Abstract
Oral contraceptives remain a popular method of contraception over 50 years after their introduction. While safe and effective for many women, the failure rate of oral contraception is about 8%. Concerns about the risk of venous thromboembolism continue to drive the search for the safest oral contraceptive formulations. The oral contraceptive NOMAC-E2 contains nomegestrol acetate (NOMAC) 2.5 mg + 17b-estradiol (E2) 1.5 mg. The approved dosing regimen is 24 days of active hormone, followed by a 4-day hormone-free interval. NOMAC is a progestin derived from testosterone, which has high bioavailability, rapid absorption, and a long half-life. Estradiol, though it has a lower bioavailability, has been successfully combined with NOMAC in a monophasic oral contraceptive. Two recently published randomized controlled trials demonstrate that NOMAC-E2 is an effective contraceptive, with a Pearl Index less than one pregnancy per 100 woman-years. The bleeding pattern on NOMAC-E2 is characterized by fewer bleeding/spotting days, shorter withdrawal bleeds, and a higher incidence of amenorrhea than the comparator oral contraceptive containing drospirenone and ethinyl estradiol. The adverse event profile appears to be acceptable. Few severe adverse events were reported in the randomized controlled trials. The most common adverse events were irregular bleeding, acne, and weight gain. Preliminary studies suggest that NOMAC-E2 does not seem to have negative effects on hemostatic and metabolic parameters. While no one oral contraceptive formulation is likely to be the optimum choice for all women, NOMAC-E2 is a formulation with effectiveness comparable with that of other oral contraceptives, and a reassuring safety profile.
Introduction
Oral contraceptives are now in their sixth decade of use. They remain the most popular method of contraception in many parts of the world.Citation1 Available products have expanded from Enovid® (mestranol-norethynodrel), the first oral contraceptive approved in 1960, into a worldwide market with dozens of brands. While their popularity has lasted through the years, adherence and satisfaction with oral contraceptives continues to pose challenges for some women. If used correctly and consistently, oral contraceptives potentially have a failure rate as low as three in 1000 women; under real-world conditions, the actual failure rate is closer to 8%,Citation2 in large part due to discontinuation of use of the product, and problems with adherence. Concerns about rare but serious side effects have also affected pill use over the years, and driven a continued search for the safest possible formulations.
Most oral contraceptives available today contain ethinyl estradiol. There is increased focus on 17b-estradiol (E2, also referred to simply as estradiol), a naturally occurring estrogen that is believed to have fewer adverse effects than ethinyl estradiol.Citation3 Attempts to introduce estradiol into oral contraceptives began decades ago, but problems with oral bioavailability and unsatisfactory bleeding profiles limited its applicability.Citation4
While estrogen options for oral contraceptives are limited, there are numerous possible progestins. Some, such as desogestrel and drospirenone, have been dogged by concerns that they raise the risk of combined oral contraceptive-related venous thromboembolism relative to other progestins.Citation5 The clinical significance of any increased risk is a subject of debate,Citation6 but the concern has motivated a search for progestins with a more favorable risk-benefit profile.
Nomegestrol acetate (NOMAC) is receiving renewed attention, in part for this reason. A progestin first developed in the 1980s, NOMAC was originally used for postmenopausal hormone therapy,Citation7 and later in an implantable contraceptive.Citation8 Most recently, NOMAC has been formulated with E2 in a combination oral contraceptive, marketed with the brand name NOMAC-E2. Zoely® (Merck & Co., Inc, Whitehouse Station, NJ, USA) was first marketed in 2011 and is now available in several countries, although the formulation has not been approved in the US. Recently published papers have focused on the pharmacology of NOMAC alone and in combination with E2.Citation9,Citation10 This review summarizes some of this information and discuss clinical outcomes of interest, including efficacy and bleeding patterns. Other clinical issues discussed include bone density, acne, thromboembolism, lipid effects, and concerns in the obese population.
Pharmacology, mechanism of action, and pharmacokinetics
Nomegestrol acetate
Like all combined oral contraceptives, Zoely contains an estrogen and a progestin component. A progestin, or progestogen, is a compound that exhibits progestational activity: most typically, the transformation of endometrium from proliferative to secretory in an estrogen-primed uterus.Citation11 The only natural progestogen is progesterone, which is inactivated when taken orally.Citation12
Synthetic contraceptive progestins are related either to testosterone or progesterone. Those first used in oral contraceptives, sometimes called first-generation or second-generation progestins, were derivatives of testosterone or 19-nortestosterone. Examples include norethynodrel, norethindrone, and levonorgestrel. One newer testosterone-derived progestin, drospirenone, is an analog of spironolactone. Many newer progestins are structurally similar to progesterone. These include cyproterone acetate, nestorone, and NOMAC.Citation11 The move toward progesterone-derived compounds was motivated in part by their greater specificity for the progesterone receptor. This may limit androgenic and other side effects, while preserving the contraceptive effect. Progesterone-derived compounds may also appeal to consumers and providers based on a perception that they are more “natural”.
Manipulations of the chemical structure of progesterone alter the potency (progestational activity) and oral bioavailability of end products.Citation9,Citation11 The chemical structures of progesterone and NOMAC are shown in , with medroxyprogesterone acetate and megestrol acetate included for comparison.
Figure 1 Progesterone, nomegestrol acetate, and selected related structures. http://pubchem.ncbi.nlm.nih.gov.
Estradiol
It was serendipity that led to the incorporation of an estrogen component in the first oral contraceptive. Mestranol, an estrogen compound used in early oral contraceptives, was a contaminant in the manufacturing process of norethynodrel.Citation13 However, its subsequent removal was followed by an unacceptable rate of irregular bleeding among participants in the early clinical trials. Mestranol was therefore added back, and estrogen found its place.
Mestranol undergoes demethylation in the liver to yield ethinyl estradiol. Ethinyl estradiol, the biologically active compound, long ago replaced mestranol as the estrogen of choice in oral contraceptive formulations. Progressively lower doses of ethinyl estradiol have been used in an attempt to eliminate dose-related adverse effects of oral contraceptives. Reducing the ethinyl estradiol dose from 50 μg to 35 μg resulted in a decreased risk of adverse cardiovascular events such as venous thromboembolism. However, it is not clear that reducing the dose more, to 20 μg or less, has further reduced this risk,Citation14 and there may be a threshold effect below which no additional risk reduction is seen. Very low doses of ethinyl estradiol are also associated with an increased incidence of irregular bleeding.Citation15
It has long been recognized that estradiol, or 17b-estradiol (E2) produced by the ovary is a potent natural estrogen with contraceptive potential. It may have a more favorable cardiovascular risk profile than ethinyl estradiol. However, estradiol is not well absorbed orally, and early attempts at E2-based pills were abandoned due to poor cycle control.Citation13,Citation16 An oral contraceptive containing E2 requires a potent progestin able to stabilize the endometrium.Citation9 The first estradiol-containing pill to be introduced to the modern market contained dienogest and estradiol valerate. An acceptable bleeding pattern was only obtainable via the creation of a four-phasic (or “dynamic”) pill, meaning that there were four different dosing regimens in a 28-day cycle.Citation17 This requires that the user follows a relatively complex set of instructions for missed pills. NOMAC is the first such progestin to succeed in a monophasic combination with E2. The structures of ethinyl estradiol and E2 are shown in .
Figure 2 Estradiol and ethinyl estradiol. http://pubchem.ncbi.nlm.nih.gov.
Mechanism of action
The contraceptive effect of birth control pills derives primarily from the progestin component, while the estrogen component contributes cycle control and potentiates contraceptive efficacy. The progestin contraceptive effects of NOMAC include antigonadotropin activity (suppression of the luteinizing and follicle-stimulating hormone surges that lead to follicle development and ovulation); thickening of cervical mucus; and thinning of the endometrium.Citation18,Citation19
NOMAC is a potent progesterone receptor agonist, with a high affinity for the progesterone receptor, being about 125% that of progesterone.Citation9 Unlike many other progestins, it does not bind to androgen, mineralocorticoid, or glucocorticoid receptors.Citation9,Citation20 Some side effects of other oral contraceptives have been attributed to this lack of specificity. NOMAC may possess mild to moderate antiandrogenic properties.Citation3,Citation21
Pharmacokinetics
The pharmacokinetics of NOMAC and E2 have recently been reviewed,Citation9,Citation10 and are briefly summarized below.
NOMAC
The pharmacokinetic profile of NOMAC provides some clues to its potential for success in an oral contraceptive. Relevant pharmacokinetic parameters include: maximum concentration (Cmax), time to reach maximum concentration (Tmax), half-life (t1/2, time needed for serum drug levels to fall to 50% of maximum), and bioavailability. Bioavailability (Fabs) is defined as the amount of orally administered drug that reaches the systemic circulation after undergoing hepatic first-pass metabolism,Citation11 and is generally determined by a comparison with intravenous administration. Some relevant pharmacokinetic parameters of NOMAC are shown in . The oral bioavailability of NOMAC is considered to be relatively high, at about 65%.Citation9 Peak serum levels of NOMAC are rapidly seen.Citation9,Citation10,Citation22 NOMAC has a long elimination half-life (45–50 hours) compared with other contraceptive progestins. This may mean that its contraceptive efficacy is more forgiving of missed pill doses than progestins with a shorter half-life.Citation22
Table 1 Selected pharmacokinetic parameters for NOMAC 2.5 mgCitation9,Citation10,Citation22
NOMAC binds to albumin but not to sex hormone binding globulin.Citation9 It is hepatically metabolized, like other contraceptive hormones, via the cytochrome P450 enzyme system. As with other oral contraceptives, there is a potential for interaction with drugs that induce hepatic enzymes. Examples include rifampin and some antiepileptic drugs.Citation10
Estradiol
The bioavailability of E2 is between 1% and 5%.Citation10,Citation22 Once ingested, estradiol is metabolized by the liver to estrone and estrone sulfate, among other compounds. Enterohepatic recirculation maintains a large circulating pool of estrogen compounds,Citation20 which results in a somewhat variable half-life,Citation10 with the half-life of estradiol after intravenous administration being 3.6 hours.Citation10
Gerrits et al studied the pharmacokinetics of NOMAC-E2 after multiple (24 days) and single dosing.Citation22 For NOMAC, the time to reach steady-state concentration was 5 days. Their results for Tmax and t1/2 were consistent with previous findings. They noted that determination of parameters for E2 was somewhat more challenging, because serum assays did not distinguish between exogenous and endogenous E2, and because the contraceptive effects of NOMAC resulted in suppression of endogenous E2 production.Citation22 The reported E2 levels at steady state were consistent with E2 levels expected for the early follicular and late luteal phases of the menstrual cycle.
Formulation, dose, and dosing schedule
NOMAC-E2 oral contraceptive formulation
Each pill contains NOMAC 2.5 mg + E2 1.5 mg. It is provided in a 24/4 dosing schedule, meaning 24 days of active pill followed by a 4-day hormone-free interval. The formulation is innovative in that it is the first monophasic oral contraceptive to use estradiol, and the only combined oral contraceptive to include NOMAC.
Dose selection
The dose of NOMAC 2.5 mg + E2 1.5 mg was identified from a dose-finding Phase IIa study conducted in France. The investigators randomized 41 healthy women aged 18–35 years to one of three doses of NOMAC (0.625, 1.25, or 2.5 mg) with ethinyl estradiol 1.5 mg or to NOMAC 2.5 mg alone. Thirty-eight women completed study treatment, which included one control cycle and one treatment cycle.Citation23 The study primarily assessed inhibition of ovulation as determined by serum assays for progesterone and luteinizing hormone. Secondary objectives were to evaluate effects on follicle-stimulating hormone and serum E2 levels, as well as cervical mucus scores.
In all groups, ovulation was suppressed during the treatment cycle, as evidenced by suppression of the midcycle luteinizing hormone peak and progesterone levels consistently less than 3 ng/mL, the level which indicates ovulation. The degree of suppression of serum progesterone was correlated with NOMAC dose in the combined groups, while daily progesterone levels were higher in the NOMAC alone group than in the group who received NOMAC 2.5 mg + E2. No follicle-stimulating hormone peak was seen in any group. The greatest suppression of follicular maturation (evidenced by lowest follicle-stimulating hormone levels) was seen in the NOMAC 2.5 mg + E2 group. Lower serum E2 values also correlated with higher doses of NOMAC-E2. Cervical mucus scores were similar among the groups, and all treatment-cycle cervical mucus scores were less than 3, indicating unfavorability for fertilization. Based on the combination of ovulation inhibition and degree of follicular suppression, the authors concluded that the formulation containing NOMAC 2.5 mg + E2 1.5 mg was optimal.Citation23
Why 24/4?
It is increasingly accepted that the original 28-day oral contraceptive cycle, consisting of 21 days of an active pill and 7 days of placebo, was based less on science and more on social considerations informed by the era in which the first oral contraceptive pills were developed.Citation24 A growing number of oral contraceptive formulations use a 24/4 regimen. Possible benefits of a shortened placebo phase include enhanced follicular suppression, improvement in premenstrual symptoms which often manifest during the placebo period, and potentially improved efficacy.Citation25,Citation26 The evidence also suggests that oral contraceptives dosed in a 24/4 regimen are associated with a shorter length of withdrawal bleeding than 21/7 dosing.Citation25
After determining the optimal dose for NOMAC-E2, the same group of researchers compared 24-day and 21-day regimens.Citation27 The primary efficacy outcome was ovarian activity assessed by evaluating ovarian follicle growth on transvaginal ultrasound. Secondary efficacy outcomes included serum measurements of follicle-stimulating hormone, luteinizing hormone, E2, and progesterone, endometrial thickness (via transvaginal ultrasound) and cervical mucus characteristics. Of 80 women randomized 35 in the 21-day group and 37 women in the 24-day group completed the study, which consisted of three 28-day cycles of the assigned pill.
No ovulations were noted in either group over the course of the study. However, mean diameter of the largest ovarian follicle was greater in the 21-day group than in the 24-day group, exceeding 10 mm in the 21-day group (13.0 ± 7.5 mm versus 9.9 ± 3.4 mm, P = 0.02). Effects on endometrial thickness and cervical mucus were similar, and consistent with the expected contraceptive effect. The authors concluded that the 24/4 regimen was superior to the 21/7 regimen for inhibition of follicular growth, and therefore preferred.Citation27
Efficacy
Two large, international, randomized controlled trials, one conducted in the AmericasCitation28 and one conducted in Europe and AustraliaCitation29 studied the efficacy of NOMAC-E2 oral contraception in comparison with pills containing drospirenone 3 mg + ethinyl estradiol 30 μg. Both trials were funded by the pill manufacturer.
The trials assessed the efficacy and tolerability of NOMAC-E2 in a 24/4 regimen versus drospirenone-ethinyl estradiol in a 21/7 regimen. Women were randomized in a 3:1 ratio to NOMAC-E2 or drospirenone–ethinyl estradiol. Participants were included if they were healthy, aged 18–50 years, had a body mass index in the range of 17.0–35.0 kg/m2, had no contraindications to oral contraceptive use per the World Health Organization’s Medical Eligibility Criteria for Contraceptive Use,Citation30 had a normal cervical (Papanicolaou) smear, and were not using exclusionary medications. Instructions for missed (>12 hours late) pills were to take the missed pill as soon as possible and continue the rest of the pack on schedule. A 7-day backup period with condoms was advised if women in the NOMAC-E2 group missed one tablet on days 1–7 or 18–24, or two tablets between days 8 and 17. Women in the drospirenone–ethinyl estradiol group were to use condoms if they missed one tablet at any time during the active pill cycle.
Pregnancy rate, or efficacy, was calculated using the Pearl Index (pregnancies per 100 women-years of exposure) and life table analyses (Kaplan–Meier estimates with confidence intervals [CI]). The latter were expressed as pregnancies per 100 women.
Of 2152 women randomized across 95 study centers by Mansour et al, 1591 received NOMAC-E2 and 535 received drospirenone–ethinyl estradiol. Approximately 72% of women in the NOMAC-E2 group completed the one-year trial, as did 76.6% of women in the drospirenone group. Completion was defined as continuing in the trial through 13 28-day cycles of pills.Citation29
For Pearl Index determinations, the trial accrued 1057 woman-years of NOMAC-E2 exposure for women aged 18–35 years. There were four in-treatment pregnancies, giving a Pearl Index of 0.38 (95% CI 0.10–0.97) for NOMAC-E2. When all women aged 18–50 years were included, the Pearl Index was 0.31 (0.08–0.79). Women in the drospirenone–ethinyl estradiol group contributed 372 woman-years for analysis among women 18–35 years of age. With three in-treatment pregnancies in the drospirenone group, the Pearl Index was 0.81 (0.17–2.35) for those aged 18–35 years, and 0.66 (0.14–1.94) among women aged 18–50 years. The difference between the groups was not statistically significant.Citation29
Life table analysis showed cumulative pregnancy rates of 0.33% among women in the NOMAC-E2 group and 0.64% among women in the drospirenone–ethinyl estradiol group. The authors concluded that cumulative pregnancy rates were lower in the NOMAC-E2 group, although the results did not achieve statistical significance.Citation29
Westhoff et alCitation28 randomized 2281 women to either NOMAC-E2 or drospirenone–ethinyl estradiol. Of those randomized, 1666 women received treatment with NOMAC-E2, 988 of whom (59%) completed the trial. Of 554 women who received drospirenone–ethinyl estradiol, 344 (62%) completed the trial.
For the Pearl Index determinations, the trial accrued 946 woman-years (12,296 cycles) of NOMAC-E2 exposure for women aged 17–35 years. There were 12 in-treatment pregnancies, giving a Pearl Index of 1.27 (95% CI 0.66–2.22) for NOMAC-E2. When all women aged 18–50 years were included, the Pearl Index was 1.13 (0.60–1.94). Women in the drospirenone–ethinyl estradiol group contributed 318 woman-years (4135 cycles) for analysis. With six in-treatment pregnancies among women aged 18–35 years, the Pearl Index for drospirenone was 1.89 (95% CI 0.69–4.11) in this group, and 1.83 (95% CI 0.74–3.77) among women aged 18–50 years. The difference between NOMAC-E2 and drospirenone–ethinyl estradiol groups was not statistically significant. Life table analysis showed cumulative pregnancy rates of 1.09% among women in the NOMAC-E2 group and 1.75% among women in the drospirenone–ethinyl estradiol group. The authors of this trial concluded that cumulative pregnancy rates were lower in the NOMAC-E2 group, while acknowledging that the results did not achieve statistical significance.Citation28
These studies indicate that NOMAC-E2 provides effective contraception, with an acceptably low pregnancy rate that is at least comparable with that of other oral contraceptives. The authors commented that the instructions for missed pills were less conservative for NOMAC-E2 than for drospirenone–ethinyl estradiol (the former group could miss two pills mid cycle without need for backup, while the latter could only miss one), but it is difficult to know whether this affected pregnancy rates.Citation28,Citation29 While the pregnancy rates were higher in the US trial, this held true for both the NOMAC and drospirenone formulations, and the reasons for this are likely varied.
Bleeding patterns
Bleeding patterns, particularly the degree of unscheduled bleeding and the length and characteristics of expected withdrawal bleeding, contribute to the tolerability of oral contraceptives. As noted, a need for a more favorable bleeding pattern led to the delay in introducing an E2-based monophasic pill, and the NOMAC-E2 formulation is the first such formulation to succeed.
The twin efficacy studies assessed several bleeding parameters.Citation28,Citation29 Primary bleeding outcomes included presence or absence of unscheduled (breakthrough) bleeding/spotting, and absence of withdrawal bleeding. Secondary parameters included occurrence of breakthrough bleeding episodes (defined as requiring more than one pad or tampon per day); occurrence of unscheduled spotting (defined as requiring no more than one pad or tampon per day); number of unscheduled bleeding/spotting days; and number of scheduled (withdrawal) bleeding days.
Both the Westhoff et alCitation28 and Mansour et alCitation29 studies assessed bleeding profiles according to standard 91-day reference periods, as well as per cycle. When compared with drospirenone–ethinyl estradiol in a 21/7 regimen, NOMAC-E2 in a 24/4 regimen was associated with fewer bleeding and spotting days in all reference periods, with statistically significant difference between groups in the later reference periods ( and ). Other bleeding parameters are presented in . The incidence of breakthrough bleeding episodes was significantly different between treatment groups in the early treatment cycles (cycles 2–4Citation29 and cycles 2–6Citation28), with a greater incidence in women on NOMAC-E2. However, after 4–6 cycles of use, these differences disappeared.
Figure 3 (A) Mean number of bleeding and spotting days for reference periods 1 (days 1–90) and 4 (days 274–364) as reported in Westhoff et al.Citation28 (B) Mean number of bleeding and spotting days for reference periods (RP) 1 (days 1–90) and 4 (days 274–364) as reported in Mansour et al.Citation29
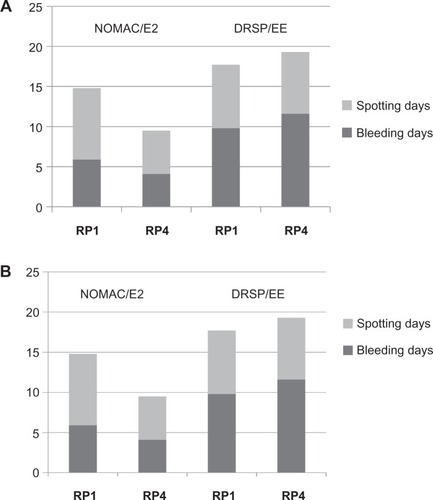
Table 2 Bleeding parameters for NOMAC-E2 versus DRSP-EE as reported in two large randomized controlled trialsCitation28,Citation29
As shown in and and , the bleeding patterns for NOMAC-E2 clearly differed from those for drospirenone–ethinyl estradiol. The reasons may relate to the estrogen dose. Differences may also be due to the different progestins, or to the 21/7 versus 24/4 dosing schedule. In their Phase II study comparing 21/7 and 24/4 regimens, Christin-Maitre et alCitation27 evaluated bleeding parameters, including incidence and duration of unscheduled (“intermenstrual”) and scheduled (withdrawal) bleeding. When compared with a 21/7 regimen, the 24/4 dosing regimen was associated with a significantly shorter mean duration of withdrawal bleeding (3.9 versus 4.8 days, P = 0.03). All other measured bleeding parameters were similar between groups.
In general, bleeding patterns on NOMAC-E2 were characterized by fewer bleeding/spotting days in a 3-month period, shorter bleeding episodes, and a similar incidence of breakthrough bleeding, when compared with drospirenone–ethinyl estradiol. The incidence of amenorrhea, or absent withdrawal bleeds, was significantly greater in the NOMAC group.Citation28,Citation29 This trend towards less bleeding would seem to be a favorable outcome. Therefore, it is interesting to note that women in the NOMAC-E2 group were more likely than those in the drospirenone–ethinyl estradiol group to discontinue the study because of bleeding irregularities. This may be due in part to the increased likelihood of breakthrough bleeding in the first months of use, or to the increased likelihood of amenorrhea at most trial time points, with NOMAC-E2 compared with drospirenone–ethinyl estradiol. Amenorrhea may be more acceptable now than it was at the time the trials were initiated.Citation31 Now that these trial results have been published, providers can offer accurate anticipatory counseling about NOMAC-E2 bleeding patterns, thus helping to ensure that women who choose NOMAC-E2 are satisfactorily informed, and that those who find such patterns unacceptable can choose a method that better suits their needs.
Safety and tolerability
Adverse events in clinical trials
There is heightened vigilance for adverse events among participants in clinical trials. For the international efficacy trials, the most common adverse events deemed related to the study contraceptive are listed in . Effects like irregular withdrawal bleeding and acne were more common in the NOMAC group, although the authors did not report tests of statistical significance for these differences. Serious adverse events were uncommon, with an overall incidence of 1.8%–2.0%.Citation28,Citation29 Not all serious adverse events had a plausible association with the study drug.
Table 3 Incidence of adverse effects reported by users of NOMAC-E2 and DRSP-EE in randomized controlled trialsCitation28,Citation29
Effects on lipids
NOMAC, alone or in combination with estrogen and at daily doses up to 5 mg, does not adversely affect lipid parameters.Citation32 A recent, randomized, controlled trial showed no significant changes in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, or triglycerides after six cycles of NOMAC-E2. In contrast, a 13% decrease in high-density lipoprotein cholesterol, a 7% increase in low-density lipoprotein cholesterol, and a 17% increase in triglycerides were seen in the comparator group receiving levonorgestrel 150 mg + ethinyl estradiol 30 μg after six cycles of use. Whether such changes are clinically relevant is uncertain.Citation33
Effects on carbohydrate metabolism
Women receiving six cycles of treatment with NOMAC-E2 demonstrated no changes in glucose or insulin levels during treatment.Citation33
Endocrine effects
A randomized trial assessed effects on endocrine function (adrenal and thyroid) among women using either NOMAC–ethinyl estradiol or levonorgestrel–ethinyl estradiol (30 μg) for six cycles. The authors reported greater increases in cortisol and corticosteroid-binding globulin for levonorgestrel–ethinyl estradiol than for NOMAC-E2, although the clinical relevance of these changes is not clear. There were no significant changes from baseline in levels of thyroid-stimulating hormone or free thyroxine (T4), in either group.Citation34
Bone mineral density
One study evaluated bone mineral density changes with contraceptive doses of NOMAC-E2.Citation35 The authors followed bone mineral density in a longitudinal study of 110 premenopausal women randomized to NOMAC-E2 or levonorgestrel–ethinyl estradiol (150 μg/30 μg) for 2 years, as measured by annual dual x-ray absorptiometry scans. The authors determined z-scores for the lumbar spine, femoral neck, hip, and trochanter (the z-score indicates the distance of the measured bone mineral density from the age-matched population mean). There were no clinically significant differences between groups or within groups over time. As has been discussed in the context of other contraceptive methods, the utility of bone mineral density testing in premenopausal women is controversial, and there are few data on the more clinically relevant outcome of fracture risk. There is no reason to suspect that fracture risk is elevated among users of any oral contraceptive formulation. A randomized trial of 176 postmenopausal women suggested that the combination of NOMAC 3.75 mg + E2 1.5 mg, given daily for 12 weeks, had “no deleterious effects” on bone health, and seemed to decrease markers of bone turnover.Citation36 Currently available data reassure us that NOMAC-E2 oral contraception does not adversely affect bone mineral density.
Weight change
In the US and European trials, women using NOMAC–ethinyl estradiol gained an average of 1.0 kg during the one-year study period, while women in the drospirenone–ethinyl estradiol group gained an average of 0.2–0.3 kg.Citation28,Citation29 The difference between treatment groups was statistically significant in both studies. The difference may be due in part to the slight diuretic effect of drospirenone. Westhoff et alCitation28 reported “weight gain” as an adverse event in 9.5% of women in the NOMAC group and 5.2% of women in the drospirenone group, while Mansour et alCitation29 found that 7.9% of women in the NOMAC group and 6.1% of women in the drospirenone group complained that their “weight increased”.
Acne
In general, oral contraceptives cause improvement in acne symptoms.Citation37 Use of oral contraceptives containing fewer androgenic progestins is often recommended, although some experts believe that the androgenicity of oral contraceptives has been overstated, due to misinterpretation of data from animal studies.Citation38 The large efficacy trialsCitation28,Citation29 included assessments of acne, and reached similar conclusions. The most common outcome, regardless of study or treatment group, was improvement in acne. However, more women in the NOMAC-E2 group complained about development or worsening of acne compared with women in the drospirenone group. Given that drospirenone is a spironolactone analog, and spironolactone is sometimes used as a treatment for acne, this is not entirely surprising.
Westhoff et alCitation28 reported that one-third of women in both groups had acne at baseline. Over the course of the study, the trend towards improvement favored drospirenone (63% versus 54% in the NOMAC group, P < 0.001). Approximately the same percentage of women saw their acne worsen (6% versus 5%), while a smaller percentage of women in the drospirenone group than in the NOMAC group reported no change in their acne (32% versus 40%). Of women with no acne at baseline, most were acne-free at the end of the study, though the proportion was greater for drospirenone than for NOMAC (95.8% versus 87.6%). There were more new cases of acne reported among the NOMAC group (12.4%) than among the drospirenone group (4.2%, P < 0.001).
Mansour et alCitation29 noted similar results. About 33% of women in both groups reported acne at baseline, and the presence of acne decreased over time. Overall, three-quarters of women reported no change in acne symptoms. For the women with acne at baseline, 61.4% in the drospirenone group and 48.4% in the NOMAC group reported improvement by the end of the study. Worsening occurred in 1.8% of women with acne in the drospirenone group and 7.2% in the NOMAC group. Similar to the study by Westhoff et al, more women in the NOMAC group than in the drospirenone group developed acne during the study (11.1% versus 5.1%).Citation28
Venous thromboembolism and markers of hemostasis
Women on oral contraceptives are at relatively increased risk for venous thromboembolism compared with women who are not. The absolute risk is still quite low for healthy women, but for women with certain health conditions or risk factors, the risk may be unacceptably high.Citation30 There have been at least two highly publicized controversies about venous thromboembolism risk with “newer” oral contraceptive progestins, ie, the 1995 desogestrel “pill scare”, and recent controversy over drospirenone-containing pills.Citation5,Citation6 Because NOMAC is so selective for the progesterone receptor, and because estradiol is believed to pose a lower thrombotic risk than ethinyl estradiol,Citation39 it has been postulated that NOMAC-E2 will be associated with a lower venous thromboembolism risk than some other oral contraceptives. Estradiol itself has a lower impact on estrogen-hepatic proteins, and is more readily metabolized by the liver than ethinyl estradiol, the ethinyl group on which slows down that process. The structure increases the bioavailability of ethinyl estradiol compared with E2, but may also contribute to an increased likelihood of estrogen-related adverse events.Citation40 Venous thromboembolism is a rare outcome, and clinical trials for efficacy are not powered to detect statistically significant differences between groups. Any difference that exists will not be known until use of the product is more widespread, and ideally if researchers can anticipate the pitfalls that have led to criticism of studies of venous thromboembolism risk done to date.
Two recent studies have compared markers of hemo-stasis in women taking NOMAC-E2 and in those taking levonorgestrel–ethinyl estradiol.Citation33,Citation41 As a component of oral contraceptives, levonorgestrel is believed to have a relatively low risk of venous thromboembolism, and is often the reference against which other oral contraceptive progestins are compared.Citation5 Gaussem et alCitation41 studied markers of coagulation and fibrinolysis in women randomized to take three cycles of either levonorgestrel–ethinyl estradiol 20 μg or NOMAC-E2. The authors reported a procoagulatory shift in levels of these markers in women taking levonorgestrel–ethinyl estradiol. In contrast, they reported significantly smaller changes from baseline in these markers among women taking NOMAC-E2. One outcome was a change in activated protein C resistance. This marker, which has been proposed as an independent risk factor for venous thromboembolism, increased to a greater degree in levonorgestrel–ethinyl estradiol users than in NOMAC-E2 users (0.46 versus 0.20, P < 0.01).Citation41 The authors noted that no laboratory values in their study were outside the normal range.
Agren et al evaluated multiple coagulatory and thrombolytic indices over six cycles of oral contraceptive use in a randomized study comparing NOMAC-E2 with levonorgestrel–ethinyl estradiol (150 μg/30 μg). They reported that NOMAC-E2 had minimal influence on markers of hemostasis, and caused less change in these parameters than the pill containing ethinyl estradiol 30 μg.Citation33 No thrombotic events were recorded among the 211 participants in both studies, who had mean ages of 27.7 and 28.7 years, and a mean body mass index of 22.7 ± 2.7 (range 18–30) kg/m2.Citation33,Citation41
These two studies also evaluated changes in sex hormone binding globulin, citing its proposed utility as a surrogate marker for venous thromboembolism risk.Citation41 Effects on sex hormone binding globulin were inconsistent between the two studies, which used different doses of ethinyl estradiol (30 μg versus 20 μg) in the levonorgestrel comparator, as well as different lengths of treatment (six versus three cycles). Agren et al reported a 44% increase in sex hormone binding globulin in the NOMAC-E2 group, and a 22% increase in the levonorgestrel-ethinyl estradiol (30 μg) group (P = 0.019).Citation33 Gaussem et alCitation41 studying a 20 μg ethinyl estradiol pill for three cycles, reported similar absolute increases in sex hormone binding globulin levels between groups over the course of the study. A study comparing NOMAC-E2 with drospirenone-ethinyl estradiol reported a significantly greater increase in sex hormone binding globulin in the drospirenone group than in the NOMAC group (308% versus 47%, P = 0.007).Citation18 Because NOMAC does not bind with sex hormone binding globulin, changes in sex hormone binding globulin levels should not affect pharmacokinetic parameters for NOMAC-E2.
Such assessments are surrogate markers of venous thromboembolism risk, and their validity as such is controversial.Citation42 One thrombotic event, a deep venous thrombosis in a drospirenone user, was reported in a clinical trial of NOMAC versus drospirenone,Citation29 while none were reported in the other drospirenone studiesCitation23,Citation28 or in studies comparing NOMAC with levonorgestrel for contraception.Citation18,Citation33,Citation41 Known risk factors for venous thromboembolism, such as morbid obesity, heavy smoking, and cardiovascular disease were generally exclusionary for these clinical studies, and it is women with these risk factors who are statistically more likely to experience a thrombotic event.
Other clinical issues
Obesity
Obesity is defined by a body mass index 30 kg/m2 or greater (Centers for Disease Control, Atlanta, GA, USA). Worldwide, the prevalence of obesity is increasing, and may exceed 40% in the US by 2030. Potential concerns for obese women and oral contraception are increased risk of thrombotic events, and decreased efficacyCitation43,Citation44 Efficacy studies of NOMAC-E2 excluded women with a body mass index greater than 35 kg/m2.Citation28,Citation29 The number of women with body mass index 30 kg/m2 or greater is not described in the primary study lpublications, although Mansour et al comment that contraceptive efficacy was independent of body mass index.Citation29 No published data are available regarding the efficacy of NOMAC-E2 in morbidly obese women (body mass index ≥ 35 kg/m2). No published studies comment on specific risks of NOMAC-E2 among obese women. The development of pills like NOMAC-E2 was motivated in part by the quest for an improved safety profile, which if true, may be beneficial for women at higher baseline risk of adverse events.
Older reproductive age
Women aged 36–50 years were enrolled in the parallel efficacy trials, and comprise approximately 20% of the study population.Citation28,Citation29 Two pregnancies were reported among women aged older than 35 years in the US trial, and none in the European trial. The inclusion of women aged 36–50 years in the overall efficacy calculation changed the Pearl Index and life table efficacy rates only minimally. The authors commented that contraceptive efficacy did not change significantly with age. Neither study reported higher rates of adverse events among participants over the age of 35 years, although safety data were not reported by age in either study.
Continuation and compliance
One challenge to successful use of oral contraceptives is adherence to a daily regimen as preventive treatment for an asymptomatic condition, ie, the desire to prevent pregnancy. The discontinuation rate in the US-based study was higher (about 40% overall) than in the European study (less than 30%).Citation28,Citation29 In the NOMAC groups, 17%–18% of women discontinued because of adverse events, compared with 10%–11% of women in the drospirenone groups. Predictors of discontinuation included younger age (18–24 years), smoking, being a first-time oral contraceptive user, and ethnicity. Treatment group was not a predictor of discontinuation.Citation28
In the European trial, the most common adverse effects leading to discontinuation among women using NOMAC were irregular bleeding (4% of participants), acne (3.3%), and weight increase (1.7%).Citation29 In all three cases, this percentage was greater than in the drospirenone group, in which less than 1% of women discontinued for each of the above reasons. The authors postulated that frequent assessments of the above effects may have heightened participants’ awareness of their occurrence, although this should have affected both groups equally in this randomized study. In the study by Westhoff et alCitation28, irregular bleeding was cited by 3.8% of NOMAC users and 1.8% of drospirenone users as reason for discontinuation. Acne, weight gain, and other adverse effects accounted for 13.5% of discontinuations in NOMAC users and 8.5% of discontinuations in drospirenone users. In both cases, the differences between groups were statistically significant in favor of drospirenone–ethinyl estradiol (P < 0.023 and P < 0.003).Citation28 Observed differences may in fact reflect different pharmacodynamic effects of different hormone regimens.
Compliance was assessed via diaries and records of pills dispensed. Mansour et al noted that most (94% in NOMAC group and 91% in drospirenone groups) participants were compliant with the study regimen, defined as taking pills on at least 95% of assigned days.Citation29 The highly monitored clinical trial environment may not reflect the level of compliance expected in everyday use, which can be affected by many factors.
Conclusion
Sixty years later, we continue to see new developments in oral contraception. We have learned not to expect one oral contraceptive to be the solution to all problems. Nonetheless, we can recognize promise in new formulations. NOMAC-E2 is an innovation that has efficacy at least comparable with that of older oral contraceptives, and is associated with fewer days of bleeding. The formulation appears to have an acceptable safety and tolerability profile. As more women choose NOMAC-E2 for contraception, further study will elucidate the answers to some outstanding questions. Will the unique combination of pharmacokinetic and pharmacodynamic properties result in potentially higher contraceptive efficacy and greater reassurance of safety than other oral contraceptives? Continued study will be needed. Future directions could also include more research into acceptability, comparison with other oral contraceptive formulations, surveillance for rare safety outcomes like venous thromboembolism, and inclusion of obese and overweight women in trials.
Disclosure
The author has received previous funding from Merck/Schering for Implanon/Nexplanon training. The author reports no other conflicts of interest in this work.
References
- United Nations Department of Economic and Social Affairs, Population DivisionWorld contraceptive use 2011 Available from: http://www.un.org/esa/population/publications/contraceptive2011/wallchart_front.pdfAccessed November 14, 2012
- TrussellJContraceptive failure in the United StatesContraception20118339740421477680
- Sitruk-WareRNathAMetabolic effects of contraceptive steroidsRev Endocr Metab Disord201112637521538049
- WenzlRBenninkHCvan BeekASponaJHuberJOvulation inhibition with a combined oral contraceptive containing 1 mg micronized 17 beta-estradiolFertil Steril1993606166198405513
- LidegaardØLøkkegaardESvendsenALAggerCHormonal contraception and risk of venous thromboembolism: national follow-up studyBMJ2009339b289019679613
- RaymondEGBurkeAEEspeyECombined hormonal contraceptives and venous thromboembolism: putting the risks into perspectiveObstet Gynecol20121191039104422525916
- ConardJBasdevantAThomasJLCardiovascular risk factors and combined estrogen-progestin replacement therapy: a placebo-controlled study with nomegestrol acetate and estradiolFertil Steril1995649579627589641
- CoutinhoEMde SouzaJCAthaydeCMulticenter clinical trial on the efficacy and acceptability of a single contraceptive implant of nomegestrol acetate, UniplantContraception1996531211258838490
- RuanXSeegerHMueckAOThe pharmacology of nomegestrol acetateMaturitas20127134535322364709
- YangLPPloskerGLNomegestrol acetate/estradiol: in oral contraceptionDrugs2012721917192822950535
- StanczykFZPharmacokinetics and potency of progestins used for hormone replacement therapy and contraceptionRev Endocr Metab Disord2002321122412215716
- DjerassiCSteroid oral contraceptivesScience1966151105510615325662
- JensenJTEvaluation of a new estradiol oral contraceptive: estradiol valerate and dienogestExpert Opin Pharmacother2010111147115720367275
- SandsetPMHøibraatenEEilertsenALDahmAMechanisms of thrombosis related to hormone therapyThromb Res2009123Suppl 2S70S7319217481
- BurkmanRBellCSerfatyDThe evolution of combined oral contraception: improving the risk to benefit ratioContraception201184193421664507
- ZeunSLuMUddinAZeilerBMorrisonDBlodeHPharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogestEur J Contracept Reprod Health Care20091422123219565420
- EndrikatJParkeSTrummerDSchmidtWDuijkersIKlippingCOvulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studiesContraception20087821822518692612
- DuijkersIJKlippingCGrobPKorverTEffects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17 beta-oestradiol on ovarian function in comparison to a monophasic combined oral contraceptive containing drospirenone and ethinylestradiolEur J Contracept Reprod Health Care20101531432520695770
- ChretienFCDuboisREffect of nomegestrol acetate on spinability, ferning and mesh dimension of midcycle cervical mucusContraception19914355652004529
- SchindlerAECampagnoliCDruckmannRClassification and pharmacology of progestinsMaturitas20086117118019434889
- LelloSNomegestrol acetate: pharmacology, safety profile and therapeutic efficacyDrugs20107054155920329803
- GerritsMGSchnabelPGPostTMPeetersPAPharmacokinetic profile of nomegestrol acetate and 17b-estradiol after multiple and single dosing in healthy womenContraception8132012 [Epub ahead of print.]
- Chabbert-BuffetNChassardDOchsenbeinEThomasJLChristin-MaitreSInhibition of ovulation by NOMAC/E2, a novel monophasic oral contraceptive combining nomegestrol acetate and 17β-oestradiol: a double-blind, randomised, dose-finding pilot studyEur J Contracept Reprod Health Care201116768421332383
- GladwellMJohn Rock’s ErrorThe New Yorker3132000 Available from: http://www.newyorker.com/archive/2000/03/13/2000_03_13_052_TNY_LIBRY_000020393Accessed November 16, 2012
- NakajimaSTArcherDFEllmanHEfficacy and safety of a new 24-day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 micro g (Loestrin 24 Fe)Contraception200775162217161118
- BaerwaldARPiersonRAOvarian follicular development during the use of oral contraception: a reviewJ Obstet Gynaecol Can200426192414715122
- Christin-MaitreSSerfatyDChabbert-BuffetNOchsenbeinEChassardDThomasJLComparison of a 24-day and a 21-day pill regimen for the novel combined oral contraceptive, nomegestrol acetate and 17β-estradiol (NOMAC/E2): a double-blind, randomized studyHum Reprod2011261338134721421664
- WesthoffCKaunitzAMKorverTEfficacy, safety, and tolerability of a monophasic oral contraceptive containing nomegestrol acetate and 17β-estradiol: a randomized controlled trialObstet Gynecol201211998999922525910
- MansourDVerhoevenCSommerWEfficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimenEur J Contracept Reprod Health Care20111643044321995590
- World Health OrganizationMedical Eligibility Criteria for Contraceptive UseFourth Edition2009 Available from: http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/enAccessed November 13, 2012
- ReadCMNew regimens with combined oral contraceptive pills–moving away from traditional 21/7 cyclesEur J Contracept Reprod Health Care201015Suppl 2S32S4121091165
- BasdevantAPelissierCConardJDegrelleHGuyeneTTThomasJLEffects of nomegestrol acetate (5 mg/d) on hormonal, metabolic and hemostatic parameters in premenopausal womenContraception1991445996051773617
- ÅgrenUMAnttilaMMäenpää-LiukkoKEffects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol compared with one containing levonorgestrel and ethinylestradiol on haemostasis, lipids and carbohydrate metabolismEur J Contracept Reprod Health Care20111644445722066891
- ÅgrenUMAnttilaMMäenpää-LiukkoKEffects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in comparison to one containing levonorgestrel and ethinylestradiol on markers of endocrine functionEur J Contracept Reprod Health Care20111645846721942708
- SørdalTGrobPVerhoevenCEffects on bone mineral density of a monophasic combined oral contraceptive containing nomegestrol acetate/17β-estradiol in comparison to levonorgestrel/ethinylestradiolActa Obstet Gynecol Scand2012911279128522762147
- Nguyên-PascalMLThomasJLBergougnouxLGarneroPDrapier-FaureEDelmasPDNomegestrol acetate may enhance the skeletal effects of estradiol on biochemical markers of bone turnover in menopausal women after a 12-week treatment periodClimacteric2005813614516096169
- ArowojoluAOGalloMFLopezLMGrimesDACombined oral contraceptive pills for treatment of acneCochrane Database Syst Rev20127CD00442522786490
- StanczykFZAll progestins are not created equalSteroids20036887989014667980
- Sitruk-WareRNathAThe use of newer progestins for contraceptionContraception20108241041720933114
- MueckAOSitruk-WareRNomegestrol acetate, a novel progestogen for oral contraceptionSteroids20117653153921335021
- GaussemPAlhenc-GelasMThomasJLHaemostatic effects of a new combined oral contraceptive, nomegestrol acetate/17β-estradiol, compared with those of levonorgestrel/ethinyl estradiol. A double-blind, randomised studyThromb .Haemost201110556056721225090
- GrimesDASchulzKFRaymondEGSurrogate end points in women’s health research: science, protoscience, and pseudoscienceFertil Steril2010931731173420153470
- AbdollahiMCushmanMRosendaalFRObesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive useThromb Haemost20038949349812624633
- HoltVLScholesDWicklundKGCushing-HaugenKLDalingJRBody mass index, weight, and oral contraceptive failure riskObstet Gynecol2005105465215625141