Abstract
Background
Lamotrigine is an anticonvulsant drug indicated for the maintenance treatment of bipolar I disorder and for various types of epilepsy. An orally disintegrating tablet (ODT) of lamotrigine was developed to provide a formulation option that might increase patient convenience and satisfaction.
Methods
Subjects with mood disorders who reported difficulty swallowing currently prescribed lamotrigine immediate-release medication (baseline) were enrolled and treated with lamotrigine ODT for three weeks (end of treatment). Subject satisfaction and convenience were measured using the Treatment Satisfaction Questionnaire for Medication (TSQM). Also measured were global psychopathology using the Clinical Global Impression severity index (CGI-S) and depressive symptoms using the Beck Depression Inventory (BDI-II).
Results
Lamotrigine ODT was found to be significantly more convenient to use than lamotrigine immediate-release (change in baseline TSQM convenience score: 23.3, n = 97, P < 0.001). The mean TSQM global satisfaction score was similar at baseline (76.3) and after treatment with lamotrigine ODT (76.0). There were no significant changes on CGI-S and BDI-II.
Conclusion
Subjects reported that lamotrigine ODT was significantly more convenient than lamotrigine immediate-release, while both formulations were associated with good satisfaction. Lamotrigine ODT may be a treatment option for patients who have difficulty swallowing medication.
Introduction
Lamotrigine is an anticonvulsant that is indicated for the maintenance treatment of bipolar I disorder and for several forms of epilepsy. Bipolar disorder affects 1.0% –1.6% of the adult population.Citation1–Citation4 Epilepsy affects 0.5%–1% of the population, with the highest incidence in young children and in the elderly.Citation5 While it is known that effective treatment for both bipolar disorder and for epilepsy may enhance patient quality of life and decrease the associated mortality and morbidity, for some patients beneficial medication effects may be reduced by suboptimal adherence.Citation6,Citation7 Poor adherence may be a particular issue in the elderly and in patients with mental illnesses.Citation8–Citation11 Factors that may improve adherence include convenience of dosing, ease of use, drug tolerability, and patient satisfaction.Citation12,Citation13 In contrast, factors that may reduce adherence include complex or multiple treatments, adverse effects, and poor tolerability.Citation9,Citation14 Difficulty swallowing is another barrier to medication adherence. Difficulty swallowing is noted to occur in nearly one third of psychiatric patients, nearly a quarter of primary care patients, and more often in women than in men.Citation10,Citation15
Alternative formulations of existing medications, such as orally disintegrating tablets (ODT) and long-acting injectable medications can potentially improve patient adherence with prescribed treatment regimens. By changing the characteristics of the medication to facilitate delivery, these alternative formulations have the potential to increase patient satisfaction with treatment and to improve adherence while maintaining efficacy and minimizing side effects.Citation16–Citation19
Lamotrigine is available in immediate-release or extended-release compressed tablets and immediate-release chewable tablets. Lamotrigine immediate-release compressed and chewable tablets have US Food and Drug Administration (FDA) indications to treat various types of epilepsy and bipolar I disorder, while the extended-release compressed tablet has an FDA indication only for the treatment of various types of epilepsy. An immediate-release ODT formulation of lamotrigine was developed to help treat patients who have difficulty swallowing the immediate-release tablets or prefer the convenience of an ODT. This formulation uses both taste-masking and oral disintegration technology, and has been shown to be bioequivalent to the lamotrigine immediate-release compressed tablet formulation (data on file, LBI108617. 2007, available from http://www.gsk-clinicalstudyregister.com). With the ODT formulation, unlike the compressed immediate-release tablet, no water is required for administration, and the tablet rapidly disintegrates without the need to swallow a whole tablet.
This paper describes a prospective, multicenter, open-label study in subjects with a mood disorder and who had difficulty or discomfort swallowing compressed lamotrigine tablets. Subjects were switched to lamotrigine ODT for 3 weeks, and dosing convenience and subject satisfaction were compared between the compressed lamotrigine tablet and lamotrigine ODT.
Materials and methods
Design
This prospective multicenter, open-label, three-week trial was conducted at 17 sites within the US. Participants were enrolled if they were diagnosed with a mood disorder and reported difficulty or discomfort swallowing currently prescribed compressed immediate-release lamotrigine tablets. Convenience and satisfaction (as measured by the Treatment Satisfaction Questionnaire for Medication [TSQM]) with current medication was evaluated by both subjects and companions/caregivers, and subjects were then switched from their current immediate-release compressed lamotrigine tablet formulation to a matching dose of lamotrigine ODT for 3 weeks. The primary efficacy/preference endpoint was change from baseline convenience score of the TSQM, version 1.4, at end of study or early withdrawal. Secondary outcomes measured the change from baseline for global satisfaction and effectiveness, change in self-reported depressive symptoms and overall psychopathology, self-reported adherence with medication, and adverse events/tolerability. Outcome measures were assessed at study baseline and at day 21 (end of study) time points. Individuals who withdrew from study prematurely (prior to day 21) had outcome assessments conducted at the time of study withdrawal.
Study population
Subjects were included in the study if they were ≥18 years of age and had a documented clinical diagnosis of mood disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV).
Other inclusion criteria required that the subjects had been on a stable dose of lamotrigine immediate-release standard tablets (≤600 mg/day) for at least 4 weeks and had self-reported difficulty or discomfort swallowing this formulation. Subjects also had to have a person willing to complete a companion/caregiver question. Female subjects had to have a negative urine pregnancy test at the time of screening and to be either of nonchildbearing potential or using medically accepted methods of birth control.
Subjects were to be excluded if they were currently participating in another clinical trial, had any clinically significant medical condition that would affect subject safety or outcome of the study, scored ≥ 1 on the suicidality item (item 9) of the Beck Depression Inventory Second Edition (BDI-II) at screening and/or baseline or had a Clinical Global Impression severity index (CGI-S) of ≥4 at screening (although baseline CGI-S scores of ≥4 were not excluded). Other exclusion criteria included diagnosis of other psychotic disorders, diagnosis of anorexia nervosa or bulimia, history of severe hepatobiliary disease, positive urine test for illicit drug use, and a history of substance abuse within the past 12 months.
Subjects were recruited by the research study teams at the 17 participating sites via a variety of methods, including review of patient records from the clinician’s own practices and self-referral in response to study flyers approved by the institutional review board. Difficulty or discomfort swallowing lamotrigine immediate-release was based upon subject self-report and the nature of the difficulty or discomfort was confirmed by the study team and documented in the clinical research assessments.
Measurements
The four subscales of the TSQM, a 14-item validated, self-reported questionnaire, were used to assess the convenience, global satisfaction, effectiveness, and side effects of both lamotrigine immediate-release (at baseline) and lamotrigine ODT (at the end of the study).Citation20 Each of four subscales of the TSQM has possible values from 0 to 100, with 100 being the maximum possible benefit. The convenience of the lamotrigine ODT formulation relative to that of the compressed tablet was quantified as the difference between the baseline score of the convenience subscale of the TSQM and the score when the subject was taking lamotrigine immediate-release. The global satisfaction, effectiveness, and side effects of lamotrigine ODT relative to lamotrigine immediate-release were assessed analogously.
Additional study questionnaires included subject preference, companion/caregiver preference (whether lamotrigine ODT or lamotrigine immediate-release is more convenient for the subject), two self-reported medication adherence questions (adapted from the Tablet Routine Questionnaire),Citation22 depressive symptom severity as assessed by the BDI-II, and global psychopathology (CGI-S). The BDI-II is a validated self-rated depression measure.Citation24 The CGI-S is a clinician-rated measure of global psychopathology which measures severity of mental illness on a seven-point scale from normal to extremely ill.Citation25
Subjects were queried as to their preference for taking the ODT or the standard tablet. Companions or caregivers were asked to observe which formulation was preferred by the subject, and the subjects who took both formulations were asked if they were more likely to adhere to taking the lamotrigine ODT formulation.
Given that any change in medication could potentially change clinical status, subjects were monitored for clinical worsening of severity of depressive symptoms using the BDI-II. Suicidality was assessed using item 9 (suicidality) from the BDI-II. If there was an occurrence of an adverse event which, in the investigator’s clinical judgment, was possibly suicidality-related, additional details were to be collected on a Possible Suicidality-Related Adverse Events (PSRAE) form.
The study protocol, any amendments, informed consent, and other information that required preapproval were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board. This study was conducted in accordance with good clinical practice and all applicable regulatory requirements including the guiding principles of the Declaration of Helsinki.
Statistical analysis
Study data were pooled across the participating centers for statistical analysis. The TSQM subscale scores and BDI-II scores were calculated using previously published methods.Citation20,Citation24 All statistical summaries and analyses were performed using SAS® version 8.2 (SAS Corporation, Cary, NC, USA). The size of the subject cohort (n = 98) allowed for ≥90% power to detect a difference from baseline of nine units in the global satisfaction score of the TSQM, assuming a standard deviation of 22.Citation20 This calculation was based on a two-sided paired t-test at a 5% significance level. It was assumed that if the global satisfaction subscale score was adequately powered, the convenience subscale (the primary endpoint) would be adequately powered as well.
The intention-to-treat population was comprised of subjects who received at least one dose of lamotrigine ODT and was used for all the analyses. Analysis of the primary endpoint was to be done via a two-sided paired t-test of the change from baseline in the convenience score from the TSQM at the end of the study or upon early withdrawal. A P value ≤ 0.05 was considered to be statistically significant. However, necessary assumptions for the t-test (ie, changes from baseline being normally distributed and symmetric) were not met. Therefore, for the convenience score, a nonparametric analysis (the sign test) was done. The sign test analysis was also performed for the global satisfaction, effectiveness, and side effect subscales. Change from baseline in CGI-S and BDI-II scores were analyzed using a paired t-test. The subject preference, companion/caregiver preference, and answers to the Tablet Routine Questionnaire were analyzed using a two-sided binomial test.
Results
Demographic and baseline characteristics
Of the 98 subjects enrolled, 97 received at least one dose of lamotrigine ODT. Eighty-nine (92%) subjects completed this study, and eight (8%) withdrew prematurely (four because of adverse events and four because of protocol deviation). Four subjects were withdrawn because of protocol deviations; each of these subjects entered the study at screen/baseline with a score of 1 on item 9 on the BDI-II, which was a violation of the exclusion criteria of the “suicidal thoughts or wishes” score limit of ≥1. At the time of withdrawal, three of these four subjects had a score of 0 and one subject maintained the baseline score of 1. A fifth subject had a score of 1 on BDI-II item 9 but was entered into the study and completed treatment.
Demographics and baseline clinical information are summarized in . Subjects were enrolled with DSM-IV clinical diagnoses of mood disorders that included major depressive disorder, bipolar I disorder (depressive, manic, mixed, unspecified, and not otherwise specified), bipolar II disorder, and mood disorder not otherwise specified. The intention-to-treat population was predominantly white (97%) and female (86%). The median age was 40.0 years; the mean (standard deviation) age was 41.0 ± 12.1 years. Baseline CGI-S scores ranged from 1 to 5, with the majority of subjects (95%) scoring between 1 (normal) and 3 (mildly ill). Four subjects entered the study who were moderately (score = 4) or markedly (score = 5) ill and all completed treatment.
Table 1 Demographic characteristics and baseline information on disease (ITT population)
Primary efficacy results
There was a mean increase of 23.3 ± 22.5 points (P < 0.001) in the primary outcome measure, ie, the TSQM convenience subscale (). According to the TSQM convenience subscale questions, 49% of subjects considered the lamotrigine ODT formulation to be extremely easy to use, whereas only 3% of subjects found lamotrigine immediate-release to be extremely easy to use (). A majority of subjects (54%) considered lamotrigine ODT to be extremely easy when planning use (versus 31% for lamotrigine immediate-release), and 44% thought that lamotrigine ODT was extremely convenient to take as instructed (versus 22% for lamotrigine immediate-release).
Figure 1 Comparison of response rate to question 9 (ease of use) on the TSQM questionnaire.
Abbreviations: TSQM, Treatment Satisfaction Questionnaire for Medication; ODT, orally disintegrating tablet; IR, immediate-release.
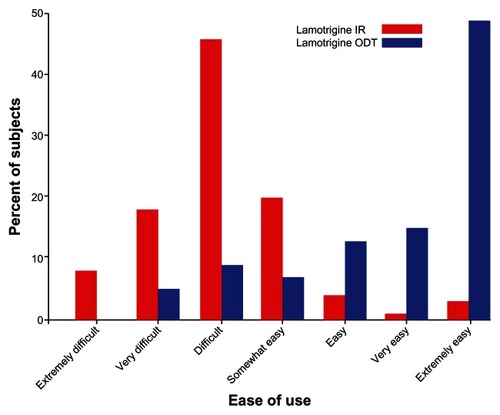
Table 2 TSQM subscale scores at baseline and endpoint
Secondary efficacy results
Secondary efficacy endpoints for the TSQM global patient satisfaction and effectiveness subscales are summarized in . Subscale scores were similar between lamotrigine immediate-release at baseline and the scores after lamotrigine ODT treatment for both global satisfaction (P = 0.060, sign test) and effectiveness (P = 0.434, sign test) subscales.
There were no significant differences between the baseline and end of trial scores, respectively, for CGI-S (2.2 and 2.3, P = 0.614) and BDI-II (13.6 and 11.9, P = 0.074). For the subject preference question, significantly more subjects (74%, P < 0.001) preferred taking lamotrigine ODT over lamotrigine immediate-release. In parallel with these results, the companion/caregiver preference question also demonstrated that significantly more caregivers considered lamotrigine ODT to be more convenient (81%; P < 0.001) than lamotrigine immediate-release. Of the 94 subjects who completed the Tablet Routine Questionnaire, a statistically significant proportion (n = 84, 89%, P < 0.001) reported that they missed fewer doses with ODT compared with those who felt that adherence was worse with ODT.
Lamotrigine ODT and adverse events
Subjects were treated with lamotrigine ODT for a period of 3 weeks at a mean prescribed daily dose of 261.3 mg. The majority of subjects (74%) took a single daily dose of lamotrigine ODT. The average number of treatment days for all subjects (n = 97) was 20.9.
Adverse events occurred in 29 of 97 (30%) of the subjects; no deaths or pregnancies occurred. The most common adverse events, with an incidence of ≥2%, were headache (5%), anxiety (3%), depression (3%), hypomania (3%), and nausea (2%). Adverse effects in 15 subjects (15%) were determined by investigators to be possibly related to the study drug.
Four subjects (4%) reported adverse events that led to premature withdrawal. Adverse effects leading to withdrawal were glossodynia (n = 1), gastrointestinal reflux (n = 1), hypomania (n = 1), and anxiety, chest pain, pain in the extremities, and restlessness (n = 1). A nonfatal serious adverse event (myocardial infarction) was reported in one subject. This subject was hospitalized and the serious adverse effect was resolved by the end of the study. This was not considered by the investigator to be drug-related and the subject remained on lamotrigine ODT until the end of the study.
Subject tolerability as assessed in the side effects subscale of the TSQM is summarized in . The difference in side effects score between baseline and end of study was 1.5, and was not significant (P = 0.458, sign test).
Suicidality as measured by item 9 on the BDI-II
There were no suicide attempts during the study. BDI-II item 9 scores were ≥1 in 13 subjects at various times throughout the study. Five subjects had a score of 1 at baseline, of whom four were withdrawn because of protocol deviation between 7 and 15 days. One of the five completed the study. Eight subjects (8/97, 8%) had baseline scores of 0 (“no thoughts of killing myself”) and later on reported scores of 1 (four subjects) or 2 (two subjects). Six of these eight subjects completed the study and two were withdrawn because of adverse events unrelated to suicidality. Extended suicidality information using the PSRAE questionnaire was collected for one subject who reached a score of 2 on BDI-II item 9.
Discussion
In this study of subjects with mood disorders and self-reported difficulty or discomfort in swallowing, lamotrigine ODT was significantly more convenient than the lamotrigine immediate-release compressed tablet formulation. While self-reported adherence appeared to be improved, subject satisfaction remained unchanged when subjects were switched from lamotrigine immediate-release to lamotrigine ODT. These data are in agreement with other studies that have shown increased preference for ODT therapy and demonstrated successful treatment of subjects with impaired ability or refusal to swallow.Citation26–Citation29
There were no significant changes in mood and overall severity of symptoms when switching subjects from compressed tablets to lamotrigine ODT. Approximately 8% of subjects had an increase from baseline suicidality ratings (scored 1 or 2 on the BDI-II item 9 suicidality question). This finding is not unexpected in a study of subjects with mood disorders.
While the package labeling for antiepileptic drugs, including lamotrigine, warns about an increase in suicidal thoughts or behavior while taking these drugs for any indication, it is not possible to conclude from this open-label study that excluded individuals at moderate risk for suicide, that switch to lamotrigine ODT would confer an increased risk in suicidality compared with lamotrigine immediate-release. Safety findings did not appear to suggest that medical risk is increased, and overall safety results are consistent with those of previous clinical trials of lamotrigine.Citation30–Citation33
Difficulty swallowing is common among individuals on pharmacologic treatments.Citation10,Citation11,Citation15,Citation34 Orally disintegrating tablets break apart rapidly on contact with saliva and remove the need to swallow tablets. This may explain the finding of perceived increased convenience with ODT compared with compressed tablet formulations.Citation34
This study had important limitations, including the relatively small number of subjects, the potential for recall bias associated with the pre-post comparison among individuals switched to a new drug formulation, and the fact that all participants were troubled enough by difficulty swallowing that they enrolled in a research study focused on this problem. It might be expected that people with mood disorders who choose to enroll in a research study are particularly motivated to engage in treatment and are likely to be adherent with medication in spite of burdens like difficulty swallowing. Most subjects entered the study with fairly high satisfaction scores, so there was not much room to improve. This could explain why there were no real changes in symptoms or overall treatment satisfaction after replacement with lamotrigine ODT. While the study was conducted at 17 sites and thus represented a geographically wide spectrum of individuals with mood disorders, the relative homogeneity of gender and ethnicity does not permit an assessment of how well lamotrigine ODT might be accepted by all individuals with mood disorders who have problems with pill-swallowing.
In conclusion, treatment of patients with chronic mood disorders is associated with challenges, such as overall tolerability, difficulty swallowing, an inconvenient dosing regimen, and side effects. These challenges may lead to lack of satisfaction with treatment and reduced adherence, and could potentially worsen outcomes and increase costs.Citation9 Individuals taking lamotrigine and who have difficulty swallowing pills may find it more acceptable to take ODT. The results of this study support the need for alternative formulations, such as ODT, that can make medication treatments more acceptable to patients with difficulty or discomfort swallowing.
Disclosure
GlaxoSmithKline funded this study. MS has received research grant funding from GlaxoSmithKline, AstraZeneca, Pfizer, Merck, and Ortho-McNeil Janssen. She is a consultant to Cognition Group, United BioSource Corporation (Bracket), and ProPhase and has received royalties from Springer Press, Johns Hopkins University Press, and Oxford Press. TRT, SE, and RM are employees of GlaxoSmithKline and own shares in the company. KN is a former employee of GSK, and owns shares in the company. Jane Saires from The WriteMedicine Inc, assisted with development of initial drafts of the manuscript in collaboration with all coauthors. The results of this study were presented at the American Epilepsy Society meeting in 2008.Citation35
References
- KesslerRCMcGonagleLAZhaoSLifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity SurveyArch Gen Psychiatry1994518198279933
- NarrowWERaeDSRobinsLNRegierDARevised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimatesArch Gen Psychiatry20025911512311825131
- GoodwinFKJamisonKRMaintenance medical treatmentGoodwinFKJamisonKRManic-Depressive IllnessNew York, NYOxford University Press1990
- RegierDANarrowWERaeDSManderscheidRWLockeBZGoodwinFKThe de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and servicesArch Gen Psychiatry19935085948427558
- HauserWAAnnegersJFKurlandLTIncidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984Epilepsia1993344534688504780
- RileyWVelliganDSajatovicMAdherence to psychiatric treatmentsPsychiatry2009208996
- VelliganDIWeidenPJSajatovicMAssessment of adherence problems in patient with serious and persistent mental illness: recommendations from the expert consensus guidelinesJ Psychiatr Pract201016343520098229
- CramerJARosenheckRCompliance with medication regimens for mental and physical disordersPsychiatr Serv1998491962019575004
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med200535348749716079372
- SchindlerJSKellyJHSwallowing disorders in the elderlyLaryngoscope200211258960212150508
- RobbinsJHamiltonJWLofGLKempsterGBOropharyngeal swallowing in normal adults of different agesGastroenterology19921038238291499933
- GoldDTSafiWTrinhHPatient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronateCurr Med Res Opin2006222383239117257452
- MasandPSNarasimhanMImproving adherence to antipsychotic pharmacotherapyCurr Clin Pharmacol20061475618666377
- PapakostasGITolerability of modern antidepressantsJ Clin Psychiatry200869Suppl E181318494538
- ReganJSowmanRWalshIPrevalence of dysphagia in acute and community mental health settingsDysphagia2006219510116763936
- NelsonJCHollanderSBBetzelJSmolenPMirtazapine orally disintegrating tablets in depressed nursing home residents 85 years of age and olderInt J Geriatr Psychiatry20062189890116955423
- SastrySVNyshadhamJRFixJARecent technological advances in oral drug delivery – a reviewPharm Sci Technol Today2000313814510754543
- GastparMMasiakMLatifMAFrazzingaroSMedoriRLombertieERSustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapineJ Psychopharmacol200519323816144784
- HanCLeeBHKimYSatisfaction of patients and caregivers with long-acting injectable risperidone and oral atypical antipsychoticsPrimary Care and Community Psychiatry200810119124
- AtkinsonMSinhaAHassSValidation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic diseaseHealth Qual Life Outcomes2004211314713317
- HarveyNSThe development and descriptive use of the Lithium Attitudes QuestionnaireJ Affect Disord1991222112191939930
- AdamsJScottJPredicting medication adherence in severe mental disordersActa Psychiatr Scand200010111912410706011
- ScottJPopeMNonadherence with mood stabilizers; prevalence and predictorsJ Clin Psychiatry20026338439012019661
- BeckATSteerRABrownGKManual for the Beck Depression Inventory-IISan Antonio, TXPsychological Corporation1996
- GuyWClinical Global ImpressionsECDEU Assessment Manual for Psychopharmacology, RevisedRockville, MDNational Institute of Mental Health1976
- Carnaby-MannGCraryMPill swallowing by adults with dysphagiaArch Otolaryngol Head Neck Surg200513197097516301368
- KeithSAdvances in psychotropic formulationsProg Neuropsychopharmacol Biol Psychiatry200630996100816678954
- ShenYCLeeMYLinCCChenCHOrally disintegrating olanzapine for the treatment of a manic patient with esophageal stricture plus chronic pharyngitisProg Neuropsychopharmacol Biol Psychiatry20073154154217029723
- Uko-EkpenyongGImproving medication adherence with orally disintegrating tabletsNursing200636202116951598
- CalabreseJRBowdenCLSachsGSAscherJAMonaghanERuddGDA double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depressionLamictal 602 Study GroupJ Clin Psychiatry199960798810084633
- MackayFJWiltonLVPearceGLFreemantleSNMannRDSafety of long-term lamotrigine in epilepsyEpilepsia1997388818869579888
- SchachterSLeppikIEMatsuoFLamotrigine: a six-month, placebo-controlled, safety and tolerance studyJ Epilepsy19958201209
- ChangKDKetterTASpecial issues in the treatment of paediatric bipolar disorderExpert Opin Pharmacother2001261362211336611
- WilkinsTGilliesRAThomasAMWagnerPJThe prevalence of dysphagia in primary care patients: a HamesNet Research Network studyJ Am Board Fam Med20072014415017341750
- SajatovicMEdwardsSManjunathRNanryKAn open-label trial measuring convenience, satisfaction, and adherence to an orally disintegrating tablet formulation of lamotriginePoster presented at the 62nd annual meeting of the American Epilepsy SocietySeattle, WADecember 5–9, 2008