is clinically available only in Japan, so there are no data available for white or black populations living in western countries. Thus, it remains unknown if the efficacy and safety of imidafenacin demonstrated in Japanese patients are applicable to other ethnic groups.
Disclosure
The author reports no conflicts of interest in this work.
References
- AbramsPCardozoLFallMThe standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence SocietyNeurourol Urodyn20022116717811857671
- HaylenBTde RidderDFreemanRMAn International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunctionNeurourol Urodyn20102942019941278
- HommaYYamaguchiOHayashiKAn epidemiological survey of overactive bladder symptoms in JapanBJU Int2005961314131816287452
- YamaguchiONishizawaOTakedaMClinical guidelines for overactive bladderInt J Urol20091612614219228224
- MarinkovicSPRovnerESMoldwinRMStantonSLGillenLMMarinkvicCMThe management of overactive bladder syndromeBMJ20123443844
- GormleyEALightnerDJBurgioKLDiagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guidelineJ Urol2012188 Suppl 62455246323098785
- NittiVAuerbachSMartinNCalhounALeeMHerschornSResults of a randomized phase III trial of mirabegron in patients with overactive bladderJ Urol2012 pii:S0022-5347(12)05216-0
- KobayashiFYagetaYSegawaMMatsuzawaSEffects of imidafenacin (KRP-197/ONO-8025), a new anti-cholinergic agent, on muscarinic acetylcholine receptors. High affinities for M3 and M1 receptor subtypes and selectivity for urinary bladder over salivary glandArzneimittelforschung2007579210017396619
- KobayashiFYagetaYYamazakiTPharmacological effects of imidafenacin (KRP-197/ONO-8025), a new bladder selective anti-cholinergic agent, in rats. Comparison of effects on urinary bladder capacity and contraction, salivary secretion and performance in the Morris water maze taskArzneimittelforschung20075714715417469649
- HommaYYamaguchiTYamaguchiOA randomized, double-blind, placebo-controlled phase II dose-finding study of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladderInt J Urol20081580981518637156
- HommaYYamaguchiOA randomized, double-blind, placebo- and propiverine-controlled trial of the novel antimuscarinic agent imidafenacin in Japanese patients with overactive bladderInt J Urol20091649950619389083
- MurakamiSYoshidaMIwashitaHPharmacological effects of KRP-197 on the human isolated urinary bladderUrol Int20037129029814512651
- YamazakiTMurakiYAnrakuTIn vivo bladder selectivity of imidafenacin, a novel antimuscarinic agent, assessed by using an effectiveness index for bladder capacity in ratsNaunyn Schmiedebergs Arch Pharmacol201138431932921814879
- YamadaSSekiMOgodaMFukataANakamuraMItoYSelective binding of bladder muscarinic receptors in relation to the pharmacokinetics of a novel antimuscarinic agent, imidafenacin, to treat overactive bladderJ Pharmacol Exp Ther201133636537121047953
- AnderssonK-EYoshidaMAntimuscarinics and the overactive detrusor. Which is the main mechanism of action?Eur Urol2003431512507537
- YokoyamaOTanakaIKusukawaNAntimuscarinics suppress adenosine triphosphate and prostaglandin E2 release from urothelium with potential improvement in detrusor overactivity in rats with cerebral infarctionJ Urol20111852392239721511278
- NishijimaSSugayaKKadekawaKNakaHMiyazonoMComparison of the effect of anti-muscarinic agents on bladder activity, urinary ATP level, and autonomic nervous system in ratsBiomed Res20093010711219420734
- AnderssonKEPotential benefits of muscarinic M3 receptor selectivityEur Urol2002Suppl 12328
- YoshidaAMaruyamaSFukumotoDTsukadaHItoYYamadaSNoninvasive evaluation of brain muscarinic receptor occupancy of oxybutynin, darifenacin and imidafenacin in rats by positron emission tomographyLife Sci20108717518020598326
- YamamotoSMaruyamaSItoYEffect of oxybutynin and imidafenacin on central muscarinic receptor occupancy and cognitive function: a monkey PET study with [11C](+)3-MPBNeuroImage2011581921712096
- ShimadaHYafuneAShibataHHiraharaYMasudaYPhase I clinical study of imidafenacin (KRP-197/ONO-8025): single-dose safety and pharmacokinetics of imidafenacin in healthy subjectsJ Clin Ther Med200723233248
- ShimadaHShibataHHiraharaYMasudaYPhase I clinical study of imidafenacin (KRP-197/ONO-8025): safety and pharmacokinetics of repeated dosage of imidafenacin in healthy subjectsJ Clin Ther Med200723249262
- ShimadaHShibataHHiraharaYMasudaYInvestigation on safety and pharmacokinetic profile of imidafenacin (KRP-197/ONO-8025) after single administration in the elderlyJ Clin Ther Med200723263272
- ShimadaHHasunumaTHiraharaYIshikawaNPharmacokinetic study of imidafenacin (KRP-197/ONO-8025): pharmacokinetics of single oral administration of imidafenacin tablet 0.1 mg and food-effect on its oral absorption in healthy male volunteersJ Clin Ther Med200723273285
- ShimadaHKobayashiHAraiMShimamotoKPharmacokinetic study of imidafenacin 0.1 mg oral disintegrating tablets: evaluation of bioequivalence of oral disintegrated tablets and conventional tablets and assessment of oral mucosal absorption in healthy male volunteersJ Clin Ther Med201127171182
- OhnoTNakadeSNakayamaKAbsolute bioavailability of imidafenacin after oral administration to healthy subjectsBr J Clin Pharmacol20076519720218251758
- OhmoriSMiuraMToriumiCSatohYOoieTAbsorption, metabolism, and excretion of [14C]imidafenacin, a new compound for treatment of overactive bladder, after oral administration to healthy male subjectsDrug Metab Dispos2007351624163317567733
- OhnoTNakadeSNakayamaKPopulation pharmacokinetic analysis of a novel muscarinic receptor antagonist, imidafenacin, in healthy volunteers and overactive bladderDrug Metab Pharmacokinet20082345646319122340
- HasegawaCOhnoTNakadeSPopulation pharmacokinetics and exposure-response relationship of a muscarinic receptor antagonist, imidafenacinDrug Metab Pharmacokinet10232012 [Epub ahead of print]
- KanayamaNKanariCMasudaYOhmoriSOoieTDrug-drug interactions in the metabolism of imidafenacin: role of the human cytochrome P450 enzymes and UDP-glucuronic acid transferases, and potential of imidafenacin to inhibit human cytochrome P450 enzymesXenobiotica20073713915417484517
- KitagawaYKuribayashiMNarimotoKKawaguchiSYaegashiHNamikiMImmediate effect on overactive bladder symptoms following administration of imidafenacinUrol Int20118633033321325789
- MitsuhashiHMatsudaHThe efficacy and safety of imidafenacin for patients with overactive bladderJ New Rem Clin20116020472053
- SakakibaraRTatenoFYanoHSafety and effects of imidafenacin on overactive bladder with neurogenic disease and dementiaRinsho Hinyokika201266775781
- ShimadaMInoueKOkumuraTEfficacy and safety of imidafenacin in female patients with urge and mixed urinary incontinenceHinyokika Kiyo2011571621304252
- TakedaMTakahashiSNishizawaOGotohMYoshidaMImidafenacin, a novel anticholinergic, significantly improves both nocturia and sleep disorders in OAB patients: EPOCH (evaluation of anticholinergics in in patients with overactive bladder and nocturia for cared-health) studyJpn J Urol Surg2009225360
- TakedaMTakahashiSNishizawaOGotohMYoshidaMMasumoriNImidafenacin, a novel anticholinergic, significantly improves nocturia, sleep disorders and quality of life in OAB patients: EVOLUTION (evaluating the value of nocturia-quality of life questionnaire utilization with treatment of imidafenacin for OAB patients suffering from nocturia) studyJpn J Urol Surg20102314431452
- AbrahamLHareendranAMillsIWDevelopment and validation of quality-of-life measure for men with nocturiaUrology20046348148615028442
- YoshidaMGotohMHommaYDevelopment and linguistic validation of the Japanese version of the nocturia quality of life questionnaire (N-QOL)NBS200920317324
- WadaNWatanabeMKitaMEffect of imidafenacin on nocturia and sleep disorder in patients with overactive bladderUrol Int20118921522122832092
- KadekawaKOnagaTShimabukuroSEffect of imidafenacin before sleeping on nocturiaLUTS20124130135
- HommaYGotohMYokoyamaOJUA clinical guidelines for benign prostatic hyperplasiaInt J Urol201118e1e33 Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1442-2042.201102861.x/pdfAccessed January 2, 2013
- OelkeMBachmannADescazeaudAGuidelines on the management of male lower urinary tract symptoms (LUTS), including benign prostatic obstruction (BPO) Available from: http://www.uroweb.org/gls/pdf/12_Male_LUTS_LR%20May%209th%202012.pdfAccessed January 2, 2013
- TakahashiSTakedaMNishizawaOGotohMYoshidaMMasumoriNClinical outcomes of imidafenacin in addition to tamsulosin for patients with overactive bladder and benign prostatic hyperplasia (ADDITION study)Abstract 440 presented at the 42nd Annual Meeting of the International Continence SocietyOctober 15–19, 2012Beijing, China Available from: http://www.icsoffice.org/Abstracts/Publish/134/000440.pdfAccessed January 2, 2013
- NishinoYKikuchiMMasueTMiwaKDeguchiTMoriyamaYCombination therapy with an α1-adrenergic antagonist and an anti-cholinergic agent for patients with prostatic hypertrophy associated with an overactive bladder: combined effects of silodosin and imidafenacinRinsho Hinyokika200963719726
- SuminoYFujitaYYamasakiMEvaluation of imidafenacin in patients with overactive bladder due to benign prostatic hyperplasiaJpn J Urol Surg2010233943
- SengokuAIshikawaJMinayoshiKAn observational study of combined effects of α1 adrenergic antagonist and imidafenacin for patients with overactive bladder associated with benign prostatic hyperplasiaJpn J Urol Surg201025345352
- YoshidaMTakedaMTakahashiSNishizawaOGotohMMasumoriNEffects of imidafenacin in OAB patients with nocturia: EVOLUTION study: analysis of sex differencesProg Med201232In press
- HommaYYamaguchiOLong-term safety, tolerability, and efficacy of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladderInt J Urol20081598699118761536
- YamaguchiOHommaYLong-term efficacy and safety of dose increase study of imidafenacin in patients with overactive bladderJpn Pharmacol Ther200937909930
- ZaitsuMMikamiKTakeuchiTComparative evaluation of the safety and efficacy of long-term use of imidafenacin and solifenacin in patients with overactive bladder: a prospective, open, randomized, parallel-group trial (the LIST study)Adv Urol2011201185469722046182
- SakakibaraRNarumotoJInfluences of imidafenacin (Staybla® tablets) on cognitive function in patients with overactive bladderJpn J Urol Surg20122513811388
- HommaYGotohMAndoTFukuharaSDevelopment of Japanese version of QOL questionnaires for urinary incontinenceJ Neurogenic Bladder Soc199910225236
- PetersenRCSmithGEWaringSCIvnikRJKokmenETangelosEGAging, memory, and mild cognitive impairmentInt Psychogeriatr19979 Suppl 165699447429
- MitchellAJShiri-FeshkiMRate of progression of mild cognitive impairment to dementia: meta-analysis of 41 robust inception cohort studiesActa Psychiatr Scand200911925226519236314
- TanakaYMasumoriNClinical follow-up of patients with propiverine hydrochloride for pollakisuria and/or urinary incontinenceJpn J Urol Surg2008217780