Abstract
Background
Adherence to pharmacological therapy is a complex and multifactorial issue that can substantially alter the outcome of treatment. Especially when using long-term medication, cancer patients have adherence rates similar to those of patients with other diseases. The consequences of poor adherence are poor health outcomes and increased health care costs. Only few studies have focused on the use of oral anticancer agents in daily practice. Information about the reasons for nonadherence is essential for the development of interventions that may improve adherence. This report presents the CAPER-capecitabine protocol, which is designed to study the adherence to capecitabine and the influence of patient attitudes towards medication and self-reported side effects. Furthermore, the relationships between patient characteristics, disease characteristics, side effects, quality of life, patient beliefs and attitudes towards disease and medication, dose adjustments, reasons for discontinuation, and plasma concentration of three of the main metabolites, including the active compound 5-fluorouracil, will be explored.
Methods
In this multicenter, prospective, observational cohort study, 90 patients aged 18 years or older starting treatment with capecitabine will be included and followed for a period up to five cycles. The main study parameters are adherence, patient attitudes towards medication, and the number and grade of patient-reported side effects. At baseline and during week 2 of cycles 1, 3 and 5, patients will be asked to donate blood and fill out a questionnaire. Blood samples will be analyzed for plasma concentration of the metabolites, 5′-deoxy-5-fluorouridine, 5-fluorouracil, and α-fluoro-β-alanine. The CAPER-capecitabine trial is closely related to the CAPER-erlotinib trial.
Discussion
The aim of the present study is to get more insight into patient experiences with the use of capecitabine in daily practice and the various aspects that govern adherence. We hypothesize that patient attitudes towards medication and the side effects experienced play an important role in the way patients use capecitabine. We expect that our findings will be useful for health care professionals in developing interventions to support patients in improving adherence and persistence with the use of capecitabine.
Background
Intravenous administration is an important route for pharmacological treatment of cancer. Over the last decade, a growing number of oral substances have been introduced in cancer treatment. Most patients prefer oral use of anticancer agents as long as it does not compromise the outcome of treatment.Citation1–Citation3 In addition, the overall costs of oral treatment are often lower than those of intravenous therapy.Citation4–Citation6 However, with the use of oral medication at home, the issue of adherence needs to be considered.
Adherence
Adherence to oral pharmacological therapy is a complex and multifactorial issue that can substantially alter the outcome of therapy.Citation7,Citation8 Adherence (synonymous with compliance) has recently been defined by the International Society for Pharmacoeconomics and Outcome Research as the extent to which a patient acts in accordance with the prescribed interval and dose of a drug regimen.Citation9 A patient is optimally adherent if no doses are missed, no extra doses are taken, and no doses are taken in the wrong quantity or at the wrong time. Adherence is measured over a period of time and reported as the adherence rate, which is the percentage of dose taken in relation to what was prescribed.Citation10 There are several methods used to measure adherence, including self-reports, pill counts, electronic monitoring systems, analyses of pharmacy dispensing records, and assessment of blood or urine samples. There is no golden standard measurement and all methods have limitations.Citation10,Citation11 The major limitation of measuring adherence is the so-called Hawthorne effect, ie, the monitoring of adherence itself influences adherence, because the awareness of patients that adherence is being monitored may influence their behavior. Adherence rates for many chronic drug therapies range between 35% and 70%.Citation7,Citation12 The consequences of poor adherence are poor health outcomes and increased health care costs.Citation7
Adherence in oncology
Cancer patients are generally thought to have higher adherence rates than other patients because they are highly motivated by the gravity of their disease.Citation13,Citation14 However, cancer patients appear to have similar adherence rates to those of patients with other diseases.Citation10,Citation15,Citation16 Treatment duration plays a role in adherence to the regimen. When the medication is continued over a longer period of time, patients become less adherent.Citation17 The measurement of adherence with medication that is used in complex cyclic schedules with stop periods and many individual adjustments during treatment, as often occurs in oncology, is challenging, since this is more difficult than the measurement of medication used on a regular daily basis.
In oncology, adherence has been studied mainly in two subpopulations, both using long-term medication. In the first population, adherence of patients with breast cancer to adjuvant hormonal therapy has been the subject of several studies.Citation10 Reported adherence rates range from 50% to 98%.Citation14,Citation17,Citation18 Several studies concerning adherence with oral medication have been published in the second population, being patients with chronic myeloid leukemia.Citation16,Citation19,Citation20 Nonadherence has been associated with a poorer response to imatinib.Citation16,Citation19 Noens et al have shown that patients with a suboptimal response had higher percentages of imatinib not taken (23%) than those with optimal response (7%).Citation16 Marin et al have also demonstrated that there was a strong correlation between adherence rate and response in patients with chronic myeloid leukemia.Citation19 Adherence was the only critical factor for achieving a molecular response. The results of these studies cannot be translated directly to adherence with other oral anticancer drugs, because their use differs mainly with respect to duration of treatment and the toxicity generated by the treatment.
Another frequently overlooked problem is over adherence, which may be more an issue in oncology than in other diseases and may lead to substantially increased toxicity.Citation11 In the study by Nilsson et al, 30% of cancer patients had an oversupply of their medication.Citation15
Medical oncologists and hematologists may not always consider the issue of adherence. As yet, suboptimal adherence may prove to be the greatest barrier to the efficacy of the various newly introduced oral anticancer agents.Citation10,Citation19
Causes of nonadherence
Several factors are associated with nonadherence. These include patient variables, such as demographic factors and patients’ beliefs, and disease variables, various aspects of treatment regimens, side effects, and quality of life.
The commonsense model of self-regulation developed by Leventhal et alCitation21 is a theoretical model for understanding patient perceptions of illness.Citation22 According to the commonsense model of self-regulation, patient perception of and beliefs about their illness are important factors in their reactions and behavior to health threats. Illness perceptions can easily be measured with the Brief Illness Perceptions Questionnaire.Citation23 Another factor which has been shown to influence adherence is patient beliefs about the medication, often measured with the Beliefs about Medicines Questionnaire (BMQ).Citation24
Few studies have focused on patients’ reasons for not adhering to oral anticancer agents. Eliasson et al have reported that finding ways to deal with side effects leads to better adherence in patients with chronic myeloid leukemia.Citation25 In clinical trials, adverse events are generally reported by clinicians using, eg, the National Cancer Institute Common Terminology Criteria for Adverse Events.Citation26 Basch et al have shown the value of patient-reported adverse events in cancer patients, which better reflect their daily health status.Citation27–Citation29
Capecitabine
Capecitabine (Xeloda®) is an oral anticancer agent, which was introduced in 2001. Capecitabine is an oral prodrug for 5-fluorouracil (5-FU). It is one of a number or oral formulations of 5-FU (UFT, S-1, doxifluridine, capecitabine) which were developed to mimic continuous infusions (defined as one week or longer), which are associated with longer survival than the commonly used intravenous bolus administration schedules (eg, the Roswell Park and Mayo schedules).Citation30,Citation31 These schedules were standard until early 2000. Continuous administration of 5-FU leads to prolonged exposure to low concentrations of 5-FU which has been shown both in vitro and in vivo to be more effective than short exposure to high concentrations. Capecitabine is registered in Europe for the treatment of advanced colorectal cancer, gastric cancer, and breast cancer.Citation32 It is also registered for the adjuvant treatment of patients following surgery of stage III (Dukes’ stage C) colon cancer, either given alone or combined with either oxaliplatin or irinotecan. Capecitabine is commonly used in a 3-week treatment cycle, with an intake of capecitabine twice daily for 14 days followed by a 7-day rest period.
The most common side effects of capecitabine are gastrointestinal disorders (especially diarrhea, nausea, vomiting, abdominal pain, and stomatitis), hand-foot syndrome (palmar-plantar erythrodysesthesia), fatigue, asthenia, anorexia, cardiotoxicity, increased renal dysfunction in those with pre-existing compromised renal function, and thrombosis/embolism.Citation32
Several studies have demonstrated that capecitabine is as effective as intravenous 5-FU. Borner et al have reported in a crossover study showing that patients preferred oral capecitabine to intravenous 5-FU.Citation3 However, using a similar study design, Pfeiffer et al observed that patients prefer the regimen with the lowest toxicity and that the route of administration is of minor importance.Citation33 The use of capecitabine has also been the subject of pharmacoeconomic analyses. Several studies have shown the cost-effectiveness of treatment with capecitabine as compared with that of 5-FU in patients with colorectal cancer or compared with intravenous taxane-based therapy in patients with breast cancer.Citation34–Citation36
Adherence with capecitabine has been the subject of a few studies. In a review published by Ruddy et al, only one study concerning adherence with capecitabine was included. Citation10 Partridge et al reported at the 2008 American Society of Clinical Oncology annual meeting that 76% of 161 breast cancer patients took at least 80% of their prescribed capecitabine doses, as measured by an electronic monitoring system during a six-cycle period.Citation37 Winterhalder et al assessed adherence to capecitabine with self-reporting in diaries.Citation38 They observed that 91% of patients were adherent with all doses. A similar rate has been reported by Simons et al using an electronic monitoring system.Citation39
Pharmacokinetics
Capecitabine is rapidly and extensively absorbed through the gastrointestinal wall as an intact molecule, and rapidly metabolized to 5-FU via a three-step enzymatic cascade. First, it is metabolized to 5′-deoxy-5-fluorocytidine (5′-DFCR), thereafter to 5′-deoxy-5-fluorouridine (5′-DFUR), and subsequently to the active compound 5-FU. 5-FU is metabolized to dihydrofluorouracil, 5-fluoro-ureido-propionic acid, and α-fluoro-β-alanine (FBAL, see ).Citation40 When taken with food at a dose of 1250 mg/m2 on day 14, peak plasma concentrations (Cmax) of capecitabine, 5′-DFCR, 5′-DFUR, 5-FU, and FBAL are 4.7, 3.1, 12.1, 1.0, and 5.5 μg/mL, respectively. Peak plasma concentrations are reached approximately 1.5–3.5 hours after administration. The elimination half-life (t1/2) of capecitabine and its metabolites in plasma is short, ie, 1–3 hours.Citation32
Figure 1 Metabolic pathways for capecitabine and tegafur. (A) Capecitabine is converted into active metabolite in situ by thymidine phosphorylase or uridine phosphorylase. Further 5-fluorouracil catabolism is initiated by dihydropyrimidine dehydrogenase, eventually yielding FBAL, a catabolite implicated in the etiology of hand-foot syndrome. (B) Tegafur is activated by cytochrome P450 2A6, forming 5-hydroxytegafur, an unstable intermediate which spontaneously converts to 5-fluorouracil. Reprinted by permission from Yen-Revollo JL, Goldberg RM, McLeod HL. Can inhibiting dihydropyrimidine dehydrogenase limit hand-foot syndrome caused by fluoropyrimidines? Clin Cancer Res. 2008;14:8–13.
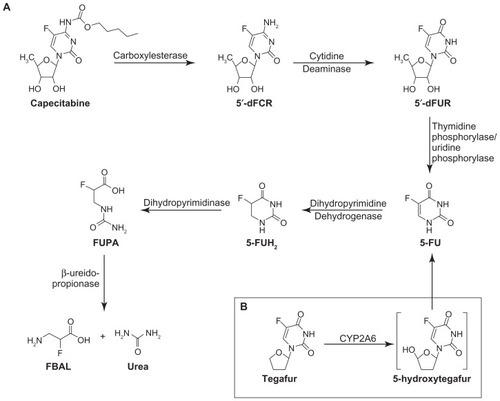
There is considerable variability in the plasma concentrations of capecitabine and its metabolites.Citation41 However, it should be realized that activation to 5-FU takes place in the tumor. The enzyme involved in the final conversion to 5-FU (thymidine phosphorylase), is found in tumor tissues, but also in normal tissues, albeit usually at lower levels; 5-FU concentrations in plasma can be considered as a reflection of the release from tumors and not as a reliable pharmacokinetic parameter for capecitabine. Moreover, it has been demonstrated that 5-FU has a relatively long tissue half-life, both in animal systems and in patients.Citation42 With use at home, variations in plasma concentration may also be influenced by adherence with capecitabine. It is hypothesized that a higher adherence rate will lead to higher plasma concentrations which, in turn, may lead to better survival rates.
Intake of food prior to or together with administration of capecitabine results in a longer time taken to reach peak plasma concentrations and a lower Cmax of capecitabine and its metabolites. The manufacturer advises in the European Public Assessment Report that “the tablets should be swallowed with water within the 30 min after a meal”.Citation32,Citation43 Renal impairment has no effect on the pharmacokinetics of capecitabine or 5-FU, but leads to an increased exposure of 5′-DFUR and FBAL and therefore requires dose modification of capecitabine.Citation44 Twelves et al have reported that plasma concentrations of capecitabine, 5′-DFUR, 5-FU, and FBAL were, in general, higher in patients with liver dysfunction, while the opposite was found for 5′-DFCR.Citation45 There were no clinical relevant differences in the adverse effects between patients with and without hepatic dysfunction.
Ethnicity may also influence the pharmacokinetics of capecitabine and its metabolites. In Caucasian patients, higher plasma concentrations of FBAL were measured than in Japanese patients.Citation46
Genetic factors may affect the efficacy and toxicity of capecitabine related to the activity of the enzymes involved in its metabolism. Mutations and polymorphisms in dihydropyrimidine dehydrogenase, resulting in decreased degradation of 5-FU (from capecitabine) is associated with severe toxicity.Citation47–Citation49 Other mutations and polymorphisms may result in altered activity of cytidine deaminase or carboxylesterase, thereby influencing the efficacy and toxicity of capecitabine.Citation50,Citation51
A small increase in plasma concentration of capecitabine and 5′-DFCR may occur when given concurrently with an antacid containing aluminum or magnesium hydroxide. This has not been shown for 5′-DFUR, 5-FU, and FBAL.Citation52
The various factors described above have to be taken into account when the relationship between plasma concentrations and adherence rates is studied in patients using capecitabine. The mutations and polymorphisms will not be evaluated in the present study, but may be part of future research.
Several studies on the efficacy, side effects, and pharmacokinetics of capecitabine have shown that plasma concentrations of 5′-DFUR, 5-FU, and FBAL are related to efficacy and toxicity. Gieschke et al have observed that the Cmax of 5′-DFUR was positively associated with survival and that the area under the concentration-time curve (AUC) of 5-FU was negatively associated with time to disease progression.Citation53 This is in contrast with data on 5-FU in which a higher AUC of 5-FU was associated with a longer survival.Citation54 In general, in 5-FU administration schedules, a clear relationship was found for the AUC of 5-FU and both hematological and gastrointestinal toxicity.Citation55,Citation56 In patients with grade 3–4 diarrhea, the AUC of FBAL was proportionately increased. Grade 3–4 hyperbilirubinemia was positively associated with the AUC of 5-FU and negatively associated with the Cmax of FBAL.Citation57 Plasma concentrations of capecitabine and 5′-DFCR were not related to toxicity.Citation53
Capecitabine can cause hand-foot syndrome, which is most likely associated with accumulation of the breakdown product FBAL, given that in formulations in which dihydropyrimidine dehydrogenase is inhibited, no hand-foot syndrome was observed.Citation40 Because side effects may be a reason for nonadherence, the plasma concentrations of 5′-DFUR, 5-FU, and FBAL are analyzed in this study.
The primary objective in this study is adherence in patients with cancer starting capecitabine and the influence of patients’ attitudes and side effects on adherence. The second part of this study contains a validation study of the adherence measurements and an explorative study on the relationships between the following parameters: patient characteristics, disease characteristics, side effects, quality of life, patients’ beliefs and attitudes towards disease and medicines, adherence, dose adjustments, and plasma 5′-DFUR, 5-FU, and FBAL concentrations.
Materials and methods
Study design
This is a prospective observational multicenter cohort study in which cancer patients starting on treatment with capecitabine will be followed for up to five 3-weekly cycles. The trial will end when the last patient has been followed for five cycles. The CAPER-capecitabine trial (NTR2324) is closely related to the CAPER-erlotinib trial (NTR1830) which is being performed simultaneously.Citation58 The protocol was approved by the medical ethics review board of the VU University Medical Center, Amsterdam, The Netherlands.
Recruitment and consent
Patients are to be recruited by the departments of medical oncology from 10 hospitals in The Netherlands. After a patient and physician have decided on treatment with capecitabine, the patient will be informed about the study and receive written information to take home. Within 2 days, the researcher contacts the patient by phone to inform him/her further about the study and to ask for his/her consent. The patient will be asked to sign the informed consent form.
Inclusion criteria
Patients on treatment in one of the participating hospitals starting with the use of capecitabine can be included. Patients younger than 18 years and those with insufficient knowledge of the Dutch language are excluded. According to the sample size calculation (see Statistical analysis) a total number of 90 patients are to be enrolled.
Measurements
Several methods are used to determine the variables. Most information is collected directly from patients, ie, they will fill out questionnaires and donate blood samples. Furthermore, medical information is retrieved from the patients’ file.
Questionnaires
Patients will be asked to fill out four questionnaires spread over a 14-week period. The first questionnaire (t = 0) contains questions about patient characteristics, comedication, side effects, quality of life, and patients’ beliefs and attitude towards disease and medication. In week 2 of cycles 1, 3 and 5, patients are asked to fill out an elaborate questionnaire containing questions determining the adherence behavior, side effects, dose adjustment, comedication, quality of life, and their beliefs and attitude towards the disease and medication. Discontinuation and reasons for discontinuation are to be canvassed in a short questionnaire when a patient discontinues capecitabine treatment prematurely.
The questionnaires include the following items:
The MARS (Medication Adherence Report Scale) questionnaireCitation59,Citation60 contains statements about adherence behavior, and the nature and grade of patient-reported side effects on a five-point scale.
Quality of life measured with the Short Form (SF)-12 Health Survey.Citation61,Citation62 The SF-12 is a short version of the SF-36 and is a validated method for measurement of quality of life.
Attitude towards disease is measured using the Brief Illness Perception Questionnaire.Citation23 The Brief Illness Perception Questionnaire is a validated method measuring attitudes towards disease.
Beliefs and attitudes towards medicines are measured with the validated BMQ questionnaire.Citation24 The BMQ is divided into two parts, ie, the BMQ general and the BMQ specific. The BMQ general measures patient beliefs and attitudes towards medicines in general, whereas the BMQ specific is specific for capecitabine.
Patient characteristics, ie, date of birth, gender, and socioeconomic status.
Dose adjustments by the patient, with dose adjustments introduced by the physician to be derived from the patient file.
Comedication.
Discontinuation and reasons for discontinuation. Discontinuation will also be determined from the patient file.
Patient file
The following items are derived from the patient treatment file:
Disease characteristics, including disease stage and line of treatment
Dose adjustments
Discontinuation and reasons for discontinuation.
Patient files-pharmacy records-pill count method
This pill count method will take into account the cyclic dosing regimen with stop periods and individual adjustments made by the physician, such as postponing a cycle or dose changes. Patients will be contacted by the researcher by telephone to count their remaining pills at that moment. The dispensing records of the pharmacies used by the patient will be assessed. Patients will be asked whether they have returned pills at the pharmacy or disposed of pills in any other way. The actual number of pills used will be calculated from this information (dispensings minus adjustments minus pill count) and compared with the prescribed number of pills, as registered in the patient’s medical file, to calculate the adherence rate.
Blood samples
Before the start of treatment with capecitabine, baseline blood samples are collected. In the second week of cycles 1, 3, and 5, blood samples are collected. At these visits, patients are asked when they ate their last meal and at what time they took their last capecitabine medication. The timing of collection of the blood sample will be recorded. The blood samples will be analyzed for plasma concentrations of 5′-DFUR, 5-FU, and FBAL using a validated liquid chromatography/tandem mass spectrometry method. Capecitabine is most commonly used in a 3-week treatment cycle, with an intake of capecitabine twice daily for 14 days followed by a 7-day rest period.Citation32 In the event of a longer rest period, the number of additional rest days will be added to the schedule to make sure the measurements always take place in the second week of each cycle.
Statistics
The primary determinant of interest is patient attitudes towards anticancer drugs at the start of therapy. We will test the hypothesis that the probability of nonadherence does not differ between those with a negative attitude and those with a positive attitude towards capecitabine, against the one-sided alternative that nonadherence is higher in those with a negative attitude. Based on previous studies, the percentage of patients who are nonadherent with drug therapy is estimated at 35% for patients with a negative attitude towards capecitabine, and at 10% for patients with a positive attitude towards capecitabine. To test the hypothesis at a significant level of 0.05, we need 33 patients with a positive attitude and 33 patients with a negative attitude to achieve 80% power. To reach the required number of evaluable patients, a total number of 90 patients will be included in the study. The statistical analyses will be performed using SPSS version 20.0 (SPSS Inc, Chicago, IL).
Discussion
The present study aims to obtain more insight into patient experiences with the use of capecitabine in daily practice. We expect variability in adherence among the general oncology population. This may be complicated by interpatient variability in pharmacokinetics. We hypothesize that patient attitudes towards anticancer drugs and side effects experienced play an important role in the way patients use capecitabine. Therefore, the relationship between attitude, self-reported side effects, and adherence is also defined as a main objective. To obtain more insight into other factors related to adherence and other aspects of use at home, the effects of the determinants are studied in an explorative manner. We expect that the present study will provide valuable knowledge on patient experiences with the use of capecitabine. This knowledge will be useful for health care professionals to develop specific interventions to support patients with the use of capecitabine in order to derive optimal benefit from the medication.
Disclosure
The authors received an unrestricted grant from Roche, The Netherlands, to perform this research. Otherwise, the authors declare that they have no competing interests in this work.
Reference
- FallowfieldLAtkinsLCattSPatients’ preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancerAnn Oncol20061720521016239231
- LiuGFranssenEFitchMIWarnerEPatient preferences for oral versus intravenous palliative chemotherapyJ Clin Oncol1997151101158996131
- BornerMScheithauerWTwelvesCMarounJWilkeHAnswering patients’ needs: oral alternatives to intravenous therapyOncologist20016Suppl 4121611585969
- KruseGBAmonkarMMSmithGSkoniecznyDCStavrakasSAnalysis of costs associated with administration of intravenous single-drug therapies in metastatic breast cancer in a U.S. populationJ Manag Care Pharm20081484485719006441
- TwelvesCJXeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial: overview of efficacy, safety, and cost-effectivenessClin Colorectal Cancer2006627828717241512
- WardSEKaltenthalerECowanJMarplesMOrrBSeymourMTThe clinical and economic benefits of capecitabine and tegafur with uracil in metastatic colorectal cancerBr J Cancer200695273416804526
- SabatéEAdherence to Long-term Therapies: Evidence for ActionGeneva, SwitzerlandWorld Health Organization2003
- TebbiCKTreatment compliance in childhood and adolescenceCancer199371344134498490895
- CramerJARoyABurrellAMedication compliance and persistence: terminology and definitionsValue Health200811444718237359
- RuddyKMayerEPartridgeAPatient adherence and persistence with oral anticancer treatmentCA Cancer J Clin200959566619147869
- PartridgeAHAvornJWangPSWinerEPAdherence to therapy with oral antineoplastic agentsJ Natl Cancer Inst20029465266111983753
- YeawJBennerJSWaltJGSianSSmithDBComparing adherence and persistence across 6 chronic medication classesJ Manag Care Pharm20091572874019954264
- KleinCEKastrissiosHMillerAAPharmacokinetics, pharmacodynamics and adherence to oral topotecan in myelodysplastic syndromes: a Cancer and Leukemia Group B studyCancer Chemother Pharmacol20065719920616158312
- WaterhouseDMCalzoneKAMeleCBrennerDEAdherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoringJ Clin Oncol199311118911978501505
- NilssonJLGAnderssonKBergkvistABjorkmanIBrismarAMoenJRefill adherence to repeat prescriptions of cancer drugs to ambulatory patientsEur J Cancer Care (Engl)20061523523716882118
- NoensLvan LierdeMADe BockRPrevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO studyBlood20091135401541119349618
- PartridgeAHWangPSWinerEPAvornJNonadherence to adjuvant tamoxifen therapy in women with primary breast cancerJ Clin Oncol20032160260612586795
- RuddyKJPartridgeAHAdherence with adjuvant hormonal therapy for breast cancerAnn Oncol20092040140219246419
- MarinDBazeosAMahonFAdherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinibJ Clin Oncol2010282381238820385986
- WuEQGuerinAYuAPBolluVKGuoAGriffinJDRetrospective real-world comparison of medical visits, costs, and adherence between nilotinib and dasatinib in chronic myeloid leukemiaCurr Med Res Opin2010262861286921062136
- LeventhalHDiefenbachMAThe active side of illness cognitionSkeltonJACroyleRTMental representation in health and illnessNew York, NYSpringer-Verlag1991247272
- McAndrewLMMusumeci-SzaboTJMoraPAUsing the common sense model to design interventions for the prevention and management of chronic illness threats: from description to processBr J Health Psychol20081319520418331667
- BroadbentEPetrieKJMainJWeinmanJThe brief illness perception questionnaireJ Psychosom Res20066063163716731240
- HorneRWeinmanJHankinsMThe beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medicationPsychology and Health199914124
- EliassonLCliffordSBarberNMarinDExploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribedLeuk Res20113562663021095002
- National Cancer InstituteCommon Terminology Criteria for Adverse Events Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed August 4, 2012
- BaschEIasonosAMcDonoughTPatient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based studyLancet Oncol2006790390917081915
- BaschEJiaXHellerGAdverse symptom event reporting by patients vs clinicians: relationships with clinical outcomesJ Natl Cancer Inst20091011624163219920223
- BaschEThe missing voice of patients in drug-safety reportingN Engl J Med201036286586920220181
- AprileGMazzerMMorosoSPuglisiFPharmacology and therapeutic efficacy of capecitabine: focus on breast and colorectal cancerAnticancer Drugs20092021722919247178
- DaviesJMGoldbergRMFirst-line therapeutic strategies in metastatic colorectal cancerOncology (Williston Park)2008221470147919133603
- European Medicines AgencyEuropean Public Assessment ReportXeloda Product Information Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000316/human_med_001157.jsp&jsenabled=trueAccessed August 4, 2012
- PfeifferPMortensenJPBjerregaardBPatient preference for oral or intravenous chemotherapy: a randomised cross-over trial comparing capecitabine and Nordic fluorouracil/leucovorin in patients with colorectal cancerEur J Cancer2006422738274317011184
- CassidyJDouillardJYTwelvesCPharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’ C colon cancer: the X-ACT trialBr J Cancer2006941122112916622438
- TseVCNgWTLeeVCost-analysis of XELOX and FOLFOX4 for treatment of colorectal cancer to assist decision-making on reimbursementBMC Cancer20111128821740590
- RugoHSKohlesJSchulmanKLCost comparison of capecitabine in patients with breast cancerAm J Clin Oncol20103355055620051811
- PartridgeAHArcherLKornblithABAdherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104J Clin Oncol2010282418242220368559
- WinterhalderRHoesliPDelmoreGSelf-reported compliance with capecitabine: findings from a prospective cohort analysisOncology201180293321606661
- SimonsSRingsdorfSBraunMEnhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical careSupport Care Cancer2011191009101820552377
- Yen-RevolloJLGoldbergRMMcLeodHLCan inhibiting dihydropyrimidine dehydrogenase limit hand-foot syndrome caused by fluoropyrimidines?Clin Cancer Res20081481318172246
- ReignerBBleschKWeidekammEClinical pharmacokinetics of capecitabineClin Pharmacokinet2001408510411286326
- PetersGJLankelmaJKokRMProlonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasmaCancer Chemother Pharmacol1993312692768422689
- ReignerBVerweijJDirixLEffect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patientsClin Cancer Res199849419489563888
- PooleCGardinerJTwelvesCEffect of renal impairment on the pharmacokinetics and tolerability of capecitabine (Xeloda) in cancer patientsCancer Chemother Pharmacol20024922523411935215
- TwelvesCGlynne-JonesRCassidyJEffect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolitesClin Cancer Res199951696170210430071
- ReignerBWatanabeTSchullerJPharmacokinetics of capecitabine (Xeloda) in Japanese and Caucasian patients with breast cancerCancer Chemother Pharmacol20035219320112783206
- van KuilenburgABDihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracilEur J Cancer20044093995015093568
- HooiveldEAMvan KuilenburgABPHaanenJBAGWestermannAMErnstige toxiciteit na behandeling met capecitabine en fluorouracil ten gevolge van een partiële dihydropyrimidine-dehydrogenasedeficiëntieSevere toxicity after treatment with capecitabine and fluorouracil caused by partial dehydropyrimidine dehydrogenase deficiencyNed Tijdschr Geneeskd2004148626628 Dutch15083629
- CiccoliniJMercierCEvrardAA rapid and inexpensive method for anticipating severe toxicity to fluorouracil and fluorouracil-based chemotherapyTher Drug Monit20062867868517038885
- MercierCDupuisCBlesiusAEarly severe toxicities after capecitabine intake: possible implication of a cytidine deaminase extensive metabolizer profileCancer Chemother Pharmacol2008631177118019107485
- RibellesNLopez-SilesJSanchezAA carboxylesterase 2 gene polymorphism as predictor of capecitabine on response and time to progressionCurr Drug Metab2008933634318473752
- ReignerBCliveSCassidyJInfluence of the antacid Maalox on the pharmacokinetics of capecitabine in cancer patientsCancer Chemother Pharmacol19994330931510071982
- GieschkeARSteimerJLReignerBGRelationships between metrics of exposure to Xeloda™ and occurrence of adverse effectsProc Am Soc Clin Oncol199817Abstr 861
- FetyRRollandFBarberi-HeyobMClinical impact of pharmacokinetically- guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomasClin Cancer Res19984203920459748117
- van GroeningenCJPinedoHMHeddesJPharmacokinetics of 5-fluorouracil assessed with a sensitive mass spectrometric method in patients on a dose escalation scheduleCancer Res198848695669613180104
- Codacci-PisanelliGPinedoHMLankelmaJPharmacokinetics of bolus 5-fluorouracil: relationship between dose, plasma concentrations, area-under-the-curve and toxicityJ Chemother20051731532016038526
- GieschkeRBurgerHUReignerBBleschKSSteimerJLPopulation pharmacokinetics and concentration-effect relationships of capecitabine metabolites in colorectal cancer patientsBr J Clin Pharmacol20035525226312630975
- TimmersLBoonsCCLMMangnusDThe use of erlotinib in daily practice: a study on adherence and patients’ experiencesBMC Cancer20111128421722354
- ButlerJAPevelerRCRoderickPHorneRMasonJCMeasuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoringTransplantation20047778678915021850
- HorneRHankinsMJenkinsRThe Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and researchQual Health Care20011013514011533420
- AaronsonNKMullerMEssink-BotMLTranslation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populationsJ Clin Epidemiol199851105510689817123
- HurstNPRutaDAKindPComparison of the MOS short form-12 (SF12) health status questionnaire with the SF36 in patients with rheumatoid arthritisBr J Rheumatol1998378628699734677