Abstract
Objective
Gender is associated with medication adherence for imatinib, but whether it is related to the prognosis of primary localized gastrointestinal stromal tumors (GISTs) is unclear. The goal of this study was to clarify the relationship between gender and prognosis in GIST patients, with differences in medication adherence considered.
Methods
The data of 320 GIST patients were retrospectively collected from the First Affiliated Hospital of Chongqing Medical University. Survival analysis was performed using the Kaplan–Meier method (Log rank test) and the risk factors of recurrence were determined using Cox multivariate analysis. Medication adherence-stratified analyses were performed to control for confounding factors.
Results
Kaplan–Meier analysis revealed that among patients who received postoperative adjuvant imatinib therapy, men had a higher recurrence rate than women (P<0.01). Pearson’s chi-square test revealed better medication adherence in women than in men (P<0.01). Cox regression analysis revealed that gender was not an independent risk factor for recurrence-free survival (RFS; P=0.25), but medication adherence was (P<0.01). Among GIST patients with a medication possession ratio (MPR) of less than 90%, 62.86% of male patients took imatinib irregularly or not at all due to limited understanding of the disease, whereas 55.74% of female patients’ took imatinib irregularly because they could not tolerate adverse drug reactions.
Conclusion
Adherence was poorer in male than in female patients, which might explain the worse prognoses of the former among patients who received adjuvant treatment with imatinib. The gender difference in the degree of adherence should be considered in postoperative pharmacotherapy for patients with primary localized GISTs.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the digestive tract. These tumors originate from interstitial cells of Cajal or their stem cell-like subset.Citation1 Surgical resection is the best radical treatment for patients with primary localized GIST.Citation2 However, some patients have recurrence and metastasis even after complete surgical resection. The reported 5-year recurrence-free survival (RFS) rate of patients with primary localized GIST after surgery is 70.5%.Citation3 Imatinib, a tyrosine kinase inhibitor (TKI), is often used for adjuvant therapy in primary localized GISTs,Citation4 and retrospective and prospective studies have demonstrated that imatinib could reduce tumor recurrence.Citation5 The prognosis in GIST can be influenced by tumor diameter, mitotic index, adherence to imatinib treatment, and other factors.Citation2,Citation6–8 However, the correlation between gender and prognosis in this tumor remains unclear despite large-scale, multi-center studies (a population-based, nationwide study in Sweden;Citation9 the Surveillance, Epidemiology and End Results [SEER] database;Citation10 and the Armed Forces Institute of Pathology studiesCitation11) and individual studies. Rong et alCitation12 and Ge et alCitation13 reported that the gender of GIST patients is an independent prognostic factor. However, tumor status and treatment (including information about targeted drug therapies such as imatinib) of patients in these studies are unclear.Citation12,Citation13 Furthermore, Wang et alCitation14 and Zhang et alCitation15 reported that gender is one of many factors associated with medication adherence for imatinib.
As described above, it is still unclear whether the prognosis of primary localized GIST may be significantly altered by gender-related factors (eg, adherence to imatinib treatment). Therefore, the goal of this study was to clearly establish the relationship between gender and prognosis in patients with different medication adherence for imatinib and identify factors that contributed to nonadherence in GIST patients of different genders.
Methods
Sampling Method
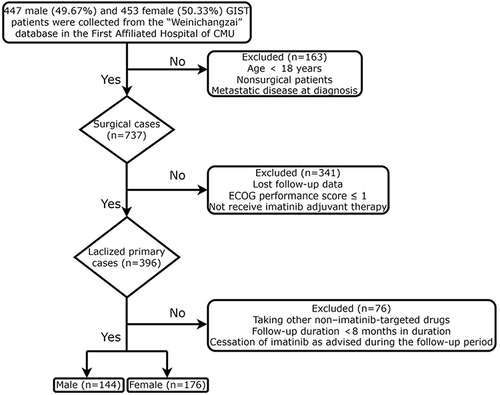
We retrospectively collected the clinical data of patients admitted to the First Affiliated Hospital of Chongqing Medical University (CMU; Chongqing, China) from January 1, 2000 to February 1, 2021. Patient follow-up ended on October 1, 2021. Inclusion criteria were as follows: (1) GIST patients who had undergone complete surgical resection and received imatinib adjuvant therapy in the standard dose (400 mg/d);Citation16 (2) GIST confirmed by postoperative pathology; (3) age equal to or greater than 18 years; (4) postoperatively, patients were followed up at regular intervals by computed tomography (CT) or magnetic resonance imaging (MRI); (5) Eastern Cooperative Oncology Group (ECOG) performance score ≤ 1.Citation17 Exclusion criteria were as follows: (1) other non–imatinib-targeted drugs were taken; (2) patients lost during follow-up or with incomplete data; (3) follow-up of ≤ 8 months in duration; and (4) cessation of imatinib as advised during the follow-up period. The patient selection process is outlined in .
Operational Definition
“Adjuvant treatment with imatinib” refers to oral intake of imatinib in accordance with physicians’ advice from 1 week to 1 month after complete surgical resection. The primary endpoint for analysis was recurrence-free survival, which was defined as the time from patient registration to the development of tumor recurrence or death resulting from any cause. “Adherence” refers to the extent to which a patient takes medication as prescribed or adheres to a treatment plan.
Assessment of Medication Adherence
There is currently no measurement approach that can be considered the gold standard for the evaluation of medication adherence for imatinib.Citation18,Citation19 To assess medication adherence for imatinib, we used the method described by Halpern et al,Citation20,Citation21 who used medication possession ratio (MPR) for the evaluation of adherence to imatinib treatment. We chose this method because it has been used in studies of patients treated with imatinib.Citation22–26 MPR is defined as the ratio of days taking oral imatinib during follow-up to the total number of days of follow-up, which is used to evaluate adherence as an increasingly accepted measure of medicines use.Citation27 To assess the impact of MPR on gender, Patients were divided into four groups based on quartiles of the MPR value. The lowest quartile (Q1) included 80 patients with the lowest MPR; Q2 extended 25% below the median MPR, which included 80 patients; Q3 and Q4 included the 160 patients with the highest MPR. Because the patients in Q3 and Q4 had a low tumor recurrence rate, they were combined into one group. Adherence was defined as follows:
Days of taking imatinib orally during follow-up ÷ days of follow-up × 100%
Adherence categories were good, Q3–Q4; medium, Q2; and poor, Q1.
Data Source
Every 3–6 months, GIST patients underwent outpatient follow-up with CT/MRI examinations to determine if their tumors had recurred. We established a database called “Weinichangzai” that included relevant clinical information for each patient and could also notify patients of follow-up visits in real time. Information collected included patients’ age, gender, primary tumor site, modified National Institutes of Health (NIH) risk grade, daily imatinib dose, tumor diameter, days of taking imatinib orally, and other factors.
From October 1, 2021 to October 29, 2021, patients with primary localized GISTs whose MPR was less than 90% were followed up in the outpatient clinic or over the telephone, and the major reasons for medication non-adherence were identified by qualitative in-depth interview.
Statistical Analysis
Patients were divided into a male group and a female group. We conducted descriptive statistics for baseline characteristics of the study population. Categorical variables were presented with frequencies (percentages) and compared via the chi-square test. Continuous variables were described as means ± standard deviations (SDs) and tested for normality using the Kolmogorov–Smirnov test, and we compared continuous variables using the Student’s t-test. A Kaplan–Meier (KM) survival curve (Log rank test) was used to analyze the relationship between gender and prognosis. We subjected variables with P < 0.2 in univariate analysis to Cox multivariate analysis. Stratified analysis was performed to control for confounding factors. We calculated all P-values as two tailed and computed all confidence intervals (CIs) at the 95% level. P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 26.0 (IBM Corp, Armonk, NY, USA).
Results
Baseline Data
A total of 320 patients with primary localized GISTs from the years 2000 to 2021 were selected from the “Weinichangzai” database of the First Affiliated Hospital of CMU based on inclusion and exclusion criteria (). There were 144 male (45%) and 176 female (55%) patients. The median follow-up time for the study population was 47 months (range, 8–259 months). There were 148 gastric (46.25%), 137 small intestinal (42.81%), 17 colorectal (5.31%), and 18 non-gastrointestinal (5.63%) primary tumors. Most tumors were high risk according to the modified NIH risk classification system,Citation28 and 61.1% had a mitotic index of <5/50 per high-power field (HPFs). The positive rates of the two principal immunohistochemistry markers DOG-1 and CD117 were 91.56% and 96.56%, respectively.
Effect of Gender on Recurrence-Free Survival of Patients with Primary Localized GISTs
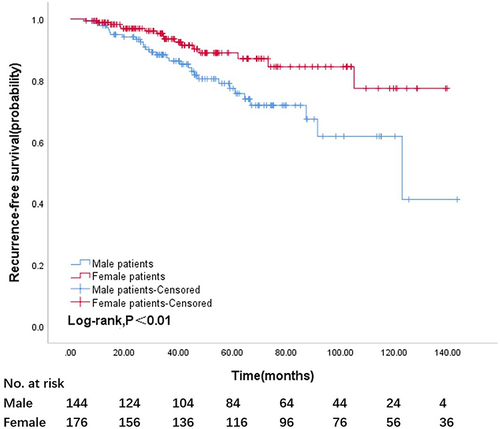
Kaplan–Meier analysis and a Log rank test revealed that among patients who received adjuvant treatment with imatinib, men had a higher tumor recurrence rate than women (log-rank, P<0.01) (). Five-year RFS rate was 58.33% in male patients and 65.91% in female patients.
Clinicopathological Characteristics of Male and Female Patients
The detailed clinicopathological characteristics of the two genders are presented in . The mean age was 54.36 years for male patients, and 53.81 years for female patients. Female patients adhered better to imatinib treatment than male patients (P<0.01). Age, tumor size, tumor site, tumor rupture, modified NIH risk classification grade, gene mutation, DOG-1 status, CD117 status, neoadjuvant therapy with imatinib, and mitotic count did not show statistically significant differences between groups ().
Table 1 Baseline Characteristics of Male and Female GIST Patients Who Received Adjuvant Treatment with Imatinib
Prognostic Factors for Recurrence
We analyzed 12 prognostic factors (age, tumor size, gender, tumor site, tumor rupture, modified NIH risk classification, gene mutation, DOG-1 status, CD117 status, mitotic count, neoadjuvant therapy with imatinib, and medication adherence for imatinib) using a Cox proportional-hazard regression analysis. In univariate analyses, 5 factors were statistically significant in determining RFS rates (P<0.05). In Cox multivariate analyses, 4 factors (tumor size, gene mutation, mitotic count, and medication adherence for imatinib) were independent factors. Gender was a significant predictive factor in univariate analysis (P = 0.01), but it was not an independent factor in multivariate analysis (P = 0.25) ().
Table 2 Univariate and Multivariate Analyses of Prognostic Factors for Recurrence-Free Survival of Primary Localized GIST Patients
Analyses Stratified by Medication Adherence for Imatinib
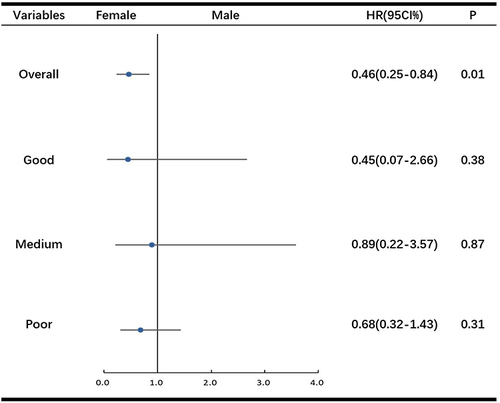
As shown in , a stratified medication adherence analysis was performed for GIST patients of different genders. The confidence interval of the overall patients lay entirely to the left of the line-of-no-effect, indicating men had a higher tumor recurrence rate than women (P = 0.01). The line of 95% confidence interval of good, medium, and poor medication adherence all crossed the line-of-no-effect, and statistical significance was not reached for any level of medication adherence (P > 0.05). The results described above establish that medication adherence may be a confounding factor between gender and prognosis.
Factors Affecting Medication Adherence for Imatinib
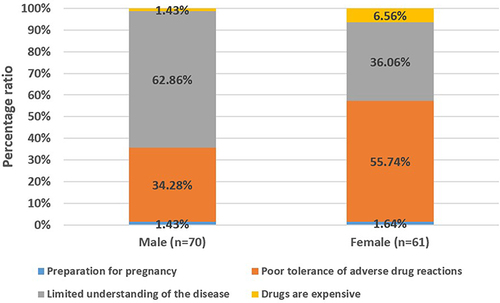
Through our follow-up survey, the major reason affecting medication adherence for imatinib in GIST patients whose MPR is less than 90% can be summarized as the following: drugs are expensive, limited understanding of the disease, poor tolerance of adverse drug reactions, and preparation for pregnancy, (). In male GIST patients, limited understanding of the disease was responsible in 62.86% of cases. In female GIST patients, poor tolerance to drug reactions was responsible for 55.74% of cases.
Discussion
GIST has a very similar frequency in male (49.67%) and female (50.33%) patients, as shown in . In the present study, about 2/5 of GIST patients had an MPR of less than 90%. Approximately 70.5% of patients with primary localized GISTs who have undergone complete surgical resection experience tumor recurrence.Citation3 In studies of GISTs, there are limited data on medication adherence and gender in relation to prognosis.Citation12,Citation29 This may be important because gender-related effects (eg, adherence to imatinib treatment) in primary localized GIST patients may contribute to GIST prognosis.
Here, we present a retrospective study, comprehensively evaluating clinicopathological features of GIST and patient outcomes to more deeply elucidate the role of patients’ medication adherence and gender on the prognosis of GIST.
First, this study was aimed at defining the relationship between gender and prognosis in GISTs. The major finding was that among patients who received adjuvant treatment with imatinib, men had a higher recurrence rate than women, but this relationship did not hold in patients who had the same level of medication adherence, and gender was not a significant predictive factor when other factors were included in the analysis. In this study, women adhered better to imatinib treatment than men. Therefore, the explanation for the higher postoperative recurrence rate in male patients might be that their medication adherence was poorer than that of female patients. Kao et alCitation30 reported the same findings in the area of diabetic retinopathy, that male patients tended to have poorer medication adherence, and, after adjustment for other factors, patients with poorer medication adherence showed a significantly higher likelihood of needing treatment for diabetic retinopathy. Hence, it is important to promote GIST patients’ medication adherence to prevent the recurrence of tumors, especially in those who are prone to have poor medication adherence (eg, male gender).
The second interesting result of our study was a gender-related difference in patients’ medication adherence. Examination of factors that could account for male and female differences in adherence to imatinib treatment revealed that male patients with an MPR of less than 90% had a limited understanding of the disease, whereas female patients had poor tolerance to adverse drug reactions. The observations of other researchersCitation31,Citation32 are consistent with ours: Kucukarslan et alCitation31 found that patients’ cognition of disease (disease harm, etiology, duration, outcome, and controllability) directly affected their medication adherence. Hyman et alCitation32 reported that female patients had a higher level of care and understanding of diseases and adherence to medical staff’s instructions than male patients, which might be why their medication adherence was better. Some studies have reported that female patients not only account for a higher proportion of adverse drug reactions in the population but have had a higher incidence of adverse drug reactions,Citation33–35 which might be why poor tolerance to drug reactions was the major factor responsible for the poor-to-medium adherence in female patients.
Third, the expense of drugs can also have an impact on patients’ medication adherence, as shown in . In 2018, a movie called Dying to Survive tells the story of chronic myelocytic leukemia patients who cannot afford the cost of imatinib and cannot take the drug regularly and consistently, resulting in a poor prognosis in patients.Citation36 Reconciling the considerable conflict between “drug”, “prognosis”, and “expense” is a problem that merits due effort.
Our findings and those of other researchers should prompt recognition of and tailoring to gender differences in the care of GIST patients receiving adjuvant therapy with imatinib. In a follow-up with male patients, perhaps more emphasis should be placed on improving their understanding of the disease. Various methods can be used to assess and encourage adherence to medications, including the use of prescription databases, patient self-reporting, questionnaires and diaries, drug dosimetry, monitoring of imatinib concentration, and electronic patient management systems.Citation37–39 In female patients, adverse drug reactions should be carefully addressed; the patient should be informed in advance that reactions from taking imatinib are possible and that these side effects might improve or resolve over an extended period.Citation40 Oral treatment with imatinib for GIST patients after surgery is a long-term process: drug discontinuation rates in the SSG XVIII/AIO trial were 13% at 1 year and 26% at 3 years.Citation16 Because imatinib can cause severe adverse reactions, some patients find that adhering to instructions for taking the medication is difficult, resulting in diminished postoperative efficacy.Citation13,Citation39
Long-term imatinib therapy, with proper and continuous dosing, is important for achieving good clinical results.Citation41 For many patients with primary localized GISTs who have undergone complete surgical resection, subsequent treatment has transformed the condition into a manageable chronic disease.Citation42–44 The safety, tolerance, and adherence rates of imatinib should be considered in long-term postoperative treatment. Patients might have doubts about the need for continued treatment in the absence of any obvious symptomatic response.Citation43 For patients with chronic diseases, poor rates of adherence can lead to diminished health-related quality of life and economic loss, which are global problems.Citation45
We acknowledge that this study had limitations. First, it was a single-center, retrospective study, meaning that selection bias could not be avoided. In addition, since our study had a single-center design, extrapolating our results to other centers requires great caution. For example, the data of many patients with poor adherence were lost, so these patients’ follow-up data were not included in the study. Second, we measured adherence according to the MPR, without other objective instruments like pill counts, an electronic monitoring system, or monitoring of imatinib concentrations during therapy, and without questionnaires on medication adherence like 8-item Morisky Medication Adherence ScaleCitation46 or 10-item validated Medication Adherence Questionnaire.Citation29 To assess the association between gender and medication adherence more precisely, we look forward to using the methods described above to evaluate adherence to imatinib treatment in future studies. Despite these limitations, our findings are important in that they identified gender differences in adherence and can help improve adherence to achieve desired clinical outcomes. A multicenter study with a larger sample size should be conducted to further investigate the relationship of gender to adherence and prognosis in the treatment of primary GISTs.
Conclusion
Male GIST patients had a higher recurrence rate than female patients among those who received adjuvant treatment with imatinib. Note that gender was not an independent risk factor for patient prognosis, but medication adherence was. Men’s medication adherence was worse than women’s, which could account for the worse prognoses among male patients. The major factor responsible for the male patients was limited understanding of the disease, whereas that of female patients could be attributed to poorer tolerance to drug resistance or drug reactions. These gender differences in the degree of adherence and the factors responsible for suboptimal adherence to imatinib adjuvant treatment should be considered in postoperative pharmacotherapy for patients with primary localized GISTs.
Abbreviations
GIST, Gastrointestinal stromal tumor; TKI, Tyrosine kinase inhibitor; RFS, recurrence-free survival; MPR, medication possession ratio; NIH, National Institutes of Health; CD117, cluster of differentiation 117; DOG-1, gastrointestinal stromal tumors protein 1; HPF, high power field; ECOG, Eastern Cooperative Oncology Group; CMU, Chongqing Medical University; HR, hazard ratio; CI, confidence interval; SD, standard deviations.
Ethics Statement
This study was approved by the Institutional Review Board of The First Affiliated Hospital of CMU (Approval number:2021-756) with a waiver for written informed consent, owing to its non-interventional, observational, and retrospective design. All clinical data collected into the “Weinichangzai” database were obtained with the patient’s verbal informed consent, in which the patient data used were kept strictly confidential. This study was performed in accordance with the standards of ethics outlined in the Declaration of Helsinki.
Consent for Publication
The authors read and approved the final manuscript.
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Additional information
Funding
References
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369(9574):1731–1741. doi:10.1016/S0140-6736(07)60780-6
- Zhang H, Liu Q. Prognostic indicators for gastrointestinal stromal tumors: a review. Transl Oncol. 2020;13(10):100812. doi:10.1016/j.tranon.2020.100812
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi:10.1016/S1470-2045(11)70299-6
- Liu X, Lin E, Sun Y, et al. Postoperative adjuvant imatinib therapy-associated nomogram to predict overall survival of gastrointestinal stromal tumor. Front Med. 2022;9:777181. doi:10.3389/fmed.2022.777181
- Sharma AK, Kim TS, Bauer S, Sicklick JK. Gastrointestinal stromal tumor: new insights for a multimodal approach. Surg Oncol Clin N Am. 2022;31(3):431–446. doi:10.1016/j.soc.2022.03.007
- Hsu KH, Yang TM, Shan YS, Lin PW. Tumor size is a major determinant of recurrence in patients with resectable gastrointestinal stromal tumor. Am J Surg. 2007;194(2):148–152. doi:10.1016/j.amjsurg.2006.10.033
- Laurent M, Brahmi M, Dufresne A, et al. Adjuvant therapy with imatinib in gastrointestinal stromal tumors (GISTs)-review and perspectives. Transl Gastroenterol Hepatol. 2019;4:24. doi:10.21037/tgh.2019.03.07
- Tetzlaff ED, Davey MP. Optimizing adherence to adjuvant imatinib in gastrointestinal stromal tumor. J Adv Pract Oncol. 2013;4(4):238–250.
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi:10.1002/cncr.20862
- Woodall CE 3rd, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg. 2009;144(7):670–678. doi:10.1001/archsurg.2009.108
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi:10.1053/j.semdp.2006.09.001
- Rong J, Chen S, Song C, et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: a propensity score matching analysis. Biol Sex Differ. 2020;11(1):43. doi:10.1186/s13293-020-00321-8
- Ge XY, Lei LW, Ge F, Jiang X. Analysis of risk factors of gastrointestinal stromal tumors in different age groups based on SEER database. Scand J Gastroenterol. 2019;54(4):480–484. doi:10.1080/00365521.2019.1604798
- Wang Y, Zhang P, Han Y, et al. Adherence to adjuvant imatinib therapy in patients with gastrointestinal stromal tumor in clinical practice: a cross-sectional study. Chemotherapy. 2019;64(4):197–204. doi:10.1159/000505177
- Zhang P, Zhang J, Zhang B, et al. 中国胃肠间质瘤患者伊马替尼服药依从性的多中心横断面调查. [Adherence to adjuvant with therapy imatinib in patients with gastrointestinal stromal tumor: a national multi-center cross-sectional study]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24(9):775–782. Chinese. doi:10.3760/cma.j.cn.441530-20210426-00174
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265–1272. doi:10.1001/jama.2012.347
- Azam F, Latif MF, Farooq A, et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12(3):728–736. doi:10.1159/000503095
- Breccia M, Efficace F, Alimena G. Adherence to treatment is a complex and multifaceted issue that can substantially alter the outcome of chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Leuk Res. 2012;36(7):804–805. doi:10.1016/j.leukres.2012.02.032
- Hall AE, Paul C, Bryant J, et al. To adhere or not to adhere: rates and reasons of medication adherence in hematological cancer patients. Crit Rev Oncol Hematol. 2016;97:247–262. doi:10.1016/j.critrevonc.2015.08.025
- Halpern R, Barghout V, Mody-Patel N, Williams D. Relationship between compliance, costs, hospitalizations for CML and GIST patients using imatinib mesylate. J Clin Oncol. 2008;26(15):6598. doi:10.1200/jco.2008.26.15_suppl.6598
- Halpern R, Barghout V, Williams D. Compliance with imatinib mesylate associated with lower health resource utilization and costs for patients with CML and GIST. BLOOD. 2007;110(11):372B–372B. doi:10.1182/blood.V110.11.5159.5159
- De Almeida MH, Pagnano K, Souza H, Souza CA. Adherence to Tyrosine Kinase Inhibitors (TKI) in Chronic Myeloid Leukemia (CML) seems to be related to duration of treatment and type. Haematologica. 2010;95:343.
- Feng W, Henk H, Thomas S, et al. Compliance and persistency with imatinib. J Clin Oncol. 2006;24(18):310S–310S. doi:10.1200/jco.2006.24.18_suppl.6038
- Guerin A, Bollu V, Guo A, et al. Non-adherence to imatinib in Chronic Myeloid Leukemia (CML) patients is associated with short- and long-term negative impacts on health care resource utilization and costs. Value Health. 2010;13(3):A32–A32. doi:10.1016/S1098-3015(10)72137-9
- StCharles M, Bollu VK, Hornyak E, Coombs J, Blanchette CM, DeAngelo DJ. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia. Blood. 2009;114(22):870. doi:10.1182/blood.V114.22.2209.2209
- Wu EQ, Guerin A, Yu AP, Bollu VK, Guo A, Griffin JD. Retrospective real-world comparison of medical visits, costs, and adherence between nilotinib and dasatinib in chronic myeloid leukemia. Curr Med Res Opin. 2010;26(12):2861–2869. doi:10.1185/03007995.2010.533648
- Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose-lowering therapies and associations with 1-year HbA(1c): a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39(2):258–263. doi:10.2337/dc15-1194
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411–1419. doi:10.1016/j.humpath.2008.06.025
- Chuah PL, Jamal NF, Siew CJ, Ahmad Bustamam RS, Jeyasingam V, Khong KC. Assessment of adherence to imatinib and health-related quality of life among patients with gastrointestinal stromal tumor: a cross-sectional study in an oncology clinic in Malaysia. Patient Prefer Adherence. 2021;15:2175–2184. doi:10.2147/PPA.S310409
- Kao CC, Hsieh HM, Lee DY, Hsieh KP, Sheu SJ. Importance of medication adherence in treatment needed diabetic retinopathy. Sci Rep. 2021;11(1):19100. doi:10.1038/s41598-021-98488-6
- Kucukarslan SN, Lewis NJ, Shimp LA, Gaither CA, Lane DC, Baumer AL. Exploring patient experiences with prescription medicines to identify unmet patient needs: implications for research and practice. Res Social Adm Pharm. 2012;8(4):321–332. doi:10.1016/j.sapharm.2011.08.003
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345(7):479–486. doi:10.1056/NEJMoa010273
- Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol. 1998;38(11):1003–1009. doi:10.1177/009127009803801103
- Hurwitz N, Wade OL. Intensive hospital monitoring of adverse reactions to drugs. Br Med J. 1969;1(5643):531–536. doi:10.1136/bmj.1.5643.531
- Böttiger LE. Adverse drug reaction: an analysis of 310 consecutive reports to the Swedish drug reaction committee. J Clin Pharmacol. 1973;13(10):373–382. doi:10.1002/j.1552-4604.1973.tb00182.x
- Lucas C. The Arts Dying to survive and cancer care in China. Lancet Oncol. 2019;20(1):30. doi:10.1016/S1470-2045(18)30921-5
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi:10.1056/NEJMra050100
- Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–457.
- Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A(1c) in type 2 diabetes. Ann Pharmacother. 2004;38(9):1357–1362. doi:10.1345/aph.1D612
- Blay JY, Rutkowski P. Adherence to imatinib therapy in patients with gastrointestinal stromal tumors. Cancer Treat Rev. 2014;40(2):242–247. doi:10.1016/j.ctrv.2013.07.005
- Le Cesne A, Ray-Coquard I, Bui BN, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised Phase 3 trial. Lancet Oncol. 2010;11(10):942–949. doi:10.1016/S1470-2045(10)70222-9
- Saponara M, Pantaleo MA, Nannini M, Biasco G. Chronic therapy in gastrointestinal stromal tumours (GISTs): the big gap between theory and practice. Target Oncol. 2012;7(4):243–246. doi:10.1007/s11523-012-0221-1
- Dirnhofer S, Leyvraz S. Current standards and progress in understanding and treatment of GIST. Swiss Med Wkly. 2009;139(7–8):90–102.
- Hauber AB, Gonzalez JM, Coombs J, Sirulnik A, Palacios D, Scherzer N. Patient preferences for reducing toxicities of treatments for gastrointestinal stromal tumor (GIST). Patient Prefer Adherence. 2011;5:307–314. doi:10.2147/PPA.S20445
- Hansen R, Seifeldin R, Noe L. Medication adherence in chronic disease: issues in posttransplant immunosuppression. Transplant Proc. 2007;39(5):1287–1300. doi:10.1016/j.transproceed.2007.02.074
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi:10.1111/j.1751-7176.2008.07572.x