Abstract
Purpose
COVID-19 vaccines are critical for containing the pandemic and preventing serious SARS-CoV-2 infections. In addition to the two main doses, a booster dose has been utilized to improve immunity. The aim of current study is to evaluate Iraqi adult population knowledge, attitudes, and practices towards COVID-19 booster dose.
Subjects and Methods
This online cross-sectional survey of adult Iraqis (n = 754) assessed the attitudes of people who have had both immunizations regarding a potential COVID-19 vaccine booster dosage and to identify potential factors that might impact these attitudes. Factors evaluated in the current study included previously received vaccine type in the first two doses, socioeconomic characteristics, health status, knowledge about COVID-19 and its vaccines and adherence to protective practices.
Results
Overall, 61.1% of participants expressed willingness to receive a COVID-19 booster dose, with a high median score of knowledge and practice toward COVID-19. Participants who did not perceive COVID-19 to be serious, p-value <0.001), participants who believed they would not be infected with COVID-19 in the next 6 months (p-value <0.001), low knowledge score group (p-value <0.001), lower education (p-value <0.001), participants who received the COVID-19 vaccine because of imposed laws (p-value <0.001), participants who received AstraZeneca vaccine (p-value <0.001), younger participants (p-value=0.003), low level of practice (p-value <0.001), participants who did not know someone who had died due to COVID-19 (p-value=0.01), low risk of developing serious side effects if infected with COVID-19 and participants in the low side effects score were significantly less frequently willing to receive a booster COVID-19 dose (p-value <0.001). The main reasons for booster dose hesitancy/refusal were the perceived lack of need for a booster shot, the uselessness of a booster shot and the conspiracy theory of boosting corporate profits through booster shots.
Conclusion
There is high hesitancy towards COVID-19 booster dose acceptance among the Iraqi population. The study identified several factors associated with vaccine hesitancy including low socioeconomic status and low knowledge about COVID-19 and its vaccines.
Introduction
The novel coronavirus, COVID-19, spread from the Chinese city of Wuhan at the end of 2019.Citation1 COVID-19 is a highly infectious disease that spreads through direct and contact transmissionCitation2 and has caused many complications that lead to hospitalization and numerous deaths.Citation3 Many measures have been taken that could reduce the risk of the rapid spread of COVID-19, including wearing a mask, using sterilizers, and maintaining social distancing. Nevertheless, these measurements were insufficient to stop the spread of the disease.Citation4 As the vaccines remain the most efficient approach in controlling infectious diseases,Citation5,Citation6 newly developed vaccines were used in an attempt to reduce the risk and the spread of COVID-19.Citation7,Citation8 Despite this vaccination program, there have been waves of infection in many countries.Citation5 These waves may be the result of the mutation of different strains of the virus, which may have led to partial vaccine resistance, and the gradual decline of the developed immunity from the vaccine over time.Citation9–Citation11 Serum antibodies (Ab) generated after vaccination give an initial response which is sustained up to 5 to 6 months followed by 5- to 10-fold-decrease in serum Abs levels which increases the susceptibility of reinfections.Citation12 The emergence of new SARS-CoV-2 variants decreased the protection provided by the initial vaccination course which is also associated with decreased Ab levels.Citation13 The booster dose showed a significant restoration of Ab levels particularly among elderly and immunocompromised individuals who had limited response to the standard vaccination course and high risk of reinfection.Citation14 Moreover, the maintenance of the Abs in vaccinated subjects by repeated vaccination suggests their ability to protect against COVID-19 variants.Citation15
A booster dose was used in addition to the two main doses to enhance immunity.Citation11 Many authorities approved the use of a booster dose that could be different from the originally used vaccine type.Citation16 The efficiency of the booster dose was evident during the Omicron variant outbreak, as the period of immunity provided by the vaccine declined to less than 180 days after receiving the second dose, while a booster dose increased the period of the provided immunity by 61%.Citation17 Nevertheless, there is a clear discrepancy in the acceptability of the booster dose between different societies and social classes, reporting different acceptable rates for COVID-19 booster dose ranging from 44.6%Citation18 to 93.7%.Citation19 Vaccination refusal/hesitancy remains a substantial obstacle in combating many infectious diseases, particularly when related to newly manufactured vaccines, like in the case of COVID-19.
There are three available types of COVID-19 vaccines approved for use in Iraq: Sinopharm,Citation20 PfizerCitation21 and AstraZeneca.Citation22 Although most of the Iraqi population is in favor of COVID-19 vaccination,Citation23 the total number of doses disbursed in Iraq at the beginning of March 2022 was 17 million doses,Citation24 out of a population of approximately 41 million people. The current study aims to evaluate vaccine hesitancy/refusal of the Iraqi adults towards receiving a booster dose and identify variables that may be associated with vaccine hesitancy.
Methods
This is a cross-sectional study that recruited Iraqi adults who were fully vaccinated (two doses) against COVID-19. A questionnaire was created on Google Forms and the link was distributed to various groups on social networking sites such as generic Facebook groups, Telegram, WhatsApp and Viber with a brief description of the study objectives. Participants’ responses were collected between November 2021 and March 2022. Inclusion criteria included being resident in Iraq, aged 18 or above and fully vaccinated (received the two doses against COVID-19). To ensure that participants met the inclusion criteria several screening questions were presented at the start of the survey regarding COVID-19 vaccination history, place of residency, and age.
Ethical Approval
All the participants agreed to the informed consent form included in the questionnaire. A confirmation of the approval of the Informed Consent Form (ICF) was obtained from all participants who agreed to take part in this study. Confidentiality of all the participants was well maintained throughout the study. Ethical approval was obtained from ethical committee of the Medical City Complex (IRB: 2021/10/15). The current study was conducted with accordance to the Declaration of Helsinki Ethical Principles.
Sample Size and Sampling Methods
Convenience sampling methodology was adopted. The minimum sample size required to conduct this study was calculated on the basis of a confidence level (Cl) of 95% and margin of error of 4%. Therefore, the minimum required sample size was found to be 601. As binary and multinomial regression model were used to analyze the data, the rule of Events Per Variable criterion (EPV) ≥10 was applied.Citation25
Study Instruments
The questionnaire utilized an instrument that was developed and validated by Al-Qerem et al.Citation18 The questionnaire consists of several parts, the first of which gives the participant an introductory overview of the study and its objectives, and also clarifies that none of the questions contained in the questionnaire reveal the identity of the participant.
The first part of the questionnaire is to ensure that the participant is 18 years or above, resides in Iraq and has had both doses of the COVID-19 vaccine to confirm that he/she meets the inclusion requirements in order to continue to complete the rest of the questions. If a respondent did not meet the inclusion criteria, then he/she was directed automatically to the form submission step.
The questionnaire consists of six different sections. In the first section, participants provide general information about themselves including age, gender, marital status, if the participant has children, smoking status, height, weight, monthly income level, educational level, and participant’s health status. Income levels were categorized in terms of household income to low (less than $500), intermediate (between $501 and $1000) and high (more than $1000). In the second section, questions are asked about the participant’s attitude towards COVID-19, such as the severity of the disease and whether he/she has been previously infected with COVID-19 and whether they think they will be infected with COVID-19 within the next six months. The third section contains general knowledge questions about COVID-19, such as symptoms of the disease, mechanisms of transmission, and whether a cure is available. This section also contains questions related to the participant’s protective practices against COVID-19. The fourth section asks about COVID-19 vaccine type received by the participants and any adverse effects that they experienced due to the vaccine. The fifth section examines knowledge and intentions related to the third booster dose of COVID-19 vaccine. This included the reasons for taking the booster dose, who should receive the booster dose and what vaccine type could be received. In this section, the participant is also asked about his/her intentions to take the booster dose of the vaccine. If the participant answers “yes” the questionnaire is submitted. However, if the answer is “no” or “not sure”, the reasons for the participant’s refusal/hesitancy to receive a booster dose will be inquired in the sixth section.
Participants were classified, based on the Centers for Disease Control and Prevention (CDC) criteria, into three groups: low, medium, and high-risk groups for developing complications during infection with COVID-19.Citation26
Knowledge scores were computed for each participant and one point was granted for each correct answer, with the maximum possible score of 22 (Table S1). Adherence to protective practices against COVID-19 scores were also computed, and it ranged from 1 point for “never” to 5 points for “all the time”, with a highest possible score of 25 (Table S1). A third score was computed for the adverse effects experienced by the participants due to the previous vaccination doses against COVID-19, and a point was granted for each reported symptom. The participants were divided into high/low-level groups based on the median of the computed scores.
Survey Validity and Reliability
A pilot study was conducted by distributing the questionnaire to 30 participants to ensure that all questions were clear and understandable to the Iraqi population. This was performed because the previous validation of the instrument was conducted in the neighboring country of Jordan and not in Iraq. The results obtained in the pilot study were not included in the final analysis. In order to assess the reliability of the results generated from the questionnaire, Cronbach’s alphas were computed for knowledge and protective practices scores, Cronbach’s alpha is deemed acceptable if higher than 0.7.
Statistical Analysis
Categorical variables were presented as frequencies and percentages, and continuous variables were presented as the means and standard deviations (SD). Bivariate analysis using Kruskal–Wallis and Chi-square analyses were used to identify variable associations with intention to receive the booster dose. Kruskal–Wallis with pairwise comparisons was applied to evaluate the differences in side effects severity scores by different vaccination type groups. Spearman correlation was used to identify the correlation between the perceived severity of side effects due to COVID-19 vaccines and the calculated side effects scores. Binary regression models were built to evaluate variables associated with side effect score levels, knowledge and practice levels. A multinomial logistic regression was conducted, the regression model included participants’ intention to receive the booster dose as the dependent variable, and the variables that were significant in the bivariate analysis were included as independent variables. IBM SPSS version 27 was used to analyze the data.
Results
Sample Characteristics
In total, 754 people participated in the study. Sample characteristics (see ) show that over half the sample were between 18 and 29 years old, with the majority unmarried, and did not have children. Three-quarters held a bachelor’s degree or higher. The majority of the sample knew someone who had died due to COVID-19, and were smokers. Almost three-quarters had a monthly household average of $1000 or less. Only 13.8% of the participants had a high risk towards developing severe COVID-19 symptoms and 44.8% of them had a normal bodyweight. The most frequently received vaccination was Pfizer’s.
Table 1 Sample Demographic Characteristics
Regarding perceived symptoms due to COVID-19 vaccination (), 14.6% reported having severe symptoms while 11.7% had no symptoms at all. More than half of the sample reported pain at the site of injection, fever, and weakness (68.2%, 56.8%, 46.9%, respectively). The mean score of the reported side effects was 3 symptoms per person. Spearman correlation showed that there was a positive significant association between the symptom severity and the computed side effect score (r = 0.62, p-value <0.001).
Table 2 Reported Side Effects Due to COVID-19 Vaccine
Sample Characteristics’ Association with Booster Dose Acceptance
The knowledge level of the participants and their adherence to protective practices against COVID-19 were evaluated by computing “knowledge” and “practice” scores (Table S1). The median for the knowledge score was 15 (quartiles = 13–18) out of a maximum possible score of 22. The median for practices was 22 (quartiles = 19–25) out of a maximum possible score of 25. Cronbach’s alpha indicated good internal consistency for the knowledge and practice scores (0.71 and 0.84, respectively). The knowledge and the practice scores were divided into two categories based on each score median.
Binary regression models were conducted to evaluate variables association with knowledge and practice levels. The results indicated that those with low household incomes (<$500) and moderate income ($500-$1000) had significantly lower odds of being in the high knowledge group (p-value >0.001, OR = 0.22 (95% CI 0.14–0.35); p-value=0.001, OR = 0.59 (95% CI 0.4–0.87), respectively). Those in the low education group had lower odds of being in a high knowledge group when compared to the higher education group (p-value <0.001, OR = 0.32 (95% CI 0.21–0.5). Factors associated with practice level included age and previous infection with COVID-19. Those aged 18–29 had lower odds of being in the high practice group compared to those aged 50 years or older (p-value=0.047, OR = 0.456 (95% CI 0.21–0.99)). Those reporting no previous infection with COVID-19 had higher odds of being in the high practice level group when compared to those previously infected (p-value=0.038, OR = 1.44 (95% CI 1.02–2.02)).
The results shown in demonstrate that more than half the sample was willing to receive a booster dose for COVID-19 vaccine (61.1%), 22.3% were unsure and only 16.3% were not intending to receive the booster dose. Bivariate analysis using Chi-square and Kruskal–Wallis tests revealed the variables associated with the intention to get the booster dose. The risk level towards developing severe COVID-19 symptoms was significantly associated with willingness to receive the booster dose (p-value <0.001). In addition to age (p-value = 0.003), education (p-value=0.001), perceived possibility of COVID-19 infection in the next 6 months (p-value <0.001), Knowledge level (p-value <0.001), Practice level (p-value <0.001), reasons for receiving COVID-19 vaccines (p-value <0.001), knowing someone who had died due to COVID-19 (p-value = 0.01), household monthly income (p-value <0.001), and finally vaccine type (p-value <0.001).
Table 3 Variables Associated with COVID-19 Booster Dose Acceptance
Multinomial regression was done to evaluate the factors related to responding “Not Sure” or “No” vs “Yes” regarding the intent to get the booster dose of the COVID-19 vaccine (). Perceived seriousness of COVID-19 decreased the odds of answering “No” (OR = 0.73 (95% CI 0.59–0.90)). Participants who believed they would not be infected with COVID-19 in the next 6 months had increased odds of responding “No” than those who believe they will have severe symptoms due to COVID −19 in the next 6 months (OR = 6.73 (95% CI 1.21–37.51)). The low knowledge level group had higher odds of answering “No” or “Not sure” than the high knowledge group (OR = 1.79 (95% CI 1.02–3.14); OR = 1.56 (95% CI 1.01–2.41), respectively). Those with lower education were less likely to answer “No” (OR = 0.49 (95% CI 0.26–0.90)).
Table 4 Multinomial Regression for Variables Association with COVID-19 Vaccine Acceptance
Participants who had received the COVID-19 vaccine because of imposed laws were more likely to respond “No” vs “Yes” (OR = 20.25 (95% CI 10.52–38.10)) and “Not sure” vs “Yes” compared to those who took the vaccine out of conviction only (OR = 4.45 (95% CI 2.41–8.24)). Those who took the vaccine out of conviction and imposed laws increased the odds of responding “No” and “Not sure” vs. “Yes” when compared to those who took the vaccine out of conviction only (OR = 3.45 (95% CI 1.75–6.81); OR= 2.61 (95% CI 1.57–4.34), respectively). Participants who received AstraZeneca vaccine had lower odds of responding “No” vs “Yes” compared to those who received Sinopharm (OR = 0.25 (95% CI 0.07–0.86)). Participants who were classified into age group (18–29 years), (30–39 years), and (40–49 years) were more likely to answer “Not sure” vs “Yes” when compared to participants aged 50 or older (OR = 6.35 (95% CI 1.36–29.53); OR = 5.49 (95% CI 1.16–25.96); OR = 5.93 (95% CI 1.14–30.88), respectively). Being in the low practice level group increased the odds of answering “Not sure” vs “Yes” compared to high practice group, with OR of 1.91 (95% CI 1.28–2.86). Participants who did not know someone who had died due to COVID-19 were more likely to answer “No” when compared to those who did, OR = 1.86 (95% CI 1.05–3.30). Those who had a low risk of developing serious side effects if infected with COVID-19 had greater odds of saying “Not sure” over “Yes” when compared to the high-risk group, OR = 1.90 (95% CI 1.01–3.58). Participants in the low side effects due to previous COVID-19 vaccination doses group were less likely to answer “Not sure” vs “Yes” than the high side effect score group, with OR of 0.91 (95% CI 0.82–0.10).
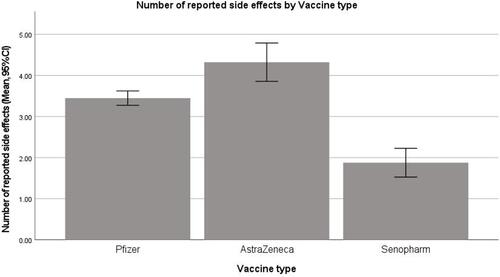
Reported COVID-19 Side Effects by Vaccine Type
Different types of COVID-19 vaccines produced different significantly different reported side effects. Kruskal–Wallis with pairwise comparison showed that there were significant differences between the three compared vaccine types (p-value <0.001). Those who received the AstraZeneca vaccine reported the highest side effects score followed by Pfizer. Those who received the Sinopharm vaccine reported the least side effects (see ). The significant variables associated with number of reported side effects were gender, previous infection with COVID-19, and the type of the vaccine received. Female participants reported more adverse effects than male participants (p-value <0.001; OR = 2.46 (95% CI 1.67–3.63)). Those who not previously infected with COVID-19 reported fewer side effects than those who were previously infected (p-value= 0.01; OR of 0.6 (95% CI 0.41–0.87)). Participants who received AstraZeneca or Pfizer vaccines reported more side effects than those who received Sinopharm (p-value <0.001 for both, OR = 8.95 (95% CI 4.22–18.1); OR= 5.79 (95% CI 3.13–10.69, respectively)).
Reported Reasons for Booster Dose Hesitancy/Refusal
Reasons for booster dose hesitancy/refusal are shown in . Over half the sample agreed with some reasons including “I took the last dose a short time ago, so there will be no need to take the booster dose for at least a year.”, “The benefits of a booster dose have not been scientifically proven.” and “The booster dose is a conspiracy to boost corporate profits.” On the other hand, the reason “I was infected with COVID-19; therefore, I do not need the booster dose” was the least reported.
Table 5 Reasons Behind Vaccine Hesitancy/Refusal
Discussion
This study was conducted to evaluate the acceptance of the Iraqi population to receive a COVID-19 vaccine booster dose after receiving initial full vaccination course. The booster dose adjunctive to the two main doses is used to increase the protection of the vaccinated individuals against the different variants of COVID-19.Citation11 However, vaccination hesitancy remains a significant obstacle against receiving the booster dose.Citation18 Vaccine resistance (refusal) is defined as those people who will definitely not be willing to get vaccinated; vaccine hesitancy is people who are uncertain whether to become vaccinated.Citation27,Citation28 There are three types of vaccines available in Iraq which were included in the present study. These are Pfizer which is an mRNA subtype vaccine, AstraZeneca is an adenovirus subtypeCitation29 and Sinopharm is an inactivated whole virus subtype.Citation30 COVID-19 vaccination coverage varies significantly between countries reaching high percentages up to 86.2% in Sint MaartenCitation31 and 97.9% in United Arab Emirates and as low as 0.1% in Burundi.Citation32
Cronbach’s alpha indicated that the questionnaire had good internal consistency which increases the confidence of the produced results. The current study results indicated acceptance rate of 61.4% among Iraqi population to receive the third booster dose of COVID-19 vaccinations. These rates indicated better acceptance when compared to those in Jordan (44.6%).Citation18 However, it remains significantly less than the required vaccination rates to produce herd immunity,Citation33 and less than the acceptance rates reported in different studies in the reign and around the world in general, including United Arabs Emirates (70.2%),Citation34 Canada (86%),Citation35 Denmark (90%),Citation36 China (91.1%),Citation37 and Poland (71%).Citation38
Variables Associated with Knowledge and Practices About COVID-19 and Its Vaccines
This study demonstrated that the average household income and education level are critical factors in the degree of knowledge level about COVID-19 and its vaccines. Where the participants with low average household income were less likely to be in the high knowledge group. Moreover, the participants who were in low education group showed less knowledge about COVID-19. These results are similar to previously published literature.Citation34,Citation39 For example, a study was conducted in Bangladesh demonstrated that low income and education levels were associated with significantly lower knowledge level.Citation39
Practice level was considerably affected by participant age and their COVID-19 infection history. The current study found those not previously infected with COVID-19 had higher practice scores compared to those who had been previously infected. This emphasizes the importance of adherence to recommended protective practices against COVID-19 to prevent and control the spread of the infection. Other studies have reported contradicting findings on the association between being previously infected and the protective practices, as some studies have shown no significant association between the practices and history of infection.Citation23 This variation may be explained by the different sample characteristics, and/or the pandemic status when the study was conducted. Those who were in the 18–29 age group had lower practice levels than the 50 and older age group. This can be explained by the higher fear of negative health outcomes in the older population, as documented in previous studies.Citation40
Side Effects Reported by the Study Sample to COVID-19 Vaccines
There was a significant correlation between the perceived side effects and the reported number of adverse effects. The most reported side effects were pain at the site of injection followed by fever and weakness. Similar findings have been reported in previous studiesCitation34,Citation35 Those are also similar to the expected side effects that are reported by different health organizations including Centers for Disease Control and Prevention.Citation41
The binary regression showed that females, participants with history of previous COVID19 infection, and participants who had received the AstraZeneca vaccine reported more adverse effects. This has also been reported by other studies,Citation18,Citation42,Citation43 which could be due to the nature of AstraZeneca (S glycoprotein) which is associated with gastrointestinal side effect.Citation42 In general, females reported more side effects than males.Citation44 This may be due to their ability to sense health-related physical changes and general discomfort.Citation44,Citation45 Participants who had not been infected with COVID-19 reported fewer side effects, consistent with previous studies,Citation46 although no association was found between infection history and side effects in randomized control studies.Citation46
Variables Associated with Participants Acceptance to Receive a Booster Dose
Willingness to take a third booster dose varied according to different factors. These factors included the type of vaccine already received, with those who received the AstraZeneca vaccine more likely to be willing to receive a booster dose than those who received Sinopharm. This may be due to the perceived effectiveness of various types of vaccines that were observed in previous studies.Citation20 Participants who think they will not be infected with COVID-19 in the next 6 months were more likely to reject taking the booster dose. This can be further confirmed by the perceived seriousness of COVID-19 that was associated with booster dose acceptance, and with the impact of knowing someone personally who had died due to COVID-19. This finding aligns with a study conducted in Jordan that found a relationship between perceived COVID-19 seriousness and vaccination intention.Citation47 This shows the importance of awareness campaigns for the possible complications of COVID-19.
Consistency with the findings in an earlier study,Citation18 participants forced to receive the initial vaccine doses by law, as well as both out of imposed laws and conviction, showed more refusal/hesitancy to take the third booster dose than those who had received the initial doses out of conviction.
Results show that the younger age groups have higher hesitancy towards the booster vaccine when compared to elderly. This supports the positive association of age with the good practice levels discussed earlier which may be due to the higher fear of the possible deteriorating health outcomes in the older group. This highlights the importance of raising awareness in younger people, perhaps by encouragement and education about the importance of the booster dose in maintaining a high level of protection against infection.
The group at high-risk of developing severe COVID-19 complications reported lower vaccine hesitancy towards the booster dose; this may be due to higher fear of being infected which has previously been associated with the presence of chronic diseases.Citation48 It has been previously reported that fear of being infected was significantly associated with decreased vaccination hesitancy.Citation49
High knowledge and practice groups had better acceptance towards receiving the booster dose. This emphasizes the role of improving knowledge within the Iraqi population to expand uptake of the COVID-19 vaccinations. This finding replicates those of several other studies in Jordan,Citation47 Ethiopia,Citation50 and United States.Citation51
The side effects associated with vaccination had an effect on the participant attitude towards accepting the booster dose. Similar findings were reported with studies conducted in Jordan,Citation18 as severe side effects may alter the perception of the participants towards the risk/benefit of the vaccine, which influence their attitude towards accepting a booster dose.Citation52
Reasons for Refusal/Hesitancy to Receive the Booster Dose
Common reasons reported by the current study for vaccination refusal/hesitancy were “I took the last dose a short time ago, so there will be no need to take the booster dose for at least a year”, “The benefits of a booster dose have not been scientifically proven” and “The booster dose is a conspiracy to boost corporate profits”. A study conducted in Jordan similar results where the most common three reasons behind vaccination refusal/hesitancy were “The benefits of a booster dose have not been scientifically proven.”, “Taking the booster dose now has no benefit, however I may receive it in the future.”, and “I took the last dose a short time ago, so there will be no need to take the booster dose for at least a year”.Citation18
Strengths
This study does not only assess the Iraqi population’s willingness to receive COVID-19 booster dose, but also explores possible determinants of hesitancy. Such data give governments and public health authorities evidence to develop interventions to improve vaccine acceptance. Also, the use of an online questionnaire gave participants the freedom to state their thoughts about COVID-19 vaccinations anonymously without the fear of social judgment.Citation53 Additionally, the sample size for this study was sufficient to minimize the influence of bias. Finally, online sampling has several advantages as it provides a private and secure environment that may reduce social desirability bias and eliminates interviewer bias.Citation54
Limitations
A possible limitation in this study is that the use of online questionnaires might be susceptible to selectivity and recall biases as participants who are more interested in the topic could be more encouraged to participate in the study. Nevertheless, the large sample size enrolled in the current study may reduce the influence of these biases. Moreover, it was previously suggested that the widespread usage of the Internet would increase the representability of the generated sample via online studies in relation to the total population.Citation54 This appears to be the case in Iraq as Internet usage reached up to 60% of the total populationCitation55 and may be still higher when excluding children under the age of 18. This was evident when comparing the generated sample characteristics to the total Iraqi population, when possible, for example the positively skewed age distribution mimics the young Iraq population age distribution.Citation56 Furthermore, although female participants were more than male participants, the sex distribution was relatively balanced. The smallest reported income group in the present study was the high-income group, which is also parallel to the general Iraqi populationCitation57. Another limitation could be that the 50 years age and older enrolled in this study is the least among other age groups representing only 5.4%. This might be due to lower use of social media platforms by elderly, and non-familiarity with Google Forms. Nevertheless, as stated earlier, the Iraqi population is a young one with a median age of 21 and only 7.7% are 55 years or older.Citation56
Conclusion
In this cross-sectional online study, a group of Iraqis were asked about their willingness and opinions toward a future COVID-19 vaccine booster dose. According to the findings, a sizable portion of population who previously opted to take a COVID-19 vaccine may be opposed to it. The main reasons for refusing a COVID-19 booster dose were side effects from earlier doses, the belief that additional vaccination is unnecessary, and safety concerns. Identifying specific groups with the lowest level of acceptance of additional COVID-19 vaccination is therefore critical for effective science communication and building general vaccine trust, especially when considering that booster COVID-19 vaccine doses may be required in the future to better control the future spread of SARS-CoV-2. Vaccination campaigns should be applied to increase the knowledge about the importance of COVID-19 vaccination to reduce vaccination hesitancy among the general population, particularly among low socioeconomic groups.
Disclosure
The authors report no conflicts of interest in this work.
References
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMOA2001017/SUPPL_FILE/NEJMOA2001017_DISCLOSURES.PDF
- Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–758. doi:10.1136/postgradmedj-2020-138234
- Gebru AA, Birhanu T, Wendimu E, et al. Global burden of COVID-19: situational analysis and review. Hum Antibodies. 2021;29(2):139–148. doi:10.3233/HAB-200420
- WHO Coronavirus (COVID-19) Dashboard. WHO coronavirus (COVID-19) dashboard with vaccination data. Available from: https://covid19.who.int/. Accessed April 5, 2022.
- Hussein T, Hammad MH, Fung PL, et al. COVID-19 pandemic development in Jordan-short-term and long-term forecasting. Vaccines. 2021;9(7):728. doi:10.3390/VACCINES9070728
- Koppaka R. Ten Great Public Health Achievements: worldwide, 2001–2010. Centers for Control Disease and Prevention (CDC). Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6024a4.htm. Accessed April 14, 2022.
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine Phase 3 trials. Nat Med. 2021;27(2):205–211. doi:10.1038/s41591-021-01230-y
- Burgos RM, Badowski ME, Drwiega E, et al. The race to a COVID-19 vaccine: opportunities and challenges in development and distribution. Drugs Context. 2021:10. doi:10.7573/DIC.2020-12-2
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326(19):1930–1939. doi:10.1001/JAMA.2021.19623
- Mallapaty S. China’s COVID vaccines have been crucial - now immunity is waning. Nature. 2021;598(7881):398–399. doi:10.1038/D41586-021-02796-W
- Wang J, Kaperak C, Sato T, Sakuraba A. COVID-19 reinfection: a rapid systematic review of case reports and case series. J Investig Med. 2021;69(6):1253–1255. doi:10.1136/JIM-2021-001853
- Vanshylla K, Di Cristanziano V, Kleipass F, et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29(6):917–929.e4. doi:10.1016/J.CHOM.2021.04.015
- Romano CM, Felix AC, de Paula AV, et al. SARS-CoV-2 reinfection caused by the P.1 lineage in Araraquara city, Sao Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2021:63. doi:10.1590/S1678-9946202163036
- Shamier MC, Tostmann A, Bogers S, et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv. 2021. doi:10.1101/2021.08.20.21262158
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi:10.1056/NEJMOA2114255/SUPPL_FILE/NEJMOA2114255_DISCLOSURES.PDF
- Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell JMB. Coronavirus pandemic (COVID-19) - our world in data. Available from: https://ourworldindata.org/coronavirus. Accessed April 5, 2022.
- Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi:10.1056/NEJMOA2119451
- Al-Qerem W, Al Bawab AQ, Hammad A, Ling J, Alasmari F. Willingness of the Jordanian population to receive a COVID-19 booster dose: a cross-sectional study. Vaccines. 2022;10(3):410. doi:10.3390/VACCINES10030410
- Qin C, Wang R, Tao L, Liu M, Liu J. Acceptance of a third dose of COVID-19 vaccine and associated factors in China based on health belief model: a national cross-sectional study. Vaccines. 2022;10(1):89. doi:10.3390/VACCINES10010089
- Sinopharm. Chinese Covid-19 Vaccine Efficacy Better than Expected; Interview with Mr. Liu Jingzhen, Chairman of Sinopharm; 2021. Available from: http://www.sinopharm.com/en/s/1395-4173-38923.html. Accessed June 21, 2022.
- US Food and Drug Administration (FDA). Pfizer-BioNTech COVID-19 Vaccine; 2021. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine. Accessed June 21, 2022.
- GOV.UK. Information for UK recipients on COVID 19 Vaccine; 2021. Available from: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca. Accessed June 21, 2022.
- Al-Qerem W, Hammad A, Alsajri AH, Al-Hishma SW, Ling J, Mosleh R. COVID-19 vaccination acceptance and its associated factors among the Iraqi population: a cross sectional study. Patient Prefer Adherence. 2022;16:307. doi:10.2147/PPA.S350917
- World Health Organization (WHO). Iraq: WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. Available from: https://covid19.who.int/region/emro/country/iq. Accessed March 30, 2022.
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–1510. doi:10.1016/0895-4356(95)00048-8
- Center of Disease Control and Prevention (CDC). People with certain medical conditions. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed April 5, 2022.
- Umakanthan S, Patil S, Subramaniam N, Sharma R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines. 2021;9(10):1064. doi:10.3390/vaccines9101064
- Umakanthan S, Lawrence S. Predictors of COVID-19 vaccine hesitancy in Germany: a cross-sectional, population-based study. Postgrad Med J. 2022. doi:10.1136/postgradmedj-2021-141365
- Francis AI, Ghany S, Gilkes T, Umakanthan S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med J. 2022;98(1159):389–394. doi:10.1136/postgradmedj-2021-140654
- Jahromi M, Al Sheikh MH. Partial protection of Sinopharm vaccine against SARS COV2 during recent outbreak in Bahrain. Microb Pathog. 2021;158:105086. doi:10.1016/j.micpath.2021.105086
- Umakanthan S, Chauhan A, Gupta MM, Sahu PK, Bukelo MM, Chattu VK. COVID-19 pandemic containment in the Caribbean Region: a review of case-management and public health strategies. AIMS Public Health. 2021;8(4):665–681. doi:10.3934/publichealth.2021053
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available from: https://ourworldindata.org/covid-vaccinations. Accessed May 20, 2022.
- Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi:10.1016/J.IMMUNI.2020.04.012
- Jairoun AA, Al-Hemyari SS, El-Dahiyat F, et al. Assessing public knowledge, attitudes and determinants of third COVID-19 vaccine booster dose acceptance: current scenario and future perspectives. J Pharm Policy Pract. 2022;15(1):1–13. doi:10.1186/S40545-022-00422-2
- The Daily. Majority of Canadians are willing to get a COVID-19 booster dose. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/220315/dq220315b-eng.htm. Accessed April 1, 2022.
- Sønderskov KM, Vistisen HT, Dinesen PT, et al. COVID-19 booster vaccine willingness. Original Article Dan Med J. 2022;69(1):10210765.
- Tung TH, Lin XQ, Chen Y, Zhang MX, Zhu JS. Willingness to receive a booster dose of inactivated coronavirus disease 2019 vaccine in Taizhou, China. Expert Rev Vaccines. 2021;21(2):261–267. doi:10.1080/14760584.2022.2016401
- Rzymski P, Poniedziałek B, Fal A. Willingness to receive the booster COVID-19 vaccine dose in Poland. Vaccines. 2021;9(11):1286. doi:10.3390/VACCINES9111286
- Banik R, Rahman M, Sikder MT, Rahman QM, Pranta MUR. Knowledge, attitudes, and practices related to the COVID-19 pandemic among Bangladeshi youth: a web-based cross-sectional analysis. J Public Health. 2021;1–11. doi:10.1007/S10389-020-01432-7/FIGURES/4
- Villalba AA, Stanley JT, Turner JR, Vale MT, Houston ML. Age differences in preferences for fear-enhancing Vs. fear-reducing news in a disease outbreak. Front Psychol. 2020;11:3661. doi:10.3389/FPSYG.2020.589390/BIBTEX
- CDC. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html. Accessed April 7, 2022.
- Abu-Hammad O, Alduraidi H, Abu-Hammad S, et al. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines. 2021;9(6):577. doi:10.3390/VACCINES9060577
- Omeish H, Najadat A, Al-Azzam S, et al. Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Hum Vaccin Immunother. 2021;18(1). doi:10.1080/21645515.2021.1981086/SUPPL_FILE/KHVI_A_1981086_SM5775.DOCX
- Ladwig KH, Marten-Mittag B, Formanek B, Dammann G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur J Epidemiol. 2000;16(6):511–518. doi:10.1023/A:1007629920752
- Gijsbers Van Wijk CMT, Kolk AM. Sex differences in physical symptoms: the contribution of symptom perception theory. Soc Sci Med. 1997;45(2):231–246. doi:10.1016/S0277-9536(96)00340-1
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination key points + supplemental content. JAMA Network Open. 2021;4(12):2140364. doi:10.1001/jamanetworkopen.2021.40364
- Al-Qerem WA, Jarab AS. COVID-19 vaccination acceptance and its associated factors among a middle eastern population. Front Public Health. 2021;9:34. doi:10.3389/FPUBH.2021.632914/BIBTEX
- Cerda AA, García LY. Factors explaining the fear of being infected with COVID‐19. Health Expect. 2022;25(2):506–512. doi:10.1111/hex.13274
- Cerda AA, García LY. Willingness to pay for a COVID-19 vaccine. Appl Health Econ Health Policy. 2021;19(3):343–351. doi:10.1007/s40258-021-00644-6
- Abebe H, Shitu S, Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resist. 2021;14:2015. doi:10.2147/IDR.S312116
- Mannan DKA, Farhana KM. Knowledge, attitude and acceptance of a COVID-19 vaccine: a Global Cross-Sectional Study. SSRN Electron J. 2020. doi:10.2139/SSRN.3763373
- Atal S, Sadasivam B, Ahmed S, Ray A. Medication concordance in modern medicine – a critical appraisal from an Indian perspective. J Family Med Prim Care. 2019;8(4):1313. doi:10.4103/JFMPC.JFMPC_176_19
- Fenner Y, Garland SM, Moore EE, et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14(1):e20. doi:10.2196/JMIR.1978
- Im EO, Chee W. Recruitment of research participants through the Internet. Comput Inform Nurs. 2004;22(5):289–297. doi:10.1097/00024665-200409000-00009
- The world Bank. Individuals using the internet (% of population)—Iraq|Data. Available from: https://data.worldbank.org/indicator/IT.NET.USER.ZS?locations=IQ. Accessed May 19, 2022.
- Iraq. The world factbook. Available from: https://www.cia.gov/the-world-factbook/countries/iraq/. Accessed April 7, 2022.
- United Nations Economic and Social Commission for Western Asia (UNESCWA). Arab middle class: measurement and role in driving change. Available from: https://archive.unescwa.org/publications/arab-middle-class-measurement-role-change. Accessed May 19, 2022.