Abstract
Purpose
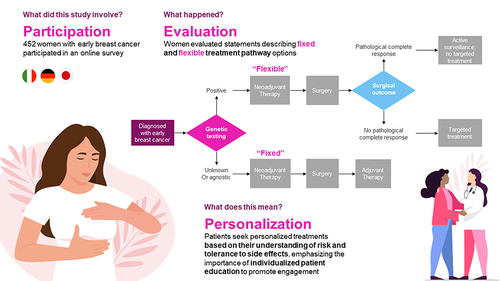
Patients with early breast cancer (eBC) are increasingly provided with different options, which may involve a sequence of different treatments and treatment modalities, and eligibility for certain adjuvant treatments depending upon pre-surgical and surgical outcomes. This study examined patient preferences around aspects of treatment decision-making in eBC.
Patients and Methods
A total of 452 patients with self-reported eBC in Germany (n=151), Italy (n=151), and Japan (n=150) completed an online survey about physician interactions and treatment side effects. The survey included best-worst scaling (BWS) to assess prioritization of 13 statements reflecting aspects of treatment decision-making. In a series of choice tasks, participants chose their most and least preferred options among subsets of 4 statements. Hierarchical Bayesian modeling was used to estimate BWS preference scores for each statement. BWS scores were based on the number of times a statement was chosen as most versus least preferred; scores total 100 for each patient.
Results
The most preferred aspects of treatment decision-making were “treatment aggressiveness matches personal risk” (mean BWS score = 13.49), “being told about what is coming” (13.18), deciding based on “own surgical outcome” (11.90), “avoiding unnecessary treatment” (10.35), and “involving in treatment decisions” (9.44). The least preferred aspects were “not being asked about treatment decisions along the way” (3.27) and “receiving the same treatment as other patients” (3.41). Patients in Japan preferred “being told about what is coming”, “deciding based on own surgical outcome”, “avoiding unnecessary treatment”, and being “involved in decisions” more than patients in Italy and Germany. Patients in Germany were more satisfied with their physician interactions and care, although their outcomes were not always better than those in Italy and Japan.
Conclusion
Patients value individualized treatment tailored to their risk of recurrence and tolerance of side effects, highlighting the need for focused patient education about options, to encourage their engagement.
Plain Language Summary
New treatment pathways based on promising biomarkers are being studied in early breast cancer. This study aimed to understand the importance that patients may place on different features describing how decisions are made along potential treatment pathways for early breast cancer. Participants in Italy, Germany, and Japan were asked to compare various aspects of treatment decision-making and choose those that were most and least important to them. Among the aspects tested, the top 4 were similar across countries: the desire to receive treatment with a level of aggressiveness that matches their individual prognosis, the need to receive adequate and timely information about their upcoming treatment, the need to tailor treatment decisions based on their individual surgery outcomes, and a desire to avoid overtreatment. Not being involved in treatment decisions was the least preferred of the aspects. Patients in Germany and Italy most valued the ability to tailor the aggressiveness of their treatment based on their individual risk of recurrence, whereas patients in Japan prioritized being knowledgeable and prepared for their treatment journey. The results from this study emphasize patients’ desire to be adequately informed about available treatment choices for early breast cancer, to avoid unnecessary treatments, and to be involved in treatment decisions.
Introduction
Breast cancer (BC) is estimated to be the most prevalent cancer worldwideCitation1 and is the second leading cause of cancer death among women.Citation1,Citation2 According to estimates from the World Health Organization, 2.3 million women were diagnosed with BC in 2020 globally, with 685,000 deaths.Citation1 An estimated 13.3% of all new cancer cases diagnosed in European Union-27 (EU-27) countries in 2020, and 28.7% of all new cancers in women, were BC.Citation3 A recent study on country-specific incidence and mortality data for BC found an increase in incidence in 16 countries, with the highest increase trend in developed countries such as Japan and Germany.Citation4
The treatment landscape for early BC (eBC) is rapidly expanding, with the characterization of promising biomarkers for prognosis and diagnosis.Citation5,Citation6 As immune checkpoint inhibitors, and the more recently approved poly (ADP-ribose) polymerase (PARP) inhibitors,Citation7 move into the early disease setting, the ways in which patients evaluate potential treatment pathways need to be understood. As treatment decisions can depend on the outcome of prior treatments, patients may be faced with choosing between a pre-determined treatment sequence where adjuvant treatment is administered regardless of the outcome of surgery, or a dynamic sequence where administration of adjuvant treatment depends on the outcome of surgery. In the dynamic sequence, the patient is spared the burden of adjuvant treatment if they are determined to be at low risk of recurrence after surgery (complete pathological response). In patients who are deemed eligible for treatment with immune checkpoint inhibitors and PARP inhibitors, the choice between a pre-determined or dynamic pathway is made before the outcome of surgery is known. We recently reported that patients with eBC may prefer a pathway allowing for a dynamic approach at the outset, with the ability to escalate treatment based on interim outcomes compared with a pre-determined approach where all treatments are pre-planned.Citation8 Here, we report characteristics of dynamic and pre-determined pathways that may influence patient preferences.
Shared decision-making is considered the gold standard of healthcare communication, especially in preference-sensitive care such as BC treatment, where patients may value the benefits and risks of treatment differently to physicians.Citation9 In the era of precision medicine, efforts are being made to improve patient outcomes by personalizing treatment regimens, primarily through a risk-stratified, biomarker-driven, de-escalated treatment approach.Citation10–12
Although there have been studies examining patient preferences in BC treatment, studies specifically focusing on the patient perspective on current pre-determined and dynamic treatment pathways and clinical management in eBC are lacking.Citation13,Citation14 Therefore, this observational study aimed to understand how patients may value the different aspects of potential treatment pathways that may be implemented in eBC. In addition, the study aimed to explore communication and shared decision-making between patients and physicians.
Materials and Methods
This observational study employed a cross-sectional, online survey of adult patients with eBC in Italy, Germany, and Japan. Patients were eligible if they had been diagnosed with stage I–IIIa eBC since 2014, with negative or unknown human epidermal growth factor receptor 2 (HER2) status, they underwent surgery for eBC (such as complete mastectomy or conservative surgery), their tumors had not extended to the chest wall, their tumors had not metastasized, they had (neo)adjuvant chemotherapy treatment, and they had not received HER2-targeted therapy. Such patients may be eligible for both immune checkpoint inhibitors or PARP inhibitor regimens, which have unique treatment factors that are the focus of this study; patients expressing HER2 are not eligible for these treatments and were excluded. Patients undergoing treatment with chemotherapy at the time of the study were excluded.
Study participants were recruited from Quarter 4 2021 to Quarter 1 2022 using both advocacy partnerships and survey panel databases of patients who had opted in to outreach. The survey panel database was Safe Harbor-certified and conformed to the privacy rules of the Market Research Society and the European Society for Opinion and Market Research, as well as adhering to the International Code on Market and Social Research. Data was collected via an online survey for all participants. Recruited patients were provided an email with a link to the survey. An online screener and informed consent statement were built into the survey. The study protocol was reviewed and received exemption status from Pearl IRB on August 4, 2020.
This research followed International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines on preference research.Citation15 To inform the content of the survey, qualitative interviews were performed with 8 patients with eBC from each participating country, in their respective languages, to identify key treatment-pathway attributes that may influence patients’ decision-making.Citation16 Based on the qualitative interview findings and the literature, the research team then collaboratively developed the survey instrument, considering the aspects of the therapeutic journey that were most important to patients, the extent to which treatment pathways differed on these factors, and the language employed by patients to describe these pathways.
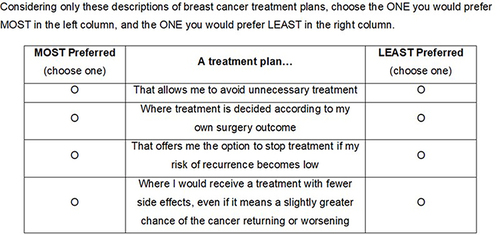
The survey included a best-worst scaling (BWS) exercise to examine how participants prioritized 13 different statements reflecting aspects of treatment decision-making that may be associated with a dynamic treatment pathway, a pre-determined pathway, or both (). The BWS exercise was an object case, designed to determine the relative importance of a set of attributes; in this study, the attributes reflected aspects of treatment decision-making.Citation17 The exercise included 13 tasks, each comprising 4 of the 13 attributes, and patients identified their most preferred and least preferred attributes. For each of the BWS tasks, the 13 attributes were mixed and matched into groups of 4 following a balanced incomplete block design. presents an example BWS item. Additional data collected included sociodemographic and clinical characteristics, as well as elements of interactions with physicians and perspectives about treatment experience.
Box 1 BWS Items
The final draft survey was translated into German, Italian, and Japanese, and cognitive interviews were conducted with 5 patients with eBC in each participating country. The interviews involved sharing the draft survey with the patient via a desktop-sharing platform and obtaining feedback on each of the items to ensure that they were easy to understand and were being interpreted as intended. The survey instrument was further refined and finalized based on the feedback.
Statistical Methods
The data were analyzed using descriptive statistics, and the results were reported in aggregate and compared at country level. Chi-square tests (categorical variables) and analyses of variance (ANOVA) tests (continuous variables) were used for comparisons between countries and subgroup analyses. Analyses were conducted using SPSS Version 25 (IBM Corporations, Armonk, NY, US) and Lighthouse Studio Version 9.12.1 (Sawtooth Software, Provo, UT, US).
With respect to the BWS exercise, the relative importance of each attribute was computed by fitting a Hierarchical Bayesian model to the BWS data and computing a parameter that behaved as a preference weight for each item. Specifically, each patient had a coefficient β for each item in the BWS. These coefficients were drawn from a multivariate normal distribution. The coefficients for a patient were used in a conditional logit model for the task, where the dependent variable was the choice made by the individual in response to the BWS, and the model estimated the probability of choosing a particular option as a function of the characteristics of the option. The estimated coefficients were transformed with the logit function and converted into selection probabilities, standardized to a 0–100% scale representing relative importance estimates.
Results
A total of 452 patients were recruited from Italy (n=151), Germany (n=151), and Japan (n=150). The mean age of the sample was 47.92 (standard deviation [SD] = 8.81; range = 19 to 78) years; the majority of the patients (76.3%) were in a committed relationship. More than three-quarters of the patients had achieved college (57.5%) or postgraduate (17.7%) education. At the time of the survey, 69.5% of the patients reported being currently employed, and 10.4% were on a temporary leave of absence. In comparison, 86.7% of the patients reported being employed prior to their BC diagnosis. Eligible patients lived mostly in an urban environment (73.9%), including 35.0% who resided in a major metropolitan area ().
Table 1 Patient Characteristics (A) Sociodemographic, (B) Employment Status, and (C) Place of Residence
presents the clinical characteristics of the sample. On average, the patients reported having “good” general health; half were of postmenopausal status. Patients had received their BC diagnosis an average of 2.95 years prior to completing the survey, although those in Japan had a longer duration since diagnosis than those in Germany and Italy (3.58 years vs 2.93 years and 2.34 years, respectively). About two-thirds (60.4%) of patients were diagnosed with stage II–IIIa BC, with the highest percentage of patients with stage II–IIIa BC being in Germany versus Japan and Italy (68.9% vs 62.7% and 49.7%, respectively). After the initial BC diagnosis, chemotherapy had been initiated within, on average, 2.14 (SD = 2.21) months; nearly three-quarters (73.7%) of patients in the overall sample had received chemotherapy in the adjuvant setting. Among patients in Japan, 68.7% had received hormone therapy or had been told by a physician that they were hormone positive/sensitive, compared with 46.7% in Germany and 40.7% in Italy. More than a third of patients with eBC included in this study reported receiving immunotherapy (37.2%), with 18.8% being received in the adjuvant setting and 10.6% in the neo-adjuvant setting. Receiving immunotherapy was reported by 9.3% of patients in Japan, compared with 55.0% of patients in Germany and 47.0% of patients in Italy. For most of the patients in Japan (96.7%), the cancer did not return after the last surgery; in Italy and Germany, 49.0% and 5.0% of patients, respectively, had cancer that did return but did not spread to the chest wall; we note that our study excluded patients with disease that recurred in later stages (stages IIIb–IV).
Table 2 Clinical Characteristics
Physician-Patient Interaction and Treatment Experience
Overall, patients were largely satisfied with their eBC treatment plan (mean score = 3.95 on a satisfaction scale of 1 to 5), with those in Germany expressing slightly higher satisfaction than those in Italy and Japan (4.07 vs 3.86 and 3.91, respectively; p=0.035). Overall, about two-thirds of patients (60.2%) were offered >1 treatment plan, although those in Germany were more likely to be offered multiple options compared with those in Italy and Japan (84.1% vs 51.0% and 45.3%, respectively; p<0.001). Patients in Germany were also more likely to think that their doctor considered their preferences regarding treatment options than patients in Italy and Japan (86.9% vs 64.9% and 78.7%, respectively; p<0.001).
Patients generally agreed that they were adequately informed about their physicians’ rationales underpinning treatment recommendations and that they were given enough time to understand treatment recommendations, with no significant differences observed across countries. However, patients in Germany were more likely to feel confident that the treatment they received was right for them, compared with patients in Italy and Japan (4.13 vs 4.11 and 3.81, respectively; p<0.001).
Overall, the most commonly self-reported side effects related to eBC treatment were fatigue (ranging from 70.9% in Germany to 77.3% in Japan; p=0.426), followed by nausea (ranging from 62.0% in Japan to 74.2% in Italy; p=0.062), and neuropathy (ranging from 37.8% in Germany to 48.7% in Japan; p=0.124). Among patients who experienced these side effects, they were either mild or moderate. On a scale of 1 to 5 (1 = extremely bad; 5 = not at all bad), the overall mean severity of nausea was 3.17 (SD = 0.78). The severity of nausea was highest among patients in Japan (mean = 2.94; SD = 0.85) and lowest among patients in Germany (mean = 3.35; SD = 0.85) (p<0.001). Patients in Japan also reported the most severe fatigue (mean = 2.72; SD = 0.94), while patients in Italy reported the least severe fatigue (mean = 3.31; SD = 0.69) (both p<0.001). Patients in Italy and Germany were more likely to be hospitalized due to side effects of treatment, relative to patients in Japan (22.5% and 18.5% vs 10.0%, respectively; p=0.013) ().
Table 3 Physician-Patient Discussions and Treatment Experience
Prioritization of Treatment Pathway Features
presents the BWS scores for the overall population. Except for being able to stop treatment if the risk of recurrence became low (p=0.751) and having sufficient time to make treatment decisions (p=0.239), the preferences for the treatment pathway features differed significantly among countries (all p<0.02) ().
Figure 2 Preferences for treatment pathway features BWS scores for the overall sample.
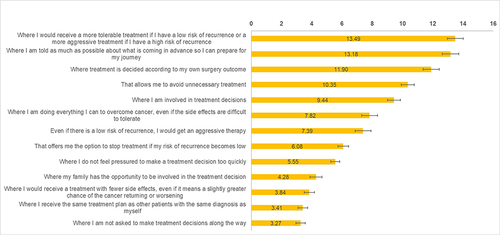
Figure 3 Preferences for treatment pathway features BWS scores by country.
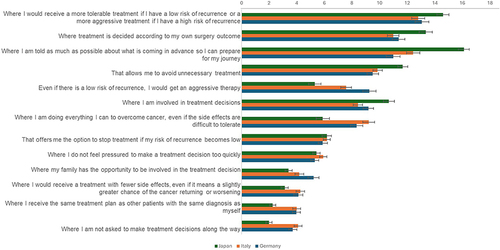
In comparison to the other countries, patients in Japan placed higher priority on being knowledgeable and prepared for their therapeutic journey and avoiding unnecessary treatment. Patients in all 3 countries valued tailoring treatment to their individual disease characteristics. While patients in Germany and Italy most valued the ability to tailor the aggressiveness of their treatment based on the individual risk of relapse compared with any other aspect of treatment, patients in Japan assigned higher importance to the ability to tailor treatment decisions based on individual surgery outcomes (Italy = 10.99 vs Germany = 11.38 vs Japan = 13.35). Consistent with this observation, patients in Japan were significantly less likely to accept overtreatment, despite the side effects (Italy = 9.22 vs Germany = 8.34 vs Japan = 5.89), and regardless of the risk of relapse (Italy = 7.58 vs Germany = 9.27 vs Japan = 5.31) (both p<0.001). However, patients in Japan were less inclined to even slightly compromise the treatment’s efficacy to reduce its side effects (Italy = 4.27 vs Germany = 4.12 vs Japan = 3.13; p=0.014) and were more reluctant to consider a standard therapeutic path offered to other patients with similar diagnosis (Italy = 4.00 vs Germany = 3.98 vs Japan = 2.25; p<0.001). Although family involvement in treatment decisions was not as important for patients in Japan (Italy = 4.19 vs Germany = 5.22 vs Japan = 3.41), they were significantly more reluctant to be excluded from treatment decisions (Italy = 4.10 vs Germany = 3.72 vs Japan = 1.99; both p<0.001) ().
Discussion
This cross-sectional, online survey examined the treatment preferences associated with systemic therapeutic pathways in the adjuvant setting in adult patients with early-stage (I–IIIa), HER2-negative BC in Italy, Germany, and Japan. According to the BWS survey findings, patients in Germany and Italy most valued the ability to tailor the aggressiveness of their treatment based on individual prognosis, whereas patients in Japan prioritized being knowledgeable and prepared for their therapeutic journey. Results highlighted the most influential factors that drive treatment decisions for patients with eBC, which may facilitate improved physician-patient communication and shared decision-making.
Shared decision-making is considered the preferred approach for preference-sensitive decisions, and is a high priority among cancer patients.Citation18–20 Elicitation of patient preferences and values is reported as a key component of shared decision-making.Citation18,Citation21 Prior studies have evaluated treatment preferences for surgery and/or chemotherapy, and systemic therapy regimens, in advanced/metastatic BC.Citation14,Citation22,Citation23 Various studies from European countries have also examined patient preferences and treatment adherence for advanced/metastatic BC.Citation13,Citation24,Citation25 However, such data from patients with eBC are limited in the adjuvant setting. Not being involved in treatment decisions was deemed the least preferred scenario in this study. This is in line with findings from recent studies, where the majority of cancer patients wanted to be involved in treatment decisions.Citation20 In our study, having sufficient time to participate in the treatment decision was viewed similarly across all countries as a lower priority compared with participation alone; this finding is expected as shared decision-making is considered a keystone in the achievement of sustainable high-quality cancer care in developed countries.Citation26 Shared decision-making conversations should take place without interruptions, disruptions, or hurry.Citation27 Among the therapeutic scenarios examined within the BWS experiment, the top 4 features, consistently rated as the most preferred across countries, included the desire to receive treatment with a level of intensity proportional to the individual prognosis, as well as the need to be informed adequately, and in a timely manner, about upcoming treatment, to tailor treatment decisions based on individual surgery outcomes, and to avoid overtreatment. Being able to stop treatment if the risk of recurrence became low was valued similarly across countries. To our knowledge, our study is unique in addressing this topic from the perspective of patients with BC. However, other priorities significantly differed between regional cohorts. For instance, while patients in Germany and Italy most valued the ability to tailor the aggressiveness of their treatment based on individual prognosis, this was deemed the second most preferred feature by patients in Japan, who prioritized being knowledgeable and prepared for the therapeutic journey. A recent European study conducted among patients with BC reported being well informed about the treatment process as one of the most important aspects of the optimal BC care pathway.Citation28 Altogether, these findings on heterogeneity in preferences advance the supposition that one therapeutic strategy does not fit all, highlighting the importance of an individualized approach for the optimal BC care pathway.Citation28,Citation29
The role of the patient in treatment decision-making, alongside their information needs, have been increasingly recognized among patients with BC in Japan.Citation30,Citation31 In this study, patients in Japan exhibited a pragmatic approach to treatment management. Although reluctant to receive unnecessary treatment, they were willing to accept a therapeutic path with a lower tolerability profile if it was justified by a better chance of success. Additionally, patients in Japan valued a treatment tailored to their individual needs and sought to actively participate in treatment management by making informed decisions. A closer examination of the treatment experience in Japan revealed that more than half of the patients in this group had not been presented with multiple treatment options, thereby limiting the information with which to participate in the treatment decision. As well, patients in Japan were less likely to agree that they had been given an appropriate amount of time to understand treatment recommendations, or to express their confidence in treatment decisions, compared with their German or Italian counterparts. The low rate of recurrence among patients in Japan in our study may have contributed to their greater preference for tailoring their treatment regimen based on surgical outcomes and avoiding unnecessary, overly aggressive treatments. For example, patients with a positive treatment experience with respect to efficacy may have less anxiety about disease progression and feel comfortable with as minimal treatment as possible. Patients in Italy and Germany, who had higher recurrence rates, may feel more anxiety about progression and be inclined to treat more aggressively. Taken together, these factors may have played a role in shaping the patients’ view regarding the ideal characteristics of a hypothetical treatment pathway. Notably, as women with BC recurring as metastatic disease were excluded from our study, our results do not speak to cancer recurrence rates overall in each country; our study used a convenience sample that is not generalizable to the BC cancer population in each country. Indeed, years since diagnosis and stage data presented in do not explain a lower recurrence rate for the Japanese patients in our study. Variation in local recurrence rates could be explained by the proportion of patients in each country with mutations in BRCA1 and/or BRCA2 genes and thus with more aggressive disease, or the proportion having breast-conserving surgery; however, we did not collect this data.
Patients in Germany were more satisfied with their physician interactions and care, even though their outcomes were not always better than those in Italy and Japan. Patients in Germany were more likely to be presented with >1 treatment option, and more likely to perceive that their physicians considered their preferences when selecting treatment. As shown in the previous research,Citation32 patients who sought information from their doctors reported greater shared decision-making, which was associated with greater short-term satisfaction. The current study indicates that discussion of multiple treatment options, and empathy, may be elements of shared decision-making that lead to greater patient satisfaction.
Altogether, results from this study underscore a strong preference of patients wanting to be adequately informed about available therapeutic choices for eBC and to be involved in decisions regarding their treatment. Cancer treatment guidelines are frequently updated, which may present a challenge for both physicians and their patients.Citation33,Citation34 However, patient preferences can support the selection and tailoring of treatment options in combination with clinical guidelines.Citation13 Furthermore, prior research noted that when treatment decisions are aligned with patient preferences, a higher level of treatment satisfaction may be achieved.Citation35,Citation36 Thus, for effective disease management, increased communication is critical for both clinicians and their patients with eBC. Results from this study contribute to the existing literature by providing additional insight into the multifaceted aspects that impact treatment decisions for patients with eBC.
The findings of this study should be interpreted within the context of relevant study limitations. The self-reported nature of the patient survey is associated with potential corresponding biases, such as inaccurate recall and false reporting (whether intentional or unintentional). For example, diagnoses are not confirmed by a physician. However, the consumer panel does take measures to minimize intentionally false reporting. Results from this study may not reflect real-world treatment decisions, which could be influenced by other factors not captured in the survey, such as physician recommendations. Another limitation was the nature of hypothetical situations that may not reflect the actual choices patients make. These choices are intended to simulate possible clinical decisions, but do not have the same clinical or emotional consequences of actual decisions. Thus, there may have been differences between stated and actual choices. Given that the focus of this research was to compare findings across countries, subgroup analyses of selected demographic or clinical characteristics were not evaluated; if performed, they should likely be compared within country, and this would result in small sample sizes.
Conclusion
Findings from this observational study bring fresh insight into the strong desire of patients to tailor therapeutic interventions to individual prognosis, to avoid unnecessary treatments, as well as a need for patients to be adequately informed about the available treatment choices for eBC, and to be involved in treatment management. As more therapeutic agents move into the early disease setting, healthcare stakeholders need to engage with patients sooner and with greater urgency, to ensure that treatment decisions are informed by their preferences, and to drive better outcomes and treatment satisfaction. Findings emphasize the importance of shared decision-making between physicians and patients, and the need for research to further understand how to empower patients in treatment decision-making.
Abbreviations
ANOVA, analyses of variance; BC, breast cancer; BWS, best-worst scaling; eBC, early breast cancer; EU-27, European Union-27; HER2, human epidermal growth factor receptor 2; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; IV, intravenous; PARP, poly (ADP-ribose) polymerase; SD, standard deviation.
Ethics Approval and Informed Consent
The authors state that this study received exemption status from full or expedited ethical review by Pearl IRB, that they have followed the principles outlined in the Declaration of Helsinki for all human or experimental investigations, and that informed consent was obtained electronically from the patients involved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
KB, EM, and DM are employees and/or stockholders of Cerner Enviza, an Oracle company, which provides consulting services to AstraZeneca. At the time of the study, XG was an employee and stockholder of Cerner Enviza. EF and SMcC are employees and/or stockholders of AstraZeneca. At the time of the study, SM was an employee and stockholder of AstraZeneca.
Acknowledgments
Medical writing support was provided by Shalini Vasantha, Ph.D., and Ramu Periyasamy, Ph.D., from Indegene Pvt. Ltd, Bangalore, India, on behalf of Cerner Enviza. The data have been presented previously as a poster at the 18th St.Gallen International Breast Cancer Conference, February 28, 2023 - March 1, 2023, Vienna, Austria.
Data Sharing Statement
Data underlying the findings described in this manuscript cannot be shared due to the content of the Informed Consent forms signed by the patients. Please visit our Disclosure Commitment page for guidance on the AstraZeneca Data Sharing Policy: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Additional information
Funding
References
- World Health Organization. Breast cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer. Accessed October 4, 2023.
- American Cancer Society. Key statistics for breast cancer. Available from: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer. Accessed October 4, 2023.
- European Cancer Information System (ECIS). Breast cancer burden in EU-27. Available from: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/2020-cancer-incidence-and-mortality-eu-27-countries-2020-07-22_en#:~:text=Over%20355%2C000%20women%20in%20the%20EU-27%20are%20estimated,prostate%20%28336%2C000%2C%2012.5%25%29%20and%20lung%20cancer%20%28318%2C000%2C%2011.9%25%29. Accessed July 11, 2024.
- Huang J, Chan PS, Lok V, et al. Global incidence and mortality of breast cancer: a trend analysis. Aging. 2021;13(4):5748–5803. doi:10.18632/aging.202502
- Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2020;11:632079. doi:10.3389/fphar.2020.632079
- Andre F, Ismaila N, Allison KH, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(16):1816–1837. doi:10.1200/jco.22.00069
- Pelosci A. Adjuvant olaparib approved in European Union for BRCA1/2-positive, HER2-negative high-risk early breast cancer. Cancer Network. Available from: https://www.cancernetwork.com/view/adjuvant-olaparib-approved-in-european-union-for-brca1-2-positive-her2-negative-high-risk-early-breast-cancer. Accessed October 4, 2023.
- Beusterien K, Will O, Flood E, McCutcheon S, Mackie DS, Mokiou S. A novel approach to computing preference estimates for different treatment pathways: an application in oncology. Patient. 2024;17(4):397–406 doi:10.1007/s40271-024-00680-z.
- Mahmoodi N, Jones GL, Muskett T, Sargeant S. Exploring shared decision making in breast cancer care: a case-based conversation analytic approach. Commun Med. 2020;16(1):40–45. doi:10.1558/cam.36775
- Goutsouliak K, Veeraraghavan J, Sethunath V, et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat Rev Clin Oncol. 2020;17(4):233–250. doi:10.1038/s41571-019-0299-9
- Aristei C, Perrucci E, Alì E, et al. Personalization in modern radiation oncology: methods, results and pitfalls. Personalized interventions and breast cancer. Front Oncol. 2021;11:616042. doi:10.3389/fonc.2021.616042
- Sakai H, Umeda M, Okuyama H, Nakamura S. Differences in perception of breast cancer treatment between patients, physicians, and nurses and unmet information needs in Japan. Support Care Cancer. 2020;28(5):2331–2338. doi:10.1007/s00520-019-05029-z
- Stamuli E, Corry S, Ross D, Konstantopoulou T. Patient preferences for breast cancer treatments: a discrete choice experiment in France, Ireland, Poland and Spain. Future Oncol. 2022;18(9):1115–1132. doi:10.2217/fon-2021-0635
- Guerra RL, Castaneda L, de Albuquerque RCR, et al. Patient preferences for breast cancer treatment interventions: a systematic review of discrete choice experiments. Patient. 2019;12(6):559–569. doi:10.1007/s40271-019-00375-w
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
- Gennari A, Shimizu C, McCutcheon S, et al. Factors influencing patient treatment decision-making in early breast cancer. Qual Life Res. 2021;30(Suppl. 1):S81 (abstract 1006). doi:10.1007/s11136-021-02976-1
- Flynn TN, Louviere JJ, Peters TJ, Coast J. Best--worst scaling: what it can do for health care research and how to do it. J Health Econ. 2007;26(1):171–189. doi:10.1016/j.jhealeco.2006.04.002
- Williams CP, Miller-Sonet E, Nipp RD, Kamal AH, Love S, Rocque GB. Importance of quality-of-life priorities and preferences surrounding treatment decision making in patients with cancer and oncology clinicians. Cancer. 2020;126(15):3534–3541. doi:10.1002/cncr.32961
- Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172–1179. doi:10.1016/j.pec.2015.06.022
- Josfeld L, Keinki C, Pammer C, Zomorodbakhsch B, Hübner J. Cancer patients’ perspective on shared decision-making and decision aids in oncology. J Cancer Res Clin Oncol. 2021;147(6):1725–1732. doi:10.1007/s00432-021-03579-6
- Rake EA, Box ICH, Dreesens D, et al. Bringing personal perspective elicitation to the heart of shared decision-making: a scoping review. Patient Educ Couns. 2022;105(9):2860–2870. doi:10.1016/j.pec.2022.05.009
- Amin S, Tolaney SM, Cambron-Mellott MJ, et al. Benefit-risk trade-offs in treatment choice in advanced HER2 negative breast cancer: patient and oncologist perspectives. Future Oncol. 2022;18(16):1927–1941. doi:10.2217/fon-2021-0761
- Maculaitis MC, Liu X, Will O, et al. Oncologist and patient preferences for attributes of CDK4/6 inhibitor regimens for the treatment of advanced/metastatic HR positive/HER2 negative breast cancer: discrete choice experiment and best–worst scaling. Patient Prefer Adherence. 2020;14:2201–2214. doi:10.2147/ppa.S254934
- Konstantopoulou T, Stamuli E, Ross D, Pacheco R. Patient preferences for breast cancer treatments: a discrete choice experiment from four European countries. Ann Oncol. 2019;30(Suppl. 5):v135 (abstract 370P). doi:10.1093/annonc/mdz242.065
- Reinisch M, Marschner N, Otto T, Korfel A, Stoffregen C, Wöckel A. Patient preferences: results of a German adaptive choice-based conjoint analysis (market research study sponsored by Eli Lilly and Company) in patients on palliative treatment for advanced breast cancer. Breast Care. 2021;16(5):491–499. doi:10.1159/000513139
- Maes-Carballo M, Muñoz-Núñez I, Martín-Díaz M, Mignini L, Bueno-Cavanillas A, Khan KS. Shared decision making in breast cancer treatment guidelines: development of a quality assessment tool and a systematic review. Health Expect. 2020;23(5):1045–1064. doi:10.1111/hex.13112
- Shickh S, Leventakos K, Lewis MA, Bombard Y, Montori VM. Shared decision making in the care of patients with cancer. Am Soc Clin Oncol Educ Book. 2023;43:e389516. doi:10.1200/EDBK_389516
- Metsälä E, Kivistik S, Straume K, Marmy L, Jorge JAP, Strom B. Breast cancer patients’ experiences on their individual care pathway: a qualitative study. Radiography. 2022;28(3):697–703. doi:10.1016/j.radi.2022.06.002
- Kimman ML, Dellaert BG, Boersma LJ, Lambin P, Dirksen CD. Follow-up after treatment for breast cancer: one strategy fits all? An investigation of patient preferences using a discrete choice experiment. Acta Oncol. 2010;49(3):328–337. doi:10.3109/02841860903536002
- Azuma K, Kawaguchi T, Yamaguchi T, et al. Development of Japanese versions of the Control Preferences Scale and Information Needs Questionnaire: role of decision-making and information needs for Japanese breast cancer patients. Patient Prefer Adherence. 2021;15:1017–1026. doi:10.2147/ppa.S295383
- Shimizu C, Sakata Y, Sakai R, Ikezawa H, Uetaki Y, Matsuoka T. Pharmacotherapy decision-making among patients with breast cancer in Japan: results of an online survey. Breast Cancer. 2019;26(6):799–807. doi:10.1007/s12282-019-00986-z
- Mandelblatt J, Kreling B, Figeuriedo M, Feng S. What is the impact of shared decision making on treatment and outcomes for older women with breast cancer? J Clin Oncol. 2006;24(30):4908–4913. doi:10.1200/jco.2006.07.1159
- Rauh S, Arnold D, Braga S, et al. Challenge of implementing clinical practice guidelines. Getting ESMO’s guidelines even closer to the bedside: introducing the ESMO Practising Oncologists’ checklists and knowledge and practice questions. ESMO Open. 2018;3(5):e000385. doi:10.1136/esmoopen-2018-000385
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8):1194–1220. doi:10.1093/annonc/mdz173
- Ruhnke GW, Tak HJ, Meltzer DO. Association of preferences for participation in decision-making with care satisfaction among hospitalized patients. JAMA Netw Open. 2020;3(10):e2018766. doi:10.1001/jamanetworkopen.2020.18766
- Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506–517. doi:10.1016/j.cpr.2014.06.002