Abstract
Purpose
Medication non-adherence is a huge concern for the medical community. For chronic, especially neurological diseases, taking medication is a central pillar of treatment. To improve adherence to these oftentimes complex medication regimens, the construct needs to be understood in more depth. The aim of this study was to investigate associations between adherence with sociodemographics, clinical variables, and coping in neurological patients.
Patients and Methods
The sample consisted of 545 patients from a German neurological clinic. Adherence was assessed with the Stendal Adherence to Medication Score (SAMS). Patients were grouped as completely adherent (SAMS = 0), non-adherent (upper 25% of the sample), and moderately adherent. Associations with coping were assessed using the Essen Coping Questionnaire.
Results
Medication adherence was low compared to other non-neurological patient samples. Differences between adherence groups were found regarding gender and facets of coping, namely “trivialisation, wishful thinking and defence” and “finding of inner stability”.
Conclusion
Interventions to improve medication adherence should focus on facets of coping with disease, increasing acceptance of disease, willpower, and confidence in treatment.
Introduction
Low adherence to prescribed medication – meaning the extent to which the behavior of patients is consistent with the medical recommendations with which they have agreedCitation1 – is an ever-present and complex problem, especially for patients with a chronic illness: about 25% of patients do not take their drugs as prescribed.Citation2,Citation3 Overall, almost 200 different variables have been studied in their ability to predict adherence, but none of them is consistently associated.Citation4
Up to now, most investigations of non-adherence in patients with chronic diseases focused on arterial hypertension, diabetes mellitus, and asthma.Citation1,Citation5 Regarding neurological diseases, medication adherence is often investigated in patients with epilepsy . Pharmacotherapy is substantial to the treatment of epilepsy, but insufficient adherence varies in patients with epilepsy from 26% to 79% between studies and is associated with a higher risk for seizures and mortality.Citation6,Citation7 Predictors of inadequate adherence include adolescence or young adulthood, the number of drugs and doses to be taken, lack of social support, a poor physician–patient relationship, and disease-related anxieties and stigmatization experiences. In addition, adequate knowledge of the disease and therapy is a premise for adherence,Citation8 although this often is insufficient in epilepsy patients.Citation9,Citation10
Medication non-adherence is also of special importance in patients with Parkinson’s disease – a disease that affects 2% of those over the age of 65.Citation11 A study revealed that 61% of patients with Parkinson’s disease were non-adherent and that the average medical cost per non-adherent patient was $15,826, compared to $9,228 for adherent patients.Citation12 People with Parkinson´s disease often have complex drug regimens, which in progressed disease stages often involve taking different drugs at different times of the day,Citation13 all of which have a negative impact on adherence.Citation14,Citation15 Studies associated non-adherence to young age,Citation14 as well as old age.Citation16 Similar to other chronic diseases, factors such as solitary living, low income, poor knowledge of the disease, cognitive impairment, and depression have a negative impact on adherence.Citation17,Citation18
Since measuring the medication blood level – as the most reliable method – is also the most expensive, self-report instruments for assessing medication adherence are often the only economic way to determine the construct. A systematic review identified 43 self-report scales to assess medication adherence.Citation19 To date, meta-analyses and reviews on the question of medication adherence have concluded that the Morisky scoreCitation20,Citation21 for blood-pressure therapies is the most frequently used self-report, but certainly not the gold standard.Citation22 Since this score only has a “yes-no” answer format and only captures the forgetting of the intake, the carelessness in handling as well as the self-determined discontinuation of the drugs, a new instrument with more items and an expanded answer format was developed. This instrument is called “Stendal Adherence to Medication Score” (SAMS). Applying the ABC Taxonomy from the European Ascertaining Barriers to Compliance ConsortiumCitation23 and World Health Organization recommendations,Citation24 a recent review on rating scales assessing adherence in Parkinson’s disease concluded that the SAMS – like the Morisky score – assesses both factors of adherence (intentional and non-intentional), and two out of five dimensions of adherence (namely patient and therapy).Citation25 Regarding the phases of adherence, the Morisky score and the SAMS assesses the implementation and discontinuation of medication, but the SAMS additionally assesses the initiation of medication.
Neurological diseases in particular can have extensive consequences on the physical, emotional and cognitive level. Patients have to cope with symptoms, treatments, functional impairment, comorbidity and uncertainty about the course of the disease.Citation26 How people with chronic diseases cope with disabilities and the impact of the disease on daily life, as well as the coping resources they use, can have an enormous impact on their quality of life.Citation27,Citation28 In the existing literature, the categorization into active and passive coping has become established to describe disease processing in several neurological disorders. For example, numerous studies showed that passive coping strategies are associated with a poorer health-related quality of life and poorer physical health in Parkinson´s disease.Citation29–Citation33 In this exploratory study, the use of the SAMS was tested in a sample of patients from a neurological clinic. Different levels of adherence were investigated regarding associations with sociodemographic factors, treatment specifics, as well as coping strategies. This is intended to deepen the understanding of medication adherence of neurological patients to develop specific interventions to improve problematic behavior.
Method
Sample
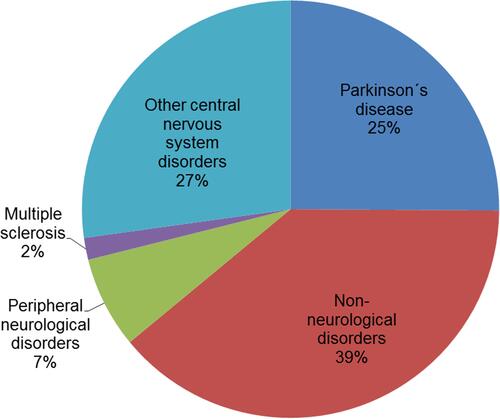
This observational study was approved by the local ethics committee of the Jena University Hospital (4572–10/15). All subjects gave written informed consent in accordance with the Declaration of Helsinki. From January to May 2018, 779 patients who were treated in the Department of Neurology at the Jena University Hospital (inpatient and outpatient) completed a questionnaire assessment. An accruing random sample was investigated in a particular time slot. After this time slot, this study was finished. Therefore, the gathered sample made it possible to draw an image of neurological patients in a single centre. Of this initial sample, which is further described in the SAMS manual,Citation34 we investigated a subsample of N = 545 who provided full questionnaire data. The patients who were excluded due to missing data did not differ in terms of level of adherence (p = 0.798) or gender (p = 0.299), but were on average 6.5 years older (p < 0.001) than the 545 subjects included in this study. Sociodemographic data of this sample are presented in . The mean age of the sample was 62.6 years (±15.94, 18–94), with the majority being male (58.7%), married (63.8%), and living with others (79.5%). Most of the participants had a middle school (37.3%) or high school degree (35.0%) and did not work (76.7%). Two-thirds of those who had a provided diagnosis reported to have a neurological disorder (68.0%). Less than 0.6% reported to have dementia. For more detailed information on the diagnoses, see .
Table 1 Sociodemopgraphic Data for Adherence Groups
Instruments
Stendal Adherence to Medication Score (α = 0.85).Citation34 The SAMS assesses adherence to medication in using a 5-point, Likert-type scale (0 = “never”, to 4 = “most of the time”). It comprises 18 items and 5 additional items that can be administered in inpatient settings (0 = “not at all”, to 4 = “very strongly”). The full SAMS and the handbook are available online (CC BY NC 3.0 licence).Citation34 SAMS was used to investigate the non-adherence to medication in patients after renal transplantation, in patients suffering from chronic painCitation35 and in neurological patients (eg, Parkinson´s disease).Citation36 The SAMS has been used as it is a promising method to detect drug adherence.Citation34 It comprehensively covers different aspects of personal reasons of nonadherence: modification of medication, missing knowledge of medication, and forgetting of medication. Using the SAMS total score (items 1–18), we can differentiate 3 levels of medication adherence:
Complete adherence: Individuals with a sum score of 0 are defined as completely adherent.
Non-adherent: Those 25% of participants with the highest sum scores are categorized as non-adherent.Citation3 The cutoff differs between samples and disease groups.Citation35 In the validation sample and the current subsample, the cutoff was a sum score of ≥10.
Moderately adherent: Participants with scores higher than 0 and lower than the cutoff for non-adherence are categorized as moderately adherent.
Essen Coping Questionnaire (α = 0.89).Citation37 The ECQ assesses emotional, cognitive and behavioral coping in chronically ill patients, using a 5-point, Likert-type scale (0 = “not at all“, to 4 = ”extremely“). It captures distinct forms of Acting, problem-oriented coping (α = 0.81), Distance and self-promotion (α = 0.68), Information seeking and exchange of experiences (α = 0.82), Trivialisation, wishful thinking and defence (α = 0.53), Depressive processing (α = 0.72), Willingness to accept help (α = 0.59), Active search for social integration (α = 0.78), Trust in medical care (α = 0.30), and Finding of inner stability (α = 0.56).
Analysis
Values are given as mean and standard deviation or median and interquartile range. Categorical variables are presented as numbers or percentages. For all analyses, the significance level was set at p < 0.05.
First, the three groups with complete, moderate, and non-adherent behaviour were determined based on the distribution form of the SAMS responses. The differences between these three groups were investigated in terms of sociodemographic and clinical variables by using Chi-squared test and univariate analysis of variance. Differences regarding psychological variables were analysed using multivariate analysis of variance (Wilks Lambda) and identified variables of further interest for univariate analysis. Post-hoc comparisons used correction after Bonferroni in case of variance homogeneity or Games-Howell correction in case variance heterogeneity, determined using Levene’s-test. After controlling for significant sociodemographic covariates, the remaining psychological variables were further investigated on the item-level.
Results
Medication Use and Adherence
Use of Medication
The clinical data of the sample are provided in . On average, this sample consumed 5.7 (± 3.6, 1–21) different drugs per day, with a pill intake of 3.5 (± 2.2, 0–11) pills in the morning, 0.9 (± 1.2, 0–5) pills at noon, 2.1 (± 1.6, 0–8) pills in the evening. On average, they took 6.7 (± 4.2, 1–19) pills per day.
Table 2 Clinical Data for Adherence Groups
Medication Adherence
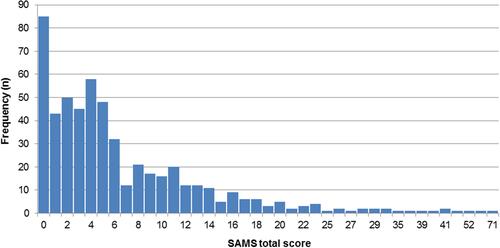
The distribution of the adherence score is depicted in . The mean sum score was 6.8 (± 8.3, 0–71). One-sixth of the sample (15.6%) reported complete adherence with a sum score of 0. A sum score between 1 and 9 was reported by 59.8% of the sample, with a mean of 4.2 (± 2.3). 24.6% of the sample were classified as non-adherent with a sum score of 10 or higher, with a mean of 17.7 (± 10.1).
Adherence Groups
Sociodemographics
The three adherence groups were compared in terms of age, sex, marital status, housing situation, school degree, occupation, and whether they had a neurological disease or not. A statistically significant difference was found regarding sex (Χ2 = 6.27, p < 0.043). Men were significantly less often classified as completely adherent (12.8% vs 19.6%), but more often classified as moderately adherent (63.7% vs 54.2%). No sex difference was found in the non-adherent group.
Use of Medication
No differences between adherence groups were found regarding the number of medications, pills, and intake times ().
Coping with Disease
The three adherence groups differed significantly regarding coping assessed with the ECQ questionnaire (F(18, 1068) = 1.88, p < 0.014). Univariate comparisons regarding the subscales of the ECQ are presented in : Completely adherent participants reported significantly higher levels of Distance and self-promotion compared to non-adherent patients, p < 0.035, higher levels of Trivialisation, wishful thinking and defence compared to moderate and non-adherent patients, p < 0.016 and p < 0.011, and Finding of inner stability, compared to moderate and non-adherent participants, p < 0.034 and p < 0.025. These subscales were further analysed with additional regard to sex, age group, school degree, and diagnosis. Besides the association of distance and self-promotion with adherence groups, an association with age group (p = 0.014) and school degree (p = 0.027) was found. The impact of the adherence group factor lost significance (p = 0.099) after controlling for age (p = 0.002) and education (p = 0.015).
Table 3 Differences Between Adherence Groups on Coping with Disease
Those two scales with no significant covariate were further analysed on the item level; differences between the adherence groups are presented in . Post hoc comparisons using Bonferroni correction revealed on the scale Trivialisation, wishful thinking and defence, that completely adherent participants more often agreed to item 13 “I refuse to accept my condition” compared to moderately adherent and non-adherent participants, p < 0.001 and p < 0.035. Also, the completely adherent participants agreed more to item 23 “I keep on living as if nothing has happened” compared to non-adherent patients, p < 0.003. Regarding the scale Finding of inner stability, completely adherent participants more often agreed to item 28 “I start to see a purpose in the disease” compared to moderately adherent and non-adherent patients, p < 0.001 and p < 0.020. Also, completely adherent participants revealed higher levels on item 42 “I regain my inner strength” compared to non-adherent participants, p < 0.030.
Table 4 Differences Between Adherence Groups on Coping with Disease Items
Discussion
This study investigated individuals from a neurological clinic regarding medication adherence and coping with disease. The sample was derived from one validation sample of SAMSCitation34 and comprised those 545 individuals who provided full data on their coping behaviour. The current sample was, on average, 63 years old and took 5.7 different drugs and 6.7 pills per day. For comparison: The average elderly German of 65+ years of age with statutory health insurance consumes 3.6 doses of long-term medication.Citation38 Polypharmacy can serve as indicator for multimorbidity. The higher number of pills per day in our cohort might therefore reflect multimorbidity. This is not surprising in cohort of people who are hospitalized or treated in specialized university outpatient units.Citation39 Polypharmacy is a common phenomenon in elderly people and was found to be associated with non-adherence to medicationCitation40 However, no association was found in this sample. This can have several reasons. Overall, the medication regimen of our sample seems rather complex compared to other samples. With this number of drugs to be taken, it seems likely that possible effects and benefits of the individual drugs were not known, which might have made non-adherence more likely. Only 15.6% were classified as fully adherent, which is lower compared to other chronically ill elderly people in Germany.Citation41 On the other hand, kidney transplant patients, for example, take about 14 drugs per day, and at the same time their drug adherence is high (29% reported SAMS = 0).Citation34,Citation42 Our sample reported a rather high rate of insufficient adherence with a mean SAMS score of 6.8 (± 8.3), which is higher than in kidney transplant patients (2.9 ± 3.334, t = 9.07, p < 0.001), but lower than in chronic pain patients (8.8 ± 7.734, t = 3.27, p < 0.01). Thus, the frequently discussed differences in medication adherence between different patient groupsCitation3 are also confirmed by the results of this study. Moreover, one has to keep in mind that we assessed self-report adherence. This might also explain the lacking association between polypharmacy and non-adherence which was found in studies using objective methods (eg, electronic pill counting) to assess adherence.
Regarding sociodemographics and clinical data, only gender was confirmed as a differentiating variable. Men were less often classified as completely adherent and more often classified as moderately adherent, which contradicts previous findings of women being less adherent than men in the USCitation43 and specifically in patients with dementia.Citation44 Contradicting previous studies in neurological patients,Citation6,Citation7,Citation14,Citation17,Citation44 age, education, employment, and medication regimen were not associated with adherence. Additionally, no differences were found comparing patients with diseases of the nervous systems with other disease groups. However, it is important to note that the classification by disease was based on what the patients themselves stated in the questionnaire. This does not necessarily correspond to the main and secondary diagnoses recorded by the doctor. A review of the diagnoses and a possibly more correct classification according to the medical record were not carried out in this study.
Regarding coping, adherence was associated with higher levels of Finding of inner stability, as well as Trivialisation and wishful thinking. This is in line with a previous finding linking adherence to higher levels of self-efficacy in patients with epilepsyCitation45 or multiple sclerosisCitation46 as well as findings of higher levels of motivation, willpower and positive attitudes towards disease and treatment in epileptic youth.Citation47 Specifically, completely adherent patients were more likely to report not accepting their condition and to keep on living as nothing has happened, which might be indicative of their perceived self-efficacy. Adherence was also associated with seeing a purpose in the disease – which could be an indicator of inner peace – as well as regaining inner strength. Both are associated with mindfulness.Citation48,Citation49 Mindfulness is positively associated with medication adherence in Alzheimer patientsCitation50 and a recent review considered mindfulness training a promising intervention to increase medication adherence.Citation51 No differences were found regarding the scales focusing on active coping strategies. Overall, it seems that fully adherent patients focus more on distraction and indulgence rather than on their illness and improving their whole lifestyle. This is in line with previous research showing that patients with epilepsy may be adherent with medication, but not with healthful lifestyle behaviours.Citation45,Citation52
According to the WHO, interventions to ensure and increase adherence must be individually tailored to the specific disease-related needs of the patient.Citation24 Common interventions such as psychoeducation and counselling might be of help to improve coping.Citation53 Because self-efficacy is a strong predictor of medication adherence,Citation54,Citation55 empowering patients to effectively cooperate with their physician might additionally increase adherence,Citation56 improving the therapeutic alliance may be one way to empower themCitation57 and to increase adherence.Citation6,Citation58 Patients could also benefit from mindfulness training.
Adherence is a dynamic process that must be followed and reviewed. The extent to which the SAMS can be used for repeat measurements, eg, after interventions to improve adherence, needs to be investigated in future studies.
This study is not free of limitations. Its monocentric design and focus on patients from a university hospital limits the generalizability of the results. Although the sample size is comparable to other studies in people with neurological disorders, results should only be generalized with caution. Multicentre studies with larger sample sizes are necessary to make confirmatory statements about the association between adherence and coping styles in different populations and cohorts. Of the initial sample, only two-thirds provided full questionnaire data, the other third had to be excluded. Analysis showed no non-response bias regarding the level of medication adherence. Nevertheless, those excluded were significantly older, which is in line with previous research identifying old age as a strong determinant of partial nonresponse.Citation59,Citation60 In addition, only self-reported questionnaires were used for analyses. No additional assessments (eg, rating scales for depression or cognition) or data from medical records were collected for this exploratory study. Therefore, cognitive impairment cannot be ruled out as limitation. However, less than 0.6% reported dementia as diagnosis and previous research showed that patients with mild to moderate dementia can provide reliable answers, nonetheless.Citation61 Future studies should confirm the association between different coping styles and adherence by incorporating relevant cofactors such as depression, multimorbidity and cognitive function.
Conclusion
Overall, self-reported medication adherence in this group of patients from a neurological hospital was low compared to other patient groups. The approach of classifying the patients with no adherence problems as fully adherent and the 25% of patients with the highest non-adherence values as non-adherent has proven to be useful. Some group differences were found, mainly regarding different facets of coping. This has several implications for clinical practice and research. Beside common predictors of nonadherence, coping with disease is a promising target for interventions to improve adherence. Following the results of this study, interventions to improve medication adherence in patients with neurological disorders should aim to improve the patients coping strategies in terms of accepting the chronic condition of the disease, strengthening willpower and self-efficacy, and enhancing a positive attitude towards the treatment. Therefore, a well-coordinated overall treatment plan and managed communication between treatment providers is essential.
Disclosure
Dr Tino Prell reports grants from Bundesministerium für Bildung und Forschung (BMBF), during the conduct of the study (01GY1804). The authors report no conflicts of interest in this work.
References
- Osterberg L, Blaschke T. Adherence to medication. N Eng J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
- Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi:10.1136/bmj.38875.675486.55
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi:10.1097/01.mlr.0000114908.90348.f9
- Vermeire E, Hearnshaw H, van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–342. doi:10.1046/j.1365-2710.2001.00363.x
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36(8):1138–1161. doi:10.1097/00005650-199808000-00004
- Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507–515. doi:10.1111/ane.12703
- Specht U. Medikamenten-Compliance bei Epilepsie [Compliance with antiepileptic drugs]. Nervenarzt. 2008;79(6):662–668. doi:10.1007/s00115-008-2408-3
- Petermann F, Tampe T. Compliance bei chronisch kranken Kindern und Jugendlichen [Compliance in children and adolescents with chronic illness]. Kindheit und Entwicklung. 2002;11(1):3–13. doi:10.1026//0942-5403.11.1.3
- Jarvie S. Epilepsy knowledge, beliefs and education. In: Pfäfflin M, Fraser RT, Thorbecke R, Specht U, Wolf P, editors. Comprehensive Care for People with Epilepsy. Eastleigh: John Libbey & Company Ltd; 2001:23–34.
- Long L, Reeves AL, Moore JL, Roach J, Pickering CT. An assessment of epilepsy patients’ knowledge of their disorder. Epilepsia. 2000;41(6):727–731. doi:10.1111/j.1528-1157.2000.tb00235.x
- Nerius M, Ziegler U, Doblhammer G, Fink A. Trends in der Prävalenz von Demenz und Parkinson – eine Analyse auf Basis aller gesetzlich versicherten Personen im Alter 65+ in Deutschland zwischen 2009 und 2012 [Trends in the Prevalence of Dementia and Parkinson’s Disease: an Analysis Based on Health Claims Data from all German Statutory Health Insurance Funds for Persons aged 65+ in Germany 2009–2012]. Gesundheitswesen. 2020;82(10):761–769. doi:10.1055/a-0829-6494
- Davis KL, Edin HM, Allen JK. Prevalence and cost of medication nonadherence in Parkinson’s disease: evidence from administrative claims data. Mov Disord. 2010;25(4):474–480. doi:10.1002/mds.22999
- Leoni O, Martignoni E, Cosentino M, et al. Drug prescribing patterns in Parkinson’s disease: a pharmacoepidemiological survey in a cohort of ambulatory patients. Pharmacoepidemiol Drug Saf. 2002;11(2):149–157. doi:10.1002/pds.682
- Grosset KA, Bone I, Grosset DG. Suboptimal medication adherence in Parkinson’s disease. Mov Disord. 2005;20(11):1502–1507. doi:10.1002/mds.20602
- Á S, Arbelo JM, Del Val JL. Treatment of Parkinson disease, time and dosage: “does simple dosage facilitate compliance and therapeutic goals?”. Neurologist. 2011;17:S43–46. doi:10.1097/NRL.0b013e31823968d3
- Wei Y-J, Palumbo FB, Simoni-Wastila L, et al. Antiparkinson drug use and adherence in medicare part d beneficiaries with Parkinson’s disease. Clin Ther. 2013;35(10):1513–1525. doi:10.1016/j.clinthera.2013.09.001
- Malek N, Grosset DG. Medication adherence in patients with Parkinson’s disease. CNS Drugs. 2015;29(1):47–53. doi:10.1007/s40263-014-0220-0
- Daley DJ, Myint PK, Gray RJ, Deane KHO. Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(10):1053–1061. doi:10.1016/j.parkreldis.2012.09.004
- Nguyen T-M-U, La Caze A, Cottrell N. What are validated self‐report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. 2014;77(3):427–445. doi:10.1111/bcp.12194
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
- Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi:10.1111/j.1751-7176.2008.07572.x
- Culig J, From LM. Morisky to Hill-bone; self-reports scales for measuring adherence to medication. Coll Antropol. 2014;38(1):55–62.
- Vrijens B, De Geest S, Hughes DA. Geest S de, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clinl Pharmacol. 2012;73(5):691–705. doi:10.1111/j.1365-2125.2012.04167.x
- Sabaté E. Adherence to long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003.
- Tosin MHS, Stebbins GT, Goetz CG, Santana RF, Leite MAA, Oliveira BGR. Measuring medication adherence in Parkinson’s disease: a systematic review of contributing components in rating scales. Mov Disord Clin Pract. 2020;7(6):607–615. doi:10.1002/mdc3.13006
- Kristofferzon M-L, Engström M, Nilsson A. Coping mediates the relationship between sense of coherence and mental quality of life in patients with chronic illness: a cross-sectional study. Qual Life Res. 2018;27(7):1855–1863. doi:10.1007/s11136-018-1845-0
- Martz E, Livneh H, eds. Coping with Chronic Illness and Disability. New York: Springer; 2007.
- Mikula P, Nagyova I, Krokavcova M, et al. Coping and its importance for quality of life in patients with multiple sclerosis. Disabil Rehabil. 2014;36(9):732–736. doi:10.3109/09638288.2013.808274
- Backer JH. Stressors, social support, coping, and health dysfunction in individuals with Parkinson’s disease. J Gerontol Nurs. 2000;26(11):6–9. doi:10.3928/0098-9134-20001101-05
- Frazier LD. Coping with disease-related stressors in Parkinson’s disease. Gerontologist. 2000;40(1):53–63.
- Schreurs KMG, De Ridder DTD, Bensing JM. A one year study of coping, social support and quality of life in Parkinson’s disease. Psychol Health. 2000;15(1):109–121. doi:10.1080/08870440008400292
- Montel S, Bonnet A-M BC. Quality of life in relation to mood, coping strategies, and dyskinesia in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2009;22(2):95–102. doi:10.1177/0891988708328219
- Bucks RS, Cruise KE, Skinner TC, Loftus AM, Barker RA, Thomas MG. Coping processes and health‐related quality of life in Parkinson’s disease. Int J Geriatr Psychiatry. 2011;26(3):247–255. doi:10.1002/gps.2520
- Franke GH, Nentzl J, Jagla-Franke M. SAMS - Stendal Adherence with Medication Score.: deutsches Manual. Psychometrikon. 2020. Available from: https://www.psychometrikon.de/inhalt/suchen/test.php?id=ff32ee9ea015021c3fb047e505e2bc45. Accessed April 17, 2021.
- Franke GH, Nentzl J, Küch D, Die J-FM. Erfassung der Medikamenten-Adhärenz bei Schmerzpatientinnen und -patienten [Assessment of adherence to medication in pain patients]. Klinische Verhaltensmedizin und Rehabilitation. 2020;33(2):27–39.
- Prell T, Grosskreutz J, Mendorf S, Franke GH, Witte OW, Kunze A. Clusters of non-adherence to medication in neurological patients. Res Social Adm Pharm. 2019;15(12):1419–1424. doi:10.1016/j.sapharm.2019.01.001
- Franke GH, Jagla M ECQ – essen Coping Questionnaire Test Manual – English version. Psychometrikon. Available from: http://psychometrikon.de/inhalt/suchen/test.php?id=b2c6285c00324df0956e23257eb0e176. Accessed April 17, 2021.
- Coca V, Nink K, Schröder H. Arzneimittelverordnungen nach Alter und Geschlecht. In: Schwabe U, Paffrath D, editors. Arzneiverordnungs-Report 2007. Berlin: Springer; 2008:919–932.
- Kruse A, Gaber E, Heuft G, Oster P, Re S, Schulz-Nieswandt F. Themenheft 10” Gesundheit im Alter”. Berlin: RKI; 2002.
- Marcum ZA, Gellad WF. Medication adherence to multidrug regimens. Clin Geriatr Med. 2012;28(2):287–300. doi:10.1016/j.cger.2012.01.008
- Jüngst C, Gräber S, Simons S, Wedemeyer H, Lammert F. Medication adherence among patients with chronic diseases: a survey-based study in pharmacies. QJM. 2019;112(7):505–512. doi:10.1093/qjmed/hcz058
- Jäger S, Franke GH, Reimer J, et al. Der Zusammenhang zwischen Medikamenten-Compliance und gesundheitsbezogener Lebensqualität bei Nierentransplantierten [Relationship between adherence to medication and health related quality of life in kidney transplanted patients]. In: Klinische Psychologie AK, im BDP, editor. Psychische Störungen in der somatischen Rehabilitation. Berlin: dpv;2009:79–93.
- Manteuffel M, Williams S, Chen W, Verbrugge RR, Pittman DG, Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt). 2014;23(2):112–119. doi:10.1089/jwh.2012.3972
- El-Saifi N, Moyle W, Jones C, Tuffaha H. Medication adherence in older patients with dementia: a systematic literature review. J Pharm Pract. 2018;31(3):322–334. doi:10.1177/0897190017710524
- Kobau R, DiIorio C. Epilepsy self-management: a comparison of self-efficacy and outcome expectancy for medication adherence and lifestyle behaviors among people with epilepsy. Epilepsy Behav. 2003;4(3):217–225. doi:10.1016/S1525-5050(03)00057-X
- Fraser C, Morgante L, Hadjimichael O, Vollmer T. A prospective study of adherence to glatiramer acetate in individuals with multiple sclerosis. J Neurosci Nurs. 2004;36(3):120–130. doi:10.1097/01376517-200406000-00002
- Kyngäs H. Compliance with health regimens of adolescents with epilepsy. Seizure. 2000;9(8):598–604. doi:10.1053/seiz.2000.0470
- Bajaj B, Pande N. Mediating role of resilience in the impact of mindfulness on life satisfaction and affect as indices of subjective well-being. Pers Individ Dif. 2016;93:63–67. doi:10.1016/j.paid.2015.09.005
- Liu X, Xu W, Wang Y, et al. Can inner peace be improved by mindfulness training: a randomized controlled trial. Stress Health. 2015;31(3):245–254. doi:10.1002/smi.2551
- Lima S, Gago M, Garrett C, Pereira MG. Medication adherence in Alzheimer’s disease: the mediator role of mindfulness. Arch Gerontol Geriatr. 2016;67:92–97. doi:10.1016/j.archger.2016.06.021
- Salmoirago-Blotcher E, Carey MP. Can mindfulness training improve medication adherence? Integrative review of the current evidence and proposed conceptual model. Explore (NY). 2018;14(1):59–65. doi:10.1016/j.explore.2017.09.010
- Dilorio C, Henry M. Self-management in persons with epilepsy. J Neurosci Nurs. 1995;27(6):338–343. doi:10.1097/01376517-199512000-00004
- Nieuwlaat R, Wilczynski N, Navarro T. et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev;2014. 11. doi:10.1002/14651858.CD000011.pub4
- Dunbar J. Predictors of patient adherence: patient characteristics. In: Shumaker SA, Schron EB, Ockene JK, Parker CT, Probstfield JL, Wolle JM, editors. The Handbook of Health Behavior Change. New York: Springer; 1990:348–360.
- Lamarche L, Tejpal A, Mangin D. Self-efficacy for medication management: a systematic review of instruments. Patient Prefer Adherence. 2018;12:1279–1287. doi:10.2147/PPA.S165749
- Náfrádi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One. 2017;12(10):e0186458. doi:10.1371/journal.pone.0186458
- Pagès-Puigdemont N, Mangues MA, Masip M, et al. Patients’ perspective of medication adherence in chronic conditions: a qualitative study. Adv Ther. 2016;33(10):1740–1754. doi:10.1007/s12325-016-0394-6
- Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med. 2017;99:269–276. doi:10.1016/j.ypmed.2017.03.008
- Eaker S, Bergström R, Bergström A, Adami HO, Nyren O. Response rate to mailed epidemiologic questionnaires: a population-based randomized trial of variations in design and mailing routines. Am J Epidemiol. 1998;147(1):74–82. doi:10.1093/oxfordjournals.aje.a009370
- Hardie JA, Bakke PS, Mørkve O. Non-response bias in a postal questionnaire survey on respiratory health in the old and very old. Scand J Public Health. 2003;31(6):411–417. doi:10.1177/140349480303100603
- Trigg R, Jones RW, Skevington SM. Can people with mild to moderate dementia provide reliable answers about their quality of life? Age Ageing. 2007;36(6):663–669. doi:10.1093/ageing/afm077