Abstract
Background
The proper development and implementation of point-of-care (POC) diagnostics requires knowledge of the perceived requirements and barriers to their implementation. To determine the current requirements and perceived barriers to the introduction of POC diagnostics in the field of medical microbiology (MM)-POC a prospective online survey (TEMPOtest-QC) was established.
Methods and results
The TEMPOtest-QC survey was online between February 2011 and July 2012 and targeted the medical community, POC test diagnostic manufacturers, general practitioners, and the general public. In total, 293 individuals responded to the survey, including 91 (31%) medical microbiologists, 39 (13%) nonmedical microbiologists, 25 (9%) employees of POC test manufacturers, and 138 (47%) members of the general public. Responses were received from 18 different European countries, with the largest percentage of these living in The Netherlands (52%). The majority (>50%) of medical specialists regarded the development of MM-POC for blood culture and hospital acquired infections as “absolutely necessary”, but were much less favorable towards their use in the home environment. Significant differences in perceptions between medical specialists and the general public included the: (1) Effect on quality of patient care; (2) Ability to better monitor patients; (3) Home testing and the doctor-patient relationship; and (4) MM-POC interpretation. Only 34.7% of the general public is willing to pay more than a€10 ($13) for a single MM-POC test, with 85.5% preferring to purchase their MM-POC test from a pharmacy.
Conclusion
The requirements for the proper implementation of MM-POC were found to be generally similar between medical specialists and POC test kit manufacturers. The general public was much more favorable with respect to a perceived improvement in the quality of healthcare that these tests would bring to the hospital and home environment.
Introduction
Progress in the field of rapid and point-of-care (POC) diagnostics has been relatively slow, especially with respect to medical microbiology (MM) and the diagnosis of infectious diseases.Citation1 In particular, the culture of microorganisms such as bacteria and fungi on solid or liquid growth medium still remains the “gold standard” by which POC diagnostic tests in MM (MM-POC) are compared.Citation2 However, the identification of pathogens by culture, as well as the confirmation of their antimicrobial sensitivity profiles, are time consuming and require the skills of dedicated and trained medical laboratory personnel. Surrogate markers of infection are becoming available as rapid diagnostics for the determination of the presence or absence of an infection, including the measurement of C reactive protein and procalcitonin. Moreover, there is evidence to suggest that these surrogate markers may be useful in the detection of an infection in seriously ill patients in the nosocomial environment.Citation3–Citation6 Additionally, specific molecular and antibody based diagnostic methods are becoming more readily available in many medical disciplines, including virology, emergency medicine, etc.Citation7,Citation8 However, POC test (POCT) manufacturers have generally been slow in developing POC devices for the detection of infectious diseases, particularly for infections caused by bacterial and fungal pathogens, even though the development of such diagnostic devices would facilitate the rapid identification of these infectious agents (if present) and allow better targeted prescribing of suitable antimicrobial therapy.Citation8 Further, the largest trend in the field of rapid microbiological diagnostics currently involves the evaluation and validation of new technologies within the medical microbiology laboratory per se, particularly the evaluation and validation of nucleic acid amplification technologies and mass spectrophotometric methods. This means that there still remains a large untapped market for the introduction of POC microbiological diagnostics for such target audiences as professional nonmicrobiologist medical professionals, general practitioners and even patients (within their own homes). The use of POC devices by the above mentioned target groups would provide tangible benefits for all concerned, including more accurate, rapid, and cheaper diagnosis of microbiological infections, whilst providing accompanying advantages with respect to national healthcare budgets; eg, by facilitating earlier discharge from hospital and helping reduce the use of staff and equipment,Citation9–Citation11 and for example by shifting the burden of healthcare from healthcare providers to the actual patients themselves. Further, such market developments could provide significant advances with respect to limiting the ever growing threat of antimicrobial resistance and its impact on patient morbidity and mortality.Citation12,Citation13 In fact, the administration of suitable antibiotic therapy in the early onset of an infectious disease has been shown to improve the outcome of critically ill patients, with guidelines being available for the prescription of empirical antimicrobial therapy; ie, the prescribing of antimicrobial therapy before culture results become available.Citation14 Moreover, the inappropriate use of antibiotics is closely linked to the development of antibiotic resistant microorganisms,Citation15 and importantly, antibiotics are ineffective when used to treat viral infections. Further, the global increasing prevalence of antimicrobial resistance has not gone unnoticed by regulatory bodies such as the National Institute of Health and the World Health Organization.
The European Union is also facing up to its responsibilities with respect to infectious disease diagnosis and the provision of improved healthcare to European citizens, having funded several projects that have investigated the development and potential of rapid and POC infectious disease diagnostics. In particular, the “TEMPOtest-QC” project (www.TEMPOtest-QC.eu) was designed to help “fill the current gap between microbiological POC testing technologies and actual clinical need”, and provide a “toolkit” (biobank of specimens, bacterial isolates, facilities and expertise, etc)Citation16 to help small and medium-sized enterprises (SME) evaluate and validate new technologies during the development of MM-POC diagnostics. In this respect, one of the main tasks of the project was to help stakeholders (medical professionals, general practitioners, developers and manufacturers, and the general public) understand the perceptions and requirements for MM-POC diagnostics within hospital, general surgery, and home environments. In turn, the knowledge from this study will help the stakeholders to better understand the requirements and potential hurdles to the introduction of MM-POC devices into healthcare environments,Citation17,Citation18 be it the hospital laboratory, by the bedside, at the general practitioner’s surgery, or even within the patient’s own home.
Methods
Survey
As part of the TEMPOtest-QC project goals, an online survey was established in order to determine the views and perceived requirements of European citizens to infectious disease (bacterial/fungal) MM-POC testing (Supplemental Data Appendix S1). For some questions multiple answers were allowed, which means that for some questions the number of responses may be greater than the number of respondents. Target groups for the survey were: (1) hospital medical microbiologists, including medical microbiology laboratory technicians; (2) nonmedical microbiology specialists (hospital doctors and nurses); (3) POC test manufacturers (employees of POC test manufacturers); (4) the general public; and (5) general practitioners. The questionnaire remained open for online respondents from 08/16/2011 to 06/22/2012; a total of 10 months. These target groups were approached using: (1) an online website (www.tempotest-qc.eu); (2) a press release (“Join the fight against resistant bacteria!” by AlphaGalileo Foundation); (3) online social media, including YouTube (http://www.youtube.com/watch?v=t1Ni8VtnFuI), and relevant discussion groups on Linked-In (www.linkedin.com); (4) an advertisement in a national Dutch free newspaper (Metro); (5) an advertisement in a local internal hospital newspaper (Ziekenhuiskrant); as well as (6) flyers and poster presentations at multiple European scientific conferences (). The online and voluntary nature of the survey meant that we were unable to record the number of nonresponders. Moreover, the survey was designed so that all questions in the questionnaire had to be completed before the opinions of respondents could be successfully submitted.
Statistical analysis
The association between the categorical variables (answer and group) was assessed using the Chi-square test or Fisher’s exact test when appropriate. Univariate logistic regression and odds ratio (OR) with 95% confidence intervals were calculated and used to compare the opinions of medical specialists and POCT manufacturers and the general public. Two sided P-values of <0.05 were considered significant. All analyses were performed using the statistical software package R (free download from http://www.R-project.org/) version 2.15.1 (The R Foundation for Statistical Computing, Vienna, Austria).Citation19 In order to increase the sample size for statistical analysis, the survey results of the hospital medical microbiologists target group (n = 91) were merged with the answers of the nonmicrobiology specialists (n = 39) and the combined group (n = 130) was referred to as “medical specialists”.
Ethical statement
This study did not involve categorizing humans by race/ethnicity, age, disease/disabilities, religion, sex/gender, sexual orientation, or other socially constructed groupings. All results were collected anonymously using an online questionnaire which was available at www.TEMPOtest-QC.eu. The questionnaire asked volunteer respondents to provide their opinions on the development and potential hurdles to MM-POC testing. Although data was collected on country of employment, country of birth, and occupation, country of employment data was utilized to provide an indication of how successful the questionnaire “promotional campaign” was, and country of birth data was used to provide an indication of the geographical diversity of the respondent’s opinions. Both country of birth and country of employment data were not used in subsequent statistical analyses of the results. Full details regarding the TEMPOtest-QC study protocol and objectives of the study were available online on the same webpage as the link to the questionnaire, and there was no compulsion for interested parties to participate in the questionnaire. For all of these reasons ethical approval for the study was not requested from the host institution (Erasmus Medical Center, Rotterdam, The Netherlands).
Results
Respondent characteristics
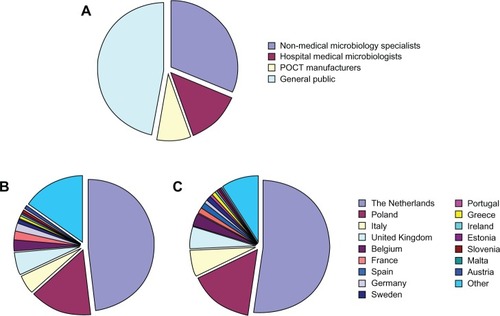
Overall, 293 individuals responded to the survey within the 10 months that the questionnaire was available online, with 91 (31%) participants responding as medical specialist (medical microbiologists), 39 (13%) as medical specialist (nonmedical microbiologists), 25 (9%) as employees of POC test manufacturers and 138 (47%) responding as members of the general public (). The largest percentage of these participants were born and living in The Netherlands, though responses were also received from respondents born or living in Poland, Italy, the United Kingdom, Belgium, and France among others ( and ). In total, responses were received from respondents born and/or currently working in 18 different European countries. Responses were also received from eleven general practitioners practicing in The Netherlands. However, these results have been omitted due to the low numbers of respondents in this target group. Of the medical microbiologist respondents, 24% reported to occasionally use a POC diagnostic device for the diagnosis of infectious diseases. The majority (68%) of medical microbiological point-of-care test (MM-POC) devices currently used were based on antibody related detection technologies.
POC infectious disease diagnostics and their specifications
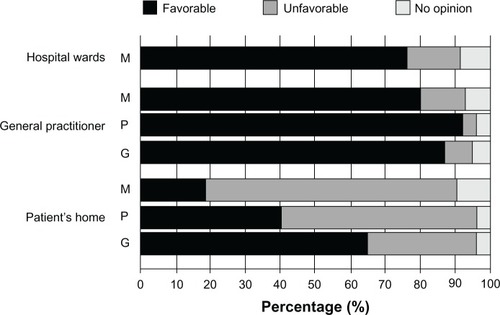
As previously mentioned, the availability of POC diagnostics for (bacterial/fungal) infectious disease testing currently lags behind the availability of POC diagnostics in other fields of medicine, such as clinical chemistry and virology. Therefore, one of the first questions asked of the medical specialist (medical microbiologists and nonmicrobiologists) target groups was designed to obtain their opinions on how important an infectious disease (bacterial/fungal) POC diagnostic device would be in helping diagnose various infectious disease-related conditions. The majority of respondents considered MM-POC devices most useful in the diagnosis of blood culture infections, followed by hospital acquired infections and respiratory infections (). There was less enthusiasm for the detection of oral and urinary tract infections. Interestingly, when asked for their opinions on the use of such MM-POC devices within various medical environments, there was a generally favorable response of medical specialists, POCT developers, and the general public for the use of MM-POC diagnostics in the hospital ward and general practitioners’ surgeries. However, there appeared a sharp division in opinions on the use of MM-POC diagnostics at the patient’s home (), with greater than 60% of the general public having a favorable opinion, compared to less than 20% of medical specialists.
Figure 3 Mean responses of medical specialists regarding the current perceived necessity for MM-POC in relationship to type of disease. The majority regarded the development of MM-POC against both hospital acquired and blood culture infections as “Absolutely Necessary”.
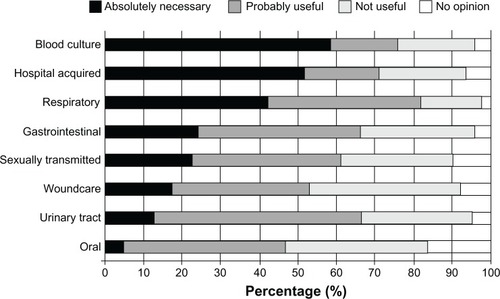
Figure 4 Opinions of target groups regarding the use of infectious disease (bacterial/fungal) POC devices in different environments. Medical specialists (hospital medical microbiologists and nonmedical microbiology specialists) (M), POCT manufacturers (P), and the general public (G) regarding the applicability of MM-POC in hospital wards, at the general practitioner or at the patient’s home.
When asked about their opinions regarding the actual specifications of bacterial/fungal POC diagnostics, medical specialists and POC manufacturers provided the responses shown in . Opinions on the most important factors with respect to an MM-POC device indicated that no single particular factor was considered most important in any of the target groups, though “reliability” and “time to diagnosis” tended to receive a large number of votes in both groups (both 60.8%). Interestingly, specificity scored high (60.0%) in the medical specialists group but was least favored by the POC manufacturer group (44.0%). Regarding the most important factors of an MM-POC device, no significant difference in the opinion of medical specialists and POCT manufacturers was observed (P = 0.54). Medical specialists opted for test specificity that could distinguish between bacteria, viruses, fungi and no infection (57.7%) and class of antibiotic resistance (72.3%). A similar result was obtained among the POCT manufacturers. Interestingly both target groups tended to agree that any such MM-POC test should possess a maximum “time-to-diagnosis” of 15 min–1 hour. In addition, according to the target groups, the maximum number of processing steps for an MM-POC device was considered as 2 to 3 processing steps, which on average, when combined with the maximum time-to-diagnosis results described above, would suggest that an MM-POC device should require an average processing time of approximately 15 minutes per step.
Table 1 Opinions of target groups regarding the most relevant specifications for bacterial or fungal point-of-care diagnostics
POC disease diagnosis at home and the quality of healthcare
shows the opinions of hospital personnel and the general public to questions relating to POC infectious disease diagnosis at home and the perceptions of these target groups regarding the quality of POC healthcare. The large majority of both medical specialists and the general public thought that the introduction of bacterial or fungal POC diagnostic testing to the general practitioner’s surgery, or within the patient’s own home, would affect the quality of patient care. However, the opinion on how patient care is affected significantly differed between the two target groups. The majority (72.5%) of the general public expects that the use of MM-POC will allow the doctor to better monitor their health compared to less than half (40.0%) of the medical specialists. The general public tended to be more positive regarding the effect of home POC testing on the doctor-patient relationship than the medical specialists. In fact, most medical specialists thought that bacterial or fungal home-testing POC technologies would indeed affect the doctor-patient relationship, with the majority of these (37/62) believing that any effect would result in a negative rather than a positive impact. Also the opinions on interpretation of the MM-POC test results differed between the two target groups. Whereas most (53.1%) of the medical specialists expect problems in the interpretation of POC test results, a significantly smaller percentage (37.0%) of the general public foresees problems.
Table 2 Opinions of target groups regarding the effect of point-of-care testing on the quality of health care
Infectious disease POC diagnostics and the general public
One of the questions in the survey was related to the effect of MM-POC on patient visits to the general practitioner. The majority of the general public respondents (46.4%) expect that the introduction of MM-POC tests will lead to a decrease in the number of visits they make to the general practitioner (). Additionally, if these MM-POC devices are to become available on the home testing market for the general public, information is required regarding the financial expectations of the general public towards the cost of such POC devices. The most favorable price for a single POC test currently lies between €5 to €10 (approximately $7.5–$12.5) per test (). Finally, 85.5% of the general public would be willing to purchase an MM-POC testing device at a pharmacy, rather than at a supermarket, at a drugstore, or over the internet ().
Table 3 Perceived effect of the introduction of bacterial or fungal point-of-care testing technologies according to the general public
Discussion
The worldwide introduction and frequent use of infectious disease POC diagnostic devices (including bacterial/fungal testing), will help reduce the global infectious disease burden and help reduce the continuing development and spread of antimicrobial resistances. The TEMPOtest-QC questionnaire revealed that the development of MM-POC devices for the diagnosis of blood borne, hospital acquired, and respiratory tract infections should have the highest priority for POCT manufacturers. Moreover we would recommend POCT manufacturers to target MM-POC devices which are able to distinguish between bacteria/fungi/viruses/ no infection, and/or class of antibiotic resistance, with a maximum “time-to-diagnosis” of 15–60 minutes, in which a maximum of 2–3 processing steps are involved. All factors regarding infectious disease POC listed in the survey were scored with an equal importance by both medical specialists and POCT manufacturers. Interestingly, the only exception was specificity; this factor scored highest in the medical specialists group but was least favored by the POCT manufacturer group. Perhaps this is a factor that needs to be re-considered by POCT developers and manufacturers?
Surprisingly, “costs” tended not to be a top priority for both groups, though this was possibly due to the fact that the medical specialists were considering the use of MM-POC devices within a medical environment (hospital or general practitioner’s surgery), rather than considering MM-POC devices intended for use in the much less mature “home-testing” environment. Perhaps encouraging for POC manufacturers, the price that the general public is willing to pay is above the current price for a single POC test as declared by POCT manufacturers (data not shown). Though of course this price may have been calculated using discounts available from high-volume sales and high-throughput sampling, as opposed to the single-use testing that will be required for consumers within their own home. The purchase of an MM-POC device is by the majority of the general public favored at a pharmacy. This may be related to “trust issues”, the general public perceiving medical products sold at pharmacies as being perhaps more “trustworthy” and of higher quality, as well as possibly valuing the expert advice available at pharmaceutical stores.
As a note of concern for POC manufacturers, respondents in the medical specialist and general public target groups were significantly different with respect to their opinions on whether there would be significant problems in interpreting the results of MM-POC diagnostic tests. In contrast, however, a clear majority of medical specialists thought that there would be significant problems in interpretation, a point of concern also raised in a previous study.Citation20 These results indicate that careful design, unambiguous result interpretation, and ready access to reliable and understandable diagnostic information is a prerequisite for building consumer confidence in the use of POC diagnostics.
The authors acknowledge there is a bias in the national reporting levels between the different European countries in this study. However, at the moment there is no evidence to suggest that the opinions of Dutch and Polish responders are different to those of other European citizens. The authors also acknowledge that the responses from POCT manufacturers may be biased towards the rapid introduction of MM-POC devices to the infectious diseases diagnostic market. In fact, the responses from POCT manufacturers were similar to the opinions of those of medical professionals, and it is the favorable opinion of the general public, which is currently the driving force behind the development and implementation of such MM-POC devices.
In conclusion, in this survey, no significant differences were observed between the opinions of medical specialists and POC manufacturers regarding the most relevant specifications for MM-POC devices. However, interesting differences were observed in the responses to the introduction of MM-POC devices and their effect on the quality of healthcare, with the general public tending to be more optimistic about the effect of MM-POC device implementation and subsequent improvement in the quality of healthcare compared to the medical specialists. All of the above mentioned issues need to be addressed, in order to successfully understand the current requirements and perceived hurdles to the implementation of MM-POC diagnostic devices into the medical, and possibly home environment.
Acknowledgments
This research was funded as part of the European Union’s Seventh Framework Programme FP7/2007–2013 (project TEMPOtest-QC, www.tempotest-qc.eu), under grant agreement no 241742. The authors would like to thank all respondents who took the time and effort to complete the online TEMPOtest-QC questionnaire. Their opinions are extremely valuable for the successful development and implementation of novel POC diagnostic devices. The authors would also like to thank B. Scharreman and F. Balvert of the Erasmus MC media group for their help and advice in making the TEMPOtest-QC video.
Supplemental data appendix S1
Nonmedical microbiology specialist (hospital doctors and nurses)
Hospital medical microbiologists including medical microbiology laboratory technicians
Point-of-care test manufacturers
General public
General practitioners
Disclosure
The authors report no conflicts of interest in this work.
References
- YagerPDomingoGJGerdesJPoint-of-care diagnostics for global healthAnnu Rev Biomed Eng20081010714418358075
- RiedelSCarrollKCBlood cultures: key elements for best practices and future directionsJ Infect Chemother201016530131620490596
- JeongSParkYChoYKimHSDiagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood cultureClin Chim Acta201241321–221731173622759977
- SakranJVMichettiCPSheridanMJThe utility of procalcitonin in critically ill trauma patientsJ Trauma Acute Care Surg2012732413418 discussion 41822846948
- NuutilaJPhagocytic Cell Surface Markers in Medical Microbiological Research and DiagnosisHaysJPvanLeeuwen WBThe Role of New Technologies in Medical Microbiological Research and DiagnosisDubaiBentham Science2012134150
- ClercOGreubGRoutine use of point-of-care tests: usefulness and application in clinical microbiologyClin Microbiol Infect20101681054106120670287
- AfshariASchrenzelJIevenMHarbarthSBench-to-bedside review: Rapid molecular diagnostics for bloodstream infection – a new frontier?Crit Care201216322222647543
- BissonnetteLBergeronMGDiagnosing infections – current and anticipated technologies for point-of-care diagnostics and home-based testingClin Microbiol Infect20101681044105320670286
- ReischlUSchmitzRPHIn search of a new gold standard – a technical review of molecular approaches to improve early diagnosis of microbial-induced sepsisEur Infect Dis2001514446
- MogensenCBBorchABrandslundIPoint of care technology or standard laboratory service in an emergency department: is there a difference in time to action? A randomised trialScand J Trauma Resusc Emerg Med2011194921906396
- PriceCPPoint of care testingBMJ200132272971285128811375233
- CharlesPGEarly diagnosis of lower respiratory tract infections (point-of-care tests)Curr Opin Pulm Med200814317618218427240
- CalsJWChappinFHHopstakenRMC-reactive protein point-of-care testing for lower respiratory tract infections: a qualitative evaluation of experiences by GPsFam Pract201027221221820022909
- SnydmanDREmpiric antibiotic selection strategies for healthcare-associated pneumonia, intra-abdominal infections, and catheter-associated bacteremiaJ Hosp Med20127S1S2S12
- OpalSMCalandraTAntibiotic usage and resistance: gaining or losing ground on infections in critically ill patients?JAMA2009302212367236819952325
- HoldenMJMadejRMMinorPKalmanLVMolecular diagnostics: harmonization through reference materials, documentary standards and proficiency testingExpert Rev Mol Diagn201111774175521902536
- PaiNPVadnaisCDenkingerCEngelNPaiMPoint-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countriesPLoS Med201299e100130622973183
- PalamountainKMBakerJCowanEPPerspectives on introduction and implementation of new point-of-care diagnostic testsJ Infect Dis2012205Suppl 2S181S19022402038
- R Core Team2012R: A language and environment for statistical computingR Foundation for Statistical ComputingVienna, Austria Available from: http://www.R-project.org/
- WoodFBrookes-HowellLHoodKA multi-country qualitative study of clinicians’ and patients’ views on point of care tests for lower respiratory tract infectionFam Pract201128666166921653924